Abstract
This review explores the multifaceted role that iron has in cancer biology. Epidemiological studies have demonstrated an association between excess iron and increased cancer incidence and risk, while experimental studies have implicated iron in cancer initiation, tumor growth, and metastasis. The roles of iron in proliferation, metabolism, and metastasis underpin the association of iron with tumor growth and progression. Cancer cells exhibit an iron-seeking phenotype achieved through dysregulation of iron metabolic proteins. These changes are mediated, at least in part, by oncogenes and tumor suppressors. The dependence of cancer cells on iron has implications in a number of cell death pathways, including ferroptosis, an iron-dependent form of cell death. Uniquely, both iron excess and iron depletion can be utilized in anticancer therapies. Investigating the efficacy of these therapeutic approaches is an area of active research that promises substantial clinical impact.
Keywords: iron, cancer, cell death, carcinogenesis
1. IRON AS A METAL: AN ESSENTIAL BUT POTENTIALLY TOXIC NUTRIENT
Trace elements are micronutrients that are necessary to sustain life. Iron is an important trace element required for the activity of proteins and enzymes that carry out a broad range of functions. For example, iron-containing proteins are required for cell respiration (e.g., cytochrome c oxidase, cytochromes, ferredoxins, and Rieske proteins), oxygen sensing, oxygen transport, oxygen metabolism (e.g., hemoglobin, peroxidase and catalase, and cytochrome P450), energy metabolism (e.g., citrate synthase, aconitase, isocitrate dehydrogenase, and succinate dehydrogenase), DNA synthesis and repair (e.g., ribonucleotide reductase and DNA helicases), and signaling (e.g., nitric oxide synthase, hydroxylases, and oxidoreductases) (39).
The biological versatility of iron results directly from its capacity to undergo oxidation–reduction (redox) reactions. A redox reaction involves the transfer of electrons between two chemical species. As a transition metal, iron possesses unpaired electrons that make it a versatile participant in redox reactions. Iron can exhibit a wide range of oxidation states, from −2 to +6, although iron is primarily limited to ferrous (+2), ferric (+3), and ferryl (+4) states in biological systems. Flexibility in oxidation states permits iron to be bound by a large variety of ligands. These attributes, combined with the abundance of iron on Earth, have led to the widespread use of iron in biological systems.
The same chemical properties, however, also enable iron to participate in harmful, unwanted reactions, such as the generation of reactive oxygen species (ROS). The Fenton reaction is a chemical reaction between ferrous iron and hydrogen peroxide that produces a hydroxyl radical:
| 1. |
In the presence of superoxide, a by-product of cellular respiration, ferric iron generated by the Fenton reaction can be reduced back to ferrous iron, which is then capable of undergoing further Fenton reactions. This cycle, known as the Haber–Weiss reaction, can produce large amounts of hydroxyl radicals, one of the most reactive ROS:
| 2. |
with the net Haber–Weiss reaction as
| 3. |
Iron can also catalyze the formation of lipid ROS:
| 4. |
ROS generation is intimately linked to carcinogenesis through the induction of damage to cellular components, including lipids, proteins, and principally DNA (161).
2. EPIDEMIOLOGICAL STUDIES OF IRON AND CANCER
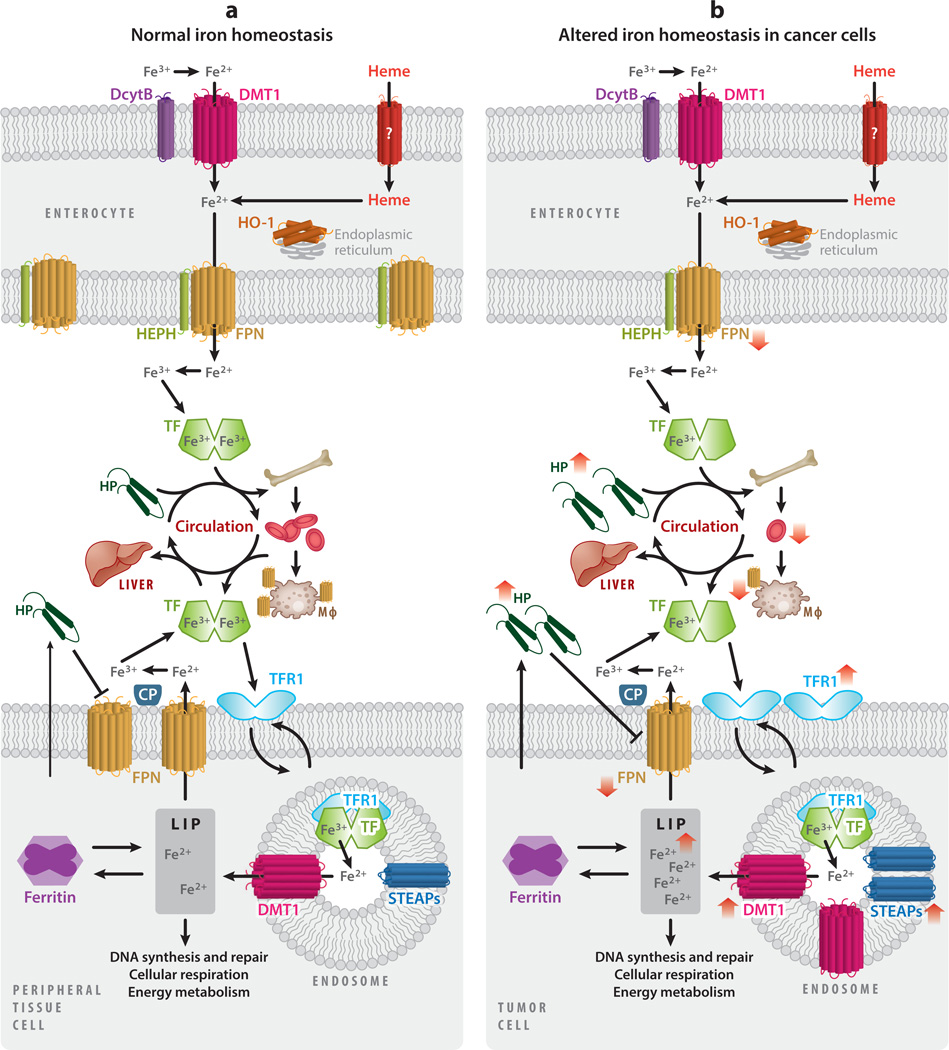
Numerous epidemiological studies have investigated the association between body iron levels and cancer. These studies rely on biomarkers to assess body iron levels: circulating iron-binding proteins [transferrin (TF) and ferritin (FT)] and transferrin saturation (i.e., the extent to which the iron-binding sites of TF are occupied by iron) (65, 80). A thorough discussion of these proteins is provided in Section 4.1 (Figure 1). Using these markers, epidemiological studies collectively support a positive association between increased body iron levels and cancer.
Figure 1.
Iron homeostasis and cancer. (a) Dietary iron is absorbed as inorganic iron or heme by enterocytes of the intestine. Dietary ferric iron (Fe3+) is reduced to ferrous iron (Fe2+) by duodenal cytochrome B (DcytB) and imported into the enterocyte by divalent metal transporter 1 (DMT1). Heme is absorbed by an unidentified heme transporter and subsequently degraded by heme oxygenase 1 (HO-1), present on the endoplasmic reticulum, liberating iron. Ferrous iron is transported out of the enterocyte by ferroportin (FPN), where it is oxidized by hephaestin (HEPH) and loaded onto transferrin (TF) for systemic circulation. A large percentage of iron is delivered to the bone marrow for the production of hemoglobin and red blood cells. Iron is recycled from senescent red blood cells through endocytosis by macrophages, reentering circulation via FPN. Alternatively, iron can be transported to the liver for storage. The liver acts as an iron-sensing organ and regulates the absorption, recycling, and tissue levels of iron by secreting hepcidin (HP), a negative regulator of FPN. Iron is imported into the cells of peripheral tissues by binding of TF to its receptor, transferrin receptor 1 (TFR1). The complex is endocytosed, and within the endosome ferric iron is released from TF, reduced by six-transmembrane epithelial antigen of prostate (STEAP) proteins, and exported to the cytosol by DMT1. Newly imported iron enters the metabolically active labile iron pool (LIP), where it can be utilized in processes including DNA synthesis and repair, cellular respiration, and energy metabolism. Iron can also be stored in ferritin or exported back into circulation by FPN, oxidized by ceruloplasmin (CP), and loaded onto TF for further rounds of iron delivery. Peripheral tissue cells also secrete HP, which, unlike HP secreted by the liver, is thought to primarily act locally. (b) As a consequence of inflammation, HP secretion by the liver is increased, thereby reducing FPN expression throughout the body. Cancer-induced HP secretion impairs iron absorption and iron recycling, restricting circulating iron levels and leading to reduced erythropoiesis and anemia. Cancer cells generally exhibit an iron-seeking phenotype, with increased levels of TFR1, STEAPs, DMT1, and HP, and decreased FPN as compared with normal cells of the same tissue. The change in direction of iron metabolic proteins in cancer is shown by red arrows.
One of the initial clinical cohort studies demonstrating an association between body iron levels and cancer was performed by Stevens et al. (150) on a cohort of 14,000 US National Health and Nutrition Examination Survey participants. A major finding from this study was that participants with higher TF saturation levels were at higher risk of cancer than participants with lower TF saturation levels (150), a finding supported by subsequent studies (188).
Further support for an association between increased body iron levels and increased incidence of cancer comes from studies of iron metabolic diseases. Hereditary hemochromatosis, the prototypical disease of excessive iron overload, can arise from mutations in a variety of genes involved in regulating systemic iron uptake, including the hemochromatosis gene itself (HFE), hepcidin (HAMP), hemojuvelin (HFE2), ferroportin (SLC40A1), and transferrin receptor 2 (TFR2) (132). The result of these mutations is markedly elevated body iron (3, 59).
Hemochromatosis patients are at an increased risk of developing several cancers, including liver, colon, rectal, prostate, and breast cancers. The risk of liver cancer is particularly high, 20 to 200 times higher than in the general population, due to the importance of the liver in iron metabolism (Figure 1) (57, 59). Another disease of iron overload, homozygous β-thalassemia, is also associated with an increased risk of hepatocellular carcinoma (124).
To understand the cause and effect relationship between body iron levels and cancer risk, investigators have examined cohorts of individuals undergoing blood donation or blood transfusion. Blood donation, which reduces body iron, is associated with a lower cancer risk (120, 195), and blood transfusion, which increases body iron, is associated with an increased cancer risk (85). Further, a multicenter, randomized controlled study in patients with advanced peripheral arterial diseases demonstrated that iron reduction by phlebotomy lowered cancer risk and mortality by 37% at 5 years’ follow-up (195). These studies suggest not only that increased iron is associated with increased cancer risk but also that increased iron promotes increased cancer risk.
3. IRON SOURCES AND CANCER
The main sources of iron in humans are dietary iron and environmental iron. Dietary iron primarily exists as either inorganic iron or heme, an iron-containing prosthetic group found in many proteins, including hemoglobin and myoglobin. Inorganic iron is commonly found in plant material and iron supplements, whereas heme iron is commonly found in foods of animal origin. Red meat is the major source of heme and contains 10-fold higher concentrations of heme than white meat. Major sources of inorganic and heme dietary iron are listed in Table 1 (40). Iron from the environment, primarily from the air and water, serves as another potential source of exposure to iron. Several epidemiological studies have attempted to understand the contribution of specific sources of iron to cancer risk. Although the results of these studies are quite consistent, they use the consumption of various foods, particularly red meat, as a proxy for iron intake, and do not directly measure iron itself.
Table 1.
Source of dietary iron
| Nonheme iron | Heme |
|---|---|
| Cereals (fortified) | Beef |
| Breads (fortified) | Liver (beef, chicken) |
| Pasta (fortified) | Oyster |
| Lentils | Turkey |
| Tofu | Chicken (dark meat) |
| White beans | Clams |
| Kidney beans | Sardines |
| Dark chocolate | Eggs |
| Certain spices (e.g., thyme, marjoram, spearmint, turmeric, bay leaf) | Shrimp |
3.1. Epidemiology of Dietary Iron and Cancer
Perhaps the largest epidemiological studies designed to understand the potential link between dietary iron uptake and cancer risk have been performed on cohort consortiums developed by the US National Cancer Institute. The NIH–AARP (National Institutes of Health–American Association of Retired Persons) Diet and Health Study is a cohort consortium developed with the aim of improving understanding of the relationship between diet and health. The NIH–AARP cohort consists of half a million retirees, between 50 and 71 years of age, recruited between 1995 and 1996 (42). Another epidemiological study cohort initiated by the National Cancer Institute is the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). This cohort comprises nearly 148,000 men and women aged 55–74 years with no prior history of prostate, lung, colorectal, or ovarian cancer (200).
In an initial NIH–AARP study, the association between iron intake and the incidence of different cancer types was assessed in approximately 500,000 participants at 8 years’ follow-up (42). A 124-item food frequency questionnaire (FFQ) was used to assess dietary iron intake. In this study, individuals in the highest quintile of red meat intake, compared with those in the lowest, exhibited a statistically significant elevated risk of several malignancies, including esophageal cancer [hazard ratio (HR), 1.51; 95% confidence interval (CI), 1.09–2.08], colorectal cancer (HR, 1.24; 95% CI, 1.12–1.36), liver cancer (HR, 1.61; 95% CI, 1.12–2.31), and lung cancer (HR, 1.20; 95% CI, 1.10–1.31), and there was borderline statistical significance for laryngeal cancer (HR, 1.43; 95% CI, 0.99–2.07). Red meat intake was positively associated with pancreatic cancer incidence only among men (HR, 1.43; 95% CI, 1.11–1.83). No association was found between increased intake of red meat and the incidence of breast, gastric, or bladder cancer, or leukemia, lymphoma, or melanoma.
In addition to large studies aimed at understanding the correlation between heme iron intake and the risk of multiple cancers, many studies have investigated the association between dietary iron and individual cancer types (Table 2). Colorectal cancer is the most extensively studied malignancy in which the association between dietary iron intake and cancer incidence has been assessed. A large, prospective study of 300,948 men and women with 2,719 cases of colorectal cancer demonstrated a positive association between colorectal cancer and red meat intake (HR, 1.24; 95% CI, 1.09–1.42) as well as processed meat intake (HR, 1.16; 95% CI, 1.01–1.32), and it concluded that increased heme iron was a likely contributor to the association between meat intake and colorectal cancer (41). In this study, the total iron intake by individuals was estimated using FFQs (41). The rationale for including processed meat intake was that heme iron, apart from being a pro-oxidant, can also catalyze the generation of N-nitroso compounds from nitrate and nitrite added to meat during processing (43). Nitroso compounds are potential carcinogens and exacerbate the risk of colon cancer (41). For example, in a combined study of two large cohorts of US health professionals [the Nurses’ Health Study (n=87,108 women; 1980–2010) and the Health Professionals Follow-up Study (n=47,389 men; 1986–2010)], processed meat intake was positively associated with an increased risk of colorectal cancer (HR, 1.15; 95% CI, 1.01–1.32) (19). In addition to these large single studies, two meta-analyses of 34 and 23 prospective studies demonstrated a positive association between heme iron and colorectal cancer, with a relative risk (RR) of 1.12 (95% CI, 1.04–1.21) and RR of 1.11 (95% CI, 1.01–1.22), respectively (8, 30). Similarly, a meta-analysis of 13 case–control studies in the Japanese population showed an association between meat consumption and an increased risk of colorectal cancer (RR, 1.34; 95% CI, 1.12–1.59) (131). Based on the overwhelming epidemiological evidence, current recommendations by the World Cancer Research Fund International for reducing colorectal cancer incidence include reducing the consumption of red and processed meat to less than 500 g per week (166).
Table 2.
Epidemiological studies of iron and cancer
| Reference | Number and type of studies | Summary relative risk or odds ratio (95% confidence interval) for cancer incidence with increased heme iron or red meat intake |
|---|---|---|
| Colorectal cancer | ||
| Alexander et al. (8) | 34 prospective studies | 1.12 (1.04–1.21) |
| Aune et al. (11) | 7 prospective studies | 1.20 (1.06–1.36) |
| 19 case–control studies | 1.34 (1.12–1.59) | |
| Pham et al. (131) | 6 cohort and 13 case-control studies | 1.16 (1.001–1.34) |
| Carr et al. (30) | 23 prospective studies | 1.11 (1.01–1.22) |
| Fonseca-Nunes et al. (65) | 21 prospective studies | 1.08 (1.00–1.17) |
| Breast cancer | ||
| Guo et al. (77) | 14 prospective studies | 1.10 (1.02–1.19) |
| Alexander et al. (7) | 8 cohort studies | 1.02 (0.98–1.07) |
| Wu et al. (186) | 46 prospective studies | 1.07 (1.01–1.14) |
| Fonseca-Nunes et al. (65) | 5 prospective and 5 case-control studies | 1.03 (0.97–1.09) |
| Esophagus cancer | ||
| Zhu et al. (201) | 7 cohort and 28 case-control studies | 1.33 (1.04–1.69) |
| Prostate cancer | ||
| Alexander et al. (6) | 15 prospective studies | 1.01 (0.94–1.09) |
| Bylsma et al. (25) | 19 prospective cohort studies | 1.02 (0.92–1.12) |
| Pancreatic cancer | ||
| Paluszkiewicz et al. (129) | 11 case-control studies | 1.48 (1.25–1.76) |
| Larsson & Wolk (105) | 11 prospective studies | 1.29 (1.08–1.53) |
| Lung cancer | ||
| Yang et al. (194) | 23 case-control and 11 cohort studies | 1.34 (1.18–1.52) |
| Xue et al. (191) | 6 cohort and 28 case–control studies | 1.44 (1.29–1.61) |
| Gnagnarella et al. (71) | 14 prospective studies | 1.24 (1.01–1.51) |
| Fonseca-Nunes et al. (65) | 1 case-control and 3 prospective studies | 1.12 (0.98–1.29) |
Epidemiological studies show possible correlations between gastroesophageal cancer subtypes and iron intake. For esophageal cancer, different histological types of esophageal cancer exhibited different correlations between cancer incidence and meat type consumed (201): An increased intake of red meat and a low intake of poultry were associated with esophageal squamous cell carcinoma, whereas an increased intake of processed meat was associated with an increased risk of adenocarcinoma of the esophagus (201). Similarly, a prospective study of 120,852 individuals aged between 55 and 69 years and followed for 16.3 years showed a positive association between increased red meat intake and a higher incidence of esophageal squamous cell carcinoma. No association was found between red meat intake and esophageal adenocarcinoma or gastric cancer subtypes (adenocarcinoma of the cardia and noncardia types). Neither study found any association for women between the incidence of esophageal cancer and dietary heme intake for any of the four subtypes (99). Further, in a large meta-analysis of the association between iron and cancer, two of five esophageal cancer studies demonstrated a positive correlation between esophageal adenocarcinoma and heme iron [RR,3.04(95%CI,1.20–7.71)andRR,3.11(95%CI,1.46–6.61)], and three studies showed opposite results (65). In a multi-institutional study performed by the Gastric and Esophageal Working Group of the European Prospective Investigation into Cancer and Nutrition (EURGAST-EPIC), a positive association was found between heme iron intake and gastric cancer incidence. The cohort included 481,419 individuals with 444 cases of gastric cancer in 8.7 years of follow-up. The dietary intake of heme was assessed by FFQs. Participants in the highest quartile of heme ingestion had a 70% higher risk of developing gastric cancer than those in the lowest quartile (93). However, a nested case–control study that investigated the relationship between body iron status and gastric cancer risk showed a decreased risk of gastric cancer with increased serum iron and FT (64). Given these variable findings, a relationship between iron and gastroesophageal cancer remains controversial.
In pancreatic cancer, however, the evidence for a relationship between iron and cancer development is more consistent. In an NIH–AARP cohort of 322,846 participants, a total of 1,471 pancreatic cancer cases was found during a 10-year follow-up study. Pancreatic cancer risk significantly increased with total intake of meat (HR, 1.20; 95% CI, 1.02–1.42) and red meat (HR, 1.22; 95% CI, 1.01–1.48). These associations remained significant in men but not women (154). Two meta-analyses of 5 case–control studies and 11 prospective cohort studies found positive associations between red meat and pancreatic cancer (105, 129).
A large NIH–AARP cohort study of men (n=278,380) and women (n=189,596) found that increased heme iron intake elevated the risk of lung carcinoma in both men (HR, 1.25; 95% CI, 1.07–1.45) and women (HR, 1.18; 95% CI, 0.99–1.42) (153). In addition to dietary heme iron, the cooking method, extent of cooking (“doneness”), and intake of specific meat mutagens were also found to influence the risk for lung carcinoma. The association between the method of cooking of red and processed meat and lung carcinoma was due to the formation of endogenous mutagens during high-temperature cooking (149). Similarly, three different meta-analyses of 34, 33, and 14 observational studies demonstrated a positive correlation between lung cancer and red meat intake (71, 191, 194). However, a recent study on the association of dietary mineral intake and lung cancer in 5,435 participants aged 55 years and older found that an increased intake of iron reduced the risk of lung cancer at 22 years’ follow-up (HR, 0.58; 95% CI, 0.37–0.92) (123). Thus, although epidemiological studies generally tend to show a positive correlation between iron and lung cancer, more studies will be required to generate a firm conclusion.
For women with breast cancer, the NIH–AARP Diet and Health Study of 193,742 postmenopausal women with 9,305 cases of breast cancer showed a positive association between heme iron intake and breast carcinoma incidence (91). In another large epidemiological study (the PLCO) of approximately 100,000 men and women (55–74 years of age), the effect of meat type, meat cooking preferences, meat mutagens, and heme iron on cancer incidence was investigated. In this study, there was a positive association between the consumption of red meat (HR, 1.23; 95% CI, 1.00–1.51) and dietary iron (HR, 1.25; 95% CI, 1.02–1.52) and breast cancer in postmenopausal women (62). Similarly, a study of a cohort of 1,205 women with invasive breast cancer showed a significant association between breast cancer and dietary iron (RR, 1.25%; 95% CI, 1.02–1.52), with heme iron intake estimated using an FFQ (62). Further, one meta-analysis of 14 prospective studies of red meat and 12 prospective studies of processed meat showed a positive association between both red and processed meat and breast carcinoma (77).
3.2. Epidemiology of Environmental Iron Exposures and Cancer
Airborne iron levels vary by location, with urban sites having higher iron levels than remote areas (approximately 1.3 μg/m3 versus 50–90 ng/m3) (182). Areas in the vicinity of iron- or steel-producing plants may have particularly high levels of iron in the air (up to 12 μg/m3). Environmental exposure to iron dust has been associated with increased incidences of lung and stomach cancer (89). An exposure–response relationship study among Finnish men (N=30,137) with a history of occupational exposure to iron fumes or dust and welding fumes found that the relative risk for lung cancer (squamous cell carcinoma) increased as the cumulative exposure to iron dust and welding fumes increased (147). Many cases have been reported of iron inhalation and acute bronchial damage, presumably due to the ability of inhaled iron to catalyze the formation of ROS (89). The ingestion of iron-containing beverages has also been linked to the increased incidence of cancer in areas of sub-Saharan Africa (97). In this case, a custom of brewing fermented beverages in iron-containing pots produces a beverage with an unusually high iron content. Combined with the prevalence of a polymorphic variant of a gene that predisposes to hemochromatosis [the Q248H allele of ferroportin (FPN)] (73), this dietary exposure is thought to underlie the increased risk for hepatocellular carcinoma in this population (66).
4. CHANGES IN IRON METABOLISM IN CANCER
Despite being the most abundant element by mass on Earth, iron availability is often regarded as a growth-limiting factor for organisms (1); iron is principally limited by the formation of nonbioavailable soluble iron oxides (4). Considering its poor bioavailability, it is not surprising that humans have no major mechanism of iron excretion. Instead, iron is principally regulated at the levels of systemic absorption and distribution and cellular utilization. Despite strict regulation of normal iron metabolism, cancers exert profound effects that alter this regulation, at both the systemic and cellular levels (Figure 1).
4.1. Systemic Iron in Cancer
Normal mechanisms of iron handling are altered in cancer in what has been termed an iron withholding response—that is, an attempt by the organism to limit the availability of this essential nutrient to the tumor, a response similar to that induced by a bacterial infection(176).Although the phenomenon of iron with holding was described decades ago, the fundamental mechanisms that underlie the decrease in systemic iron in malignant disease have only recently begun to be uncovered.
4.1.1. Physiological mechanisms of systemic iron regulation.
In humans, dietary iron is absorbed mainly through intestinal absorptive cells, enterocytes, in the duodenum and upper jejunum (171). The efficiency of iron absorption is relatively low and depends on oxygen state and form; heme is more bioavailable and is absorbed at a significantly higher rate (two- to sevenfold higher) than iron from nonheme sources (178). At normal physiological pH, ferrous iron (Fe2+) is readily oxidized to the insoluble ferric (Fe3+) form (1). Fe3+ is reduced back to Fe2+ by the ferric reductase duodenal cytochrome B (DcytB) and transported across the apical membrane of enterocytes by divalent metal transporter 1 (DMT1) (Figure 1) (12). Alternatively, heme is imported into the enterocytes through an unknown mechanism, where it is degraded by heme oxygenase 1 (HO-1), liberating Fe2+. Fe2+ from heme and nonheme sources is then transported to the basal surface of the enterocyte, reoxidized to Fe3+ by hephaestin (HEPH), exported through their on exporter FPN (119), and loaded onto the circulating iron transport protein TF for systemic delivery (Figure 1).
Peripheral tissues require iron for essential cellular processes that include DNA synthesis, cell cycle progression, energy generation, heme synthesis, and iron–sulfur (Fe–S) cluster biogenesis. However, the majority of systemic iron is utilized in the bone marrow for hemoglobin and red blood cell synthesis. Iron is recycled from senescent erythrocytes by macrophages, which export recycled iron back into circulation via FPN (Figure 1) (14).
Excess systemic iron that is not utilized by the bone marrow or peripheral tissues is stored in the liver. The liver also acts as an iron-sensing organ and controls systemic iron through the secretion of the peptide hormone hepcidin, a master regulator of organismal iron homeostasis. When systemic levels of iron are high, hepcidin is produced by the liver and secreted into the circulation (Figure 1). Hepcidin binds to FPN and triggers its degradation (127, 134). This results in decreased iron efflux into the systemic circulation from enterocytes and macrophages, and restores systemic iron to normal levels. Conversely, when systemic iron levels are low, hepcidin production is reduced, allowing FPN to efflux iron from enterocytes and macrophages and increase systemic iron levels. Thus, FPN and hepcidin function as an essential regulatory axis for maintaining systemic iron homeostasis.
4.1.2. Dysregulation of systemic iron metabolism in cancer.
Dysregulation of systemic iron homeostasis is commonly seen in cancer patients and is manifested as a decreased red blood cell count, or anemia. Cancer-induced anemia is generally considered an anemia of inflammation (also known as anemia of chronic disease). It is characterized by reduced erythropoiesis and iron restriction (Figure 1) (125). The cause of cancer-induced anemia is multifactorial, including the presence of comorbidities and the location and extent of the disease. Mechanistically, cancer-induced anemia is mediated by the secretion of inflammatory factors, such as tumor necrosis factor-α(TNFα)andinterleukin-6(IL-6).TNF α is instrumental in inhibiting erythropoiesis (24), while IL-6 drives iron restriction through upregulation of hepcidin (24). Therapy-induced anemia is distinct and can be induced by cytotoxic agents and other therapies that impair hematopoiesis (76). Treatment options include blood transfusions, erythropoiesis-stimulating agents, and iron supplementation.
4.2. Cellular Iron Metabolism in Cancer
The fundamental mechanisms through which iron is absorbed, stored, and effluxed from cells have largely been studied in nonmalignant cells. Cancer cells retain these basic pathways; however, in many instances cancer cells exhibit a trend toward enhanced iron acquisition and retention.
4.2.1. Physiological mechanisms of cellular iron regulation.
TF-bound iron destined for peripheral cells binds to transferrin receptor 1 (TFR1), receptor present on the surface of most cells, with the exception of mature red blood cells (118) (Figure 1). Binding triggers endocytosis of the holo–TF–TFR1complex, release of Fe3+, and subsequent reduction to Fe2+ by six-transmembrane epithelial antigen of prostate (STEAP)-family reductases. Fe2+ is then transported out of the endosome into the cytosol by DMT1, where it becomes part of the metabolically active labile iron pool (LIP). Excess iron that is not utilized by the cell either is stored in the iron storage protein FT and can later be accessed via autophagy (known as ferritinophagy) (52) or is exported out of the cell by FPN, where it is reloaded onto TF for further circulation. Locally produced hepcidin degrades FPN on nearby cells and decreases iron export (Figure 1) (135, 157, 198).
Cellular iron homeostasis is controlled by a posttranscriptional system involving the iron responsive element-binding proteins (IRPs) IRP1 and IRP2 and corresponding iron-responsive elements (IREs) on untranslated regions of messenger RNAs (mRNAs) encoding proteins important to cellular iron regulation (159). In response to iron levels, cytosolic IRPs bind to stem-loop IREs present in either the 5’ or 3’ untranslated region of these mRNAs to control the synthesis of proteins involved in iron import (TFR1, DMT1), iron storage (FT), or iron export (FPN1). For example, when cellular iron levels are low, IRPs bind to the 5’ IREs of FPN1 and FT mRNA, resulting in translation repression. Additionally, IRPs bind to 3’ IREs in TFR1 mRNA to stabilize the mRNA and increase TFR1 protein synthesis. Ultimately, these changes result in increased intracellular iron. In contrast, surplus intracellular iron leads to IRP destabilization, preventing IRP1 and IRP2 from binding to the IREs of these mRNAs. This permits unimpeded translation of FPN1 and FT and fosters degradation of TFR1 mRNA, leading to decreased cytosolic iron. IRPs, therefore, act as the master regulators that maintain intracellular iron homeostasis.
4.2.2. Dysregulation of cellular iron metabolism in cancer.
Cancer cells frequently shift intracellular iron metabolism in ways that favor iron accumulation: toward increased iron uptake and storage, decreased iron export, or both (Figure 1). Interestingly, increased iron accumulation is also characteristic of tumor-initiating cells (18) and cancer stem cells (142). Thus, the iron exporter FPN is downregulated in breast (135), prostate (157), and ovarian cancers (18), while its negative regulator, hepcidin, is upregulated (135, 157). IRP2 is also upregulated in prostate cancer (46), resulting in an increase in TF receptor and a decrease in FT and FPN. In several cancers, the iron import protein TFR1 (18, 86) is increased and correlates with poor prognosis (79). STEAPs are also upregulated in several cancers (72), as is DMT1 in colon cancer (23).
Lipocalin 2 (LCN2) is a secreted protein that mediates an alternative form of iron transport and is upregulated in some tumors (107). LCN2 binds siderophores, iron-binding ligands of low molecular weight that are produced by pathogens (63), as well as siderophore-like catechols of mammalian origin (14). Knockdown of LCN2 suppresses prostate cancer cell growth, while overexpression promotes growth (165). Further, depending on the iron saturation of the LCN2-bound ligand, LCN2 can mediate either iron import or iron export from cells and subsequently support cell survival and growth (60, 158) or induce apoptosis (47), respectively. The iron storage protein FT facilitates increased iron storage while limiting increased ROS generation. FT is upregulated in a number of cancers including breast cancer (116), glioblastoma (142), Hodgkin’s lymphoma (58), and pancreatic cancer (116). Although it has not been studied, ferritinophagy may also contribute to an increased LIP in cancer, since autophagy is upregulated in cancer and contributes to tumor growth and aggressiveness (180).
4.3. Dysregulation of Iron by Oncogenes and Tumor Suppressors
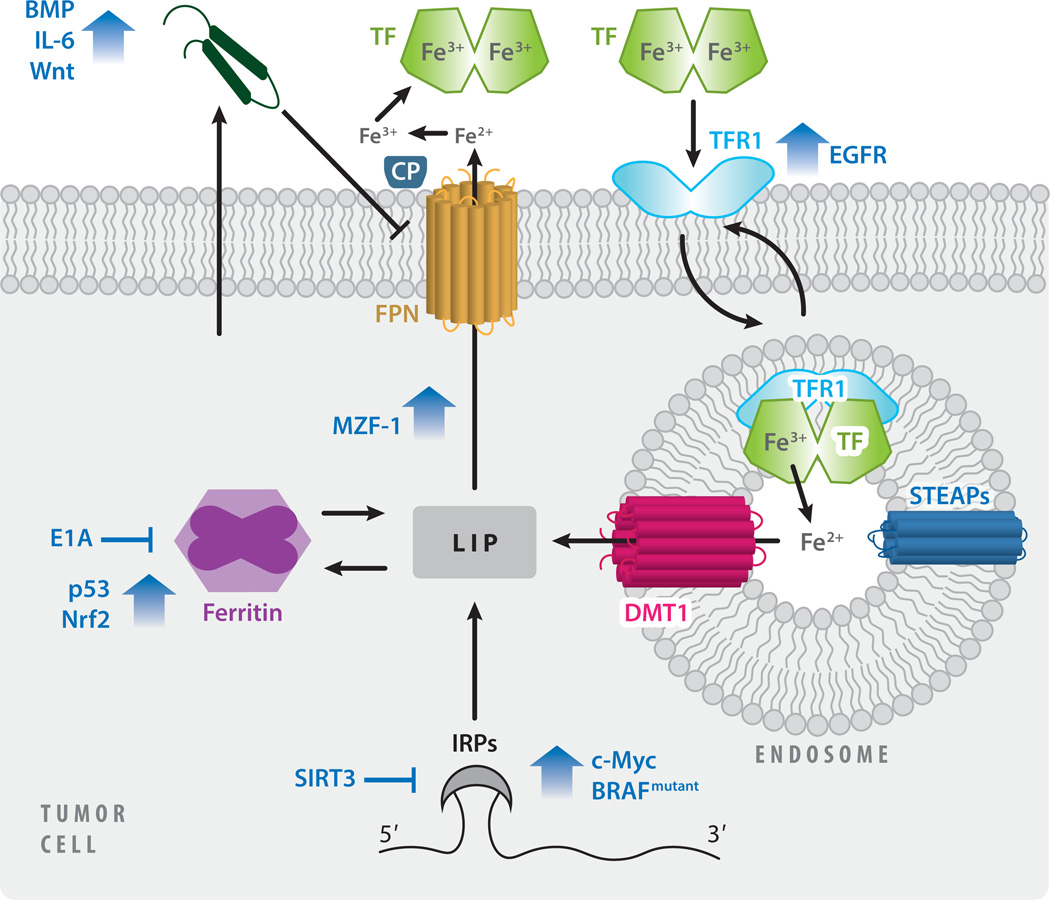
Subversion of normal iron metabolism in cancer cells is achieved through the regulation of iron metabolism by oncogenes and tumor suppressors (Figure 2). C-Myc, a proto-oncogene and transcription factor, induces IRP2 (187), which subsequently induces TFR1 (136) and inhibits FT, thus increasing the metabolically available LIP. In colorectal cancer, activating BRAF mutations, important oncogenic drivers, are associated with increased IRP2 levels (88). BRAF has an important role in promoting MEK (mitogen-activated protein kinase kinase) activation, and the inhibition of MEK signaling by trametinib suppressed IRP2 and TFR1, and reduced intracellular iron. Iron metabolism is also regulated by SIRT3, a multifunctional protein in cancer (9), through ROS-mediated regulation of IRP1 (94). Similarly, the oncogenic driver epidermal growth factor receptor (EGFR) increases iron import by promoting cell surface expression of TFR1 (172), and the adenoviral oncogene E1 A suppresses FT to decrease iron storage and increase intracellular iron availability (164). Bone morphogenetic protein (BMP) signaling, IL-6, and Wnt signaling induce hepcidin production in prostate cancer (157), contributing to an increase in intracellular iron by reducing iron efflux. Wnt is also activated in an iron-dependent manner in colon cancer, with mutations in adenomatous polyposis coli (APC), a tumor suppressor (22, 136). Tumor suppressors exert an opposing effect: The tumor suppressor p53 induces FT (197), asdoes NRF2 (nuclear factor erythroid 2-related factor 2) (133). In prostate cancer cells, the transcription factor myeloid zinc finger 1 (MZF-1) functions as a tumor suppressor, in part by promoting FPN-mediated iron export (34).
Figure 2.
Regulation of cellular iron metabolism in cancer. Dysregulated iron metabolism in cancer cells is driven by oncogenes and tumor suppressors. The localization of TFR1 to the cell surface, and therefore its ability to bind TF and import iron, is promoted by EGF. Iron storage is altered by the suppression of ferritin by E1A and its induction by p53 and nuclear factor Nrf2. Iron export through FPN is promoted by MZF-1, but inhibited by increased hepcidin expression, driven by BMPs, IL-6, and Wnt signaling. Some factors (c-Myc, mutated BRAF, and SIRT3) dysregulate multiple phases of cellular iron metabolism (import, storage, and export) by targeting IRPs. Abbreviations: BMPs, bone morphogenetic proteins; CP, ceruloplasmin; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; FPN, ferroportin; IL-6, interleukin-6; IRPs, iron regulatory proteins; LIP, labile iron pool; MZF-1, myeloid zinc finger 1; Nrf2, erythroid 2-related factor 2; STEAP, six-transmembrane epithelial antigen of prostate; TF, transferrin; TFR1, transferrin receptor 1.
5. DIVERSE ROLES OF IRON IN CANCER
As discussed above, the subversion of iron metabolism in cancer cells leads to increased intracellular iron. Increased intracellular iron in turn has diverse roles in the initiation, growth, and metastasis of cancer cells.
5.1. Role of Iron as a Tumor Initiator
The role of iron in carcinogenesis was initially reported in 1959 by Richmond (138). Richmond showed that repeated intramuscular injection of iron dextran complex induced sarcoma in rats. Subsequently, numerous animal models have demonstrated the ability of iron to induce cancer. Fenton chemistry, the generation of ROS, and subsequent DNA damage are believed to be the main underlying mechanisms by which iron is carcinogenic (161). A particularly compelling series of studies demonstrated that ferric nitrilotriacetate is sufficient to induce kidney tumors in rats. DNA isolated from these tumors exhibits oxidized bases, consistent with oxidative stress–induced DNA damage, as well as a pattern of genetic alterations remarkably reminiscent of human kidney cancer (5).
Additional mechanistic details explaining the link between dietary iron and cancer have been investigated. In the 1980s, Graf & Eaton (74) proposed that phytic acid compounds in dietary fiber reduced the risk of colorectal cancer by chelation of dietary iron. Rats fed red meat exhibited increased colonic epithelium proliferation and the formation of cytotoxic agents in colonic mucosa (145, 146). Further, long-term administration of an iron compound in drinking water induced and enhanced ulcerative colitis and significantly increased colorectal tumor incidence. In a mouse model, dietary heme induced gut dysbiosis, lipid peroxidation, and aggravated colitis, thereby potentiating the development of adenoma (16). Three major mechanisms are proposed to explain the association between red meat and colorectal cancer. First, the iron present in heme generates oxidative cytotoxic agents that can promote colorectal cancer (17). Second, carcinogenic N-nitroso compounds can form in the intestinal tract in the presence of iron by N-nitrosation of peptide-derived amines or by nitrosylation, yielding S-nitrosothiols and nitrosyl iron (20). Third, meat cooked at high temperatures generates mutagenic heterocyclic amines, such as 2-amino-1-methyl-6-phenylimidazo(4,5-b) pyridine and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (149). These results highlight the role of the heme iron present in red meat in promoting colon cancer.
5.2. Role of Iron as a Tumor Growth Factor
Iron excess fosters tumor growth (83, 84, 136). Conversely, iron depletion mediated by reduced dietary intake (83) or iron chelators (111, 199) inhibits tumor growth. Tumor growth can also be modulated by manipulating the proteins of iron metabolism. Thus, decreasing cellular iron import by blocking TF decreases tumor growth (181), as does increasing cellular iron export through FPN overexpression (45, 135). Disrupting IRPs can also reduce tumor growth (46, 114, 174).
Iron’s function as a tumor growth factor can be understood in the context of its cellular roles. Iron and iron-containing cofactors (heme, Fe–S) are integral to DNA metabolism, the cell cycle, and cellular energy generation. DNA replication requires the synthesis of new deoxyribonucleotides. The rate-limiting step in DNA synthesis is catalyzed by the iron-dependent protein ribonucleotide reductase (55). Increased intracellular iron in cancer is therefore essential to facilitate the enhanced rate of cellular proliferation and DNA replication. Indeed, iron depletion by iron chelators (111), FPN overexpression (18, 45, 135), and IRP2 knockdown (46) exerts its inhibitory effect on tumor growth by reducing cancer cell proliferation. DMT1 knockdown also inhibits proliferation in colorectal cancer (190). Reduced proliferation in the presence of iron depletion is mediated by both a G1/G0 cell cycle arrest and, in some cases, induction of cell death (see Section 6). The tumor suppressor p53, which is induced following iron depletion, is a key mediator of cell cycle inhibition and cell death following iron depletion (45, 108). However, iron depletion also induces p53-independent mechanisms of cell cycle inhibition, including the regulation of cyclins and cyclin-dependent kinases (45, 108). It has also been proposed that iron may contribute to tumor growth by promoting mitochondrial oxygen consumption and adenosine triphosphate (ATP) generation through oxidative phosphorylation and citric acid cycling (26), pathways that have a role in cancer progression.
5.3. Role of Iron in Metastasis
A role for iron in metastasis is relatively unexplored. Iron depletion by iron chelation (173) and FPN overexpression (78) has been shown to inhibit metastasis. Mechanistically, iron depletion appears to inhibit cancer cell motility (152), invasion (18), and the transforming growth factor-β-induced epithelial-to-mesenchymal transition (35). The metastasis suppressor N-Myc downstream-regulated gene 1 (NDRG1) (35, 152, 173), as well as IL-6 (18), appear to mediate these effects, in part by regulating matrix metalloproteinases (18, 32).
6. ROLE OF IRON IN CELL DEATH
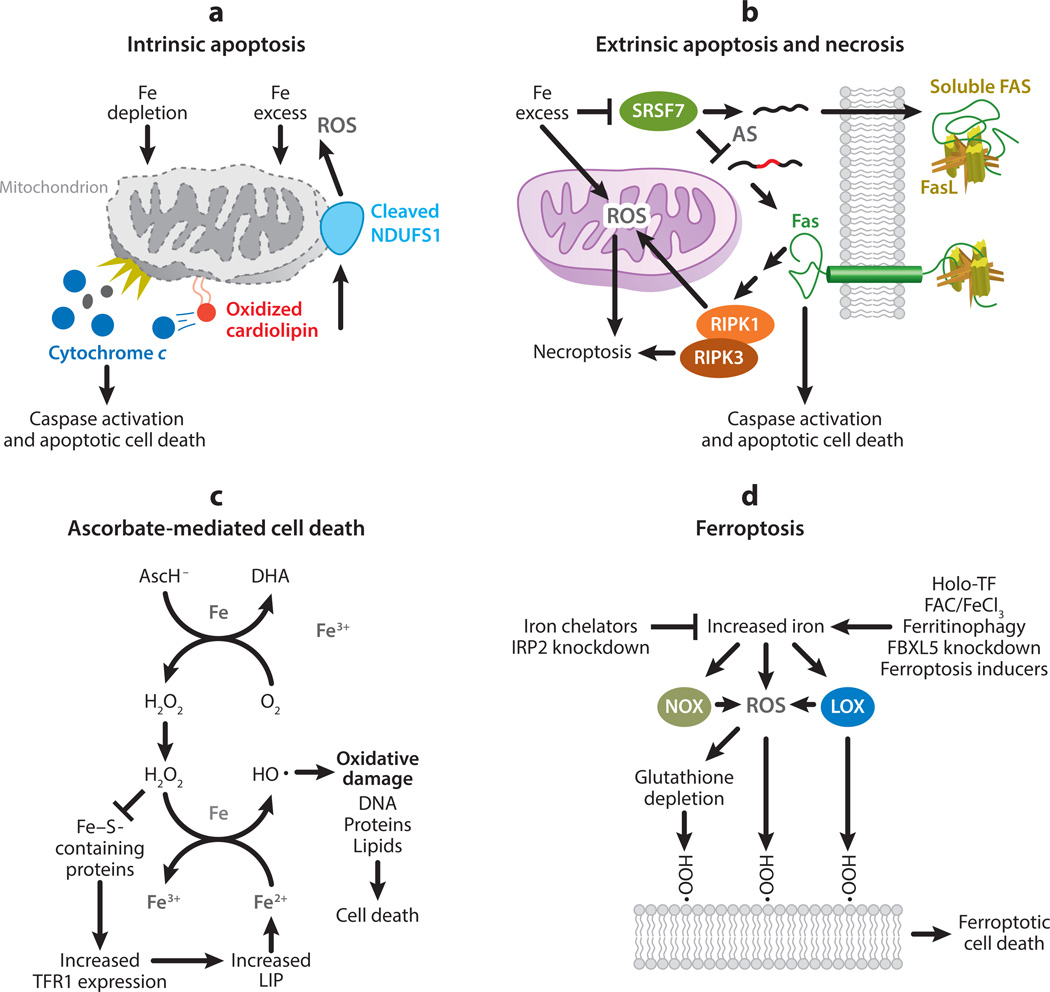
Considering the importance of iron in the growth and progression of cancer, it is not surprising that iron plays a prominent part in multiple cell death pathways, including apoptosis, necroptosis, ascorbate-mediated death, and ferroptosis (Figure 3). While these cell death pathways play various partsinotherpathologiesandinnormaldevelopment,theyalsohaveimportantrolesinthecontext of cancer. In particular, both the depletion and repletion of iron can be used to foster cancer cell death, identifying a role for iron manipulation in the armamentarium of anticancer strategies.
Figure 3.
Role of iron in cell death. (a) Iron depletion disrupts cellular processes, while iron excess causes cellular damage through the generation of reactive oxygen species (ROS). If excessive, these processes initiate the intrinsic apoptosis pathway and trigger permeabilization of the outer mitochondrial membrane and the release of cytochrome c. Through the generation of ROS, excess iron may promote the release of cytochrome c through the oxidation of cardiolipin. Cytochrome c release leads to the activation of caspases and apoptotic cell death. Caspase activation further contributes to mitochondrial ROS generation through cleavage of the iron–sulfur cluster-containing protein NADH:ubiquinone oxidoreductase core subunit S1 (NDUFS1). (b) Excess iron can promote extrinsic apoptosis or necrosis, or both, through regulating the death receptor FAS. Alternative splicing (AS) results in a soluble, decoy FAS and a membrane-bound Fas capable of initiating cell death. Excess iron promotes the production of membrane-bound FAS by interfering with messenger RNA binding of serine- and arginine-rich splicing factor 7 (SRSF7). The binding of FAS ligand (FasL) to membrane-bound FAS can initiate either caspase activation and apoptosis or necroptosis in a receptor-interacting protein kinase-1 and -3 (RIPK1 and RIPK3)-dependent manner. RIPK1 and RIPK3 induce necroptosis, in part, by initiating the generation of ROS in the mitochondria. (c) Ascorbate (AscH−) is oxidized to dehydroascorbic acid (DHA) in the presence of iron (Fe) and oxygen (O2) to generate hydrogen peroxide (H2O2). Through Fenton chemistry, H2O2 generates hydroxyl radicals (), which can cause oxidative damage to cellular components (including DNA, protein, and lipids) and induce cell death. H2O2 can also inhibit the activity of iron–sulfur (Fe–S)-containing proteins, including cytosolic aconitase, increasing transferrin receptor 1 (TFR1) expression and the labile iron pool (LIP). As the LIP is predominately ferrous iron (Fe2+), increased LIP further promotes production via Fenton chemistry. (d) Ferroptosis is a form of cell death caused by the iron-mediated generation of lipid peroxides. The reduction of intracellular iron, through iron chelation and knockdown of iron-responsive element-binding protein 2 (IRP2), protects against ferroptosis. Conversely, several methods to increase intracellular iron promote ferroptotic cell death: iron treatment with holo-transferrin (holo-TF), ferric ammonium citrate (FAC), and iron chloride hexahydrate (FeCl3); ferritinophagy; and knockdown of FBXL5, an inhibitor of IRP2. Furthermore, ferroptosis inducers (such as erastin and RSL3) have been reported to directly increase LIP. Increased iron is thought to promote ferroptosis through an increase in ROS, particularly lipid ROS, and a reduction in glutathione, an antioxidant important in reducing lipid peroxides. Fenton chemistry, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), and lipoxygenases (LOX) contribute to ROS generation, lipid peroxidation, and ferroptotic cell death.
6.1. Apoptosis
Apoptosis is a form of programmed cell death characterized by several factors: loss of adhesion, cell shrinkage, membrane blebbing, and the fragmentation and condensation of the nucleus (karyorrhexis and pyknosis) (155). Apoptosis can be induced by cell-intrinsic or extrinsic factors (90).
6.1.1. Iron and intrinsic apoptosis.
The intrinsic apoptotic pathway can be initiated by cellular stresses, including prolonged nutrient deprivation, endoplasmic reticulum stress, and DNA damage (90). These stressors initiate apoptosis by triggering permeabilization of the mitochondrial outer membrane, release of cytochrome c, and induction of caspase activity. Interestingly, both iron depletion and iron overload can induce intrinsic apoptosis, demonstrating the importance of maintaining strict iron homeostasis.
The induction of apoptosis by iron depletion was demonstrated following treatment with iron chelators (75, 148) or the widespread disruption of iron homeostasis by IRP2 knockdown (46). While FPN overexpression in prostate cancer cells induced endoplasmic reticulum stress and DNA damage (45), two established inducers of apoptosis, no increase in apoptosis was observed. Therefore, the induction of apoptosis by iron depletion likely depends on the extent and duration of iron depletion. Apoptosis induced by iron depletion occurs by both p53-dependent (183) and p53-independent (2) mechanisms. Iron depletion has further been shown to augment apoptosis induced by the deprivation of other factors, such as IL-3 (47).
Elevated iron levels also induce apoptosis (175) and enhance apoptosis induced by other agents (168). Iron is involved not only in initiating apoptosis but also in its execution through the generation of ROS (Figure 3). ROS-mediated oxidation of cardiolipin, a mitochondrial inner membrane lipid that sequesters cytochrome c, is thought to contribute to the release of cytochrome c in response to apoptotic stimuli (95). Further, caspase activation induces mitochondrial ROS generation through the cleavage of the Fe–S containing protein NADH:ubiquinone oxidoreductase core subunit S1 (NDUFS1), which amplifies apoptotic signaling (137).
6.1.2. Iron and extrinsic apoptosis.
The extrinsic apoptotic pathway is initiated by the binding of a ligand to a death receptor (90). Fas (CD95), which is bound by Fas ligand (FASL; CD95L), is a death receptor regulated by iron. Alternative splicing of Fas mRNA results in the expression of multiple Fas proteins (31). One Fas protein remains membrane bound and is proapoptotic, while another, generated by deletion of exon 6, is soluble and antiapoptotic (36). A genome wide screen of small interfering RNA (siRNA) knockdowns identified 200 regulators of Fas alternative splicing (156). While a majority of the identified regulators were known to be involved in RNA processing, four genes involved in iron metabolism were also identified: ACO1 (iron-responsive element-binding protein 1), FTL (ferritin light chain), B2M (beta-2-microglobulin), and PCBP2 [poly(rC) binding protein 2]. SiRNA knockdown of ACO1, as well as the use of iron chelators (deferoxamine and ciclopirox), resulted in iron depletion and favored the production of the soluble, antiapoptotic form of FAS. Conversely, siRNA knockdown of FTL, as well as hemin treatment, resulted in iron accumulation and favored the production of the membrane-bound, proapoptotic form of FAS. Consistent with the effects of iron levels on Fas alternative splicing, iron depletion protected T lymphocytes from Fas-induced apoptosis while iron excess enhanced it.
Using a functional splicing network model (130), serine- and arginine-rich splicing factor 7 (SRSF7) was identified as the mediator of the iron-dependent alternative splicing of Fas. SRSF7 activity promotes production of the soluble, anti apoptotic form of FAS. Elevated iron levels disrupt the RNA binding properties and activity of SRSF7 by interfering with the zinc knuckle domain. Considering the elevated iron levels in cancer compared with normal tissue, this study suggests an iron-dependent mechanism by which cancer cells may be more susceptible to apoptosis-inducing agents.
6.2. Iron and Necroptosis
Necroptosis is a type of programmed cell death characterized by cellular swelling, plasma membrane rupture, and a dependence on receptor-interacting protein kinase 3 (RIPK3) (109). Iron overload can induce necroptosis in vitro (44, 189). Although necroptosis is distinct from apoptosis, they are similar in that either can be induced by Fas–FasL binding (87). Therefore, iron-mediated regulation of Fas (described in Section 6.1.2) regulates the initiation not only of extrinsic apoptosis but also of necroptosis. Apoptosis may be the preferred mechanism of Fas-induced cell death over necroptosis (167), although this is likely context dependent. There is also some evidence that mitochondrial ROS may have a role in necroptosis (15). Necroptosis-inducing agents offer an opportunity for novel iron-mediated cancer therapies, particularly for treating apoptosis-resistant forms of cancer.
6.3. Iron and Ascorbate-Mediated Cell Death
Pharmacological doses of ascorbate induce cell death selectively in cancer cells and increase sensitivity to chemotherapeutic agents (113). Ascorbate has shown promise as an anticancer therapy in preliminary clinical trials in patients with metastatic pancreatic cancer (177), as well as in patients with glioblastoma and non-small-cell lung cancer (141). Ascorbate toxicity depends on H2O2 generation (33), which reacts with the increased LIP in cancer cells via Fenton chemistry (Equation 1) (141) to cause cellular damage, including DNA damage (Figure 3) (113). Interestingly, through disruption of Fe–S cluster proteins, ascorbate-generated H2O2 induces additional increases in the LIP, further contributing to the increased toxicity of ascorbate in cancer cells versus normal cells (141). Although the mechanism of ascorbate-mediated cell death is becoming clearer, there is no consensus as to the exact type of resulting cell death. Different groups have reported apoptotic (29) and nonapoptotic cell death (169), with the specific mechanism possibly dependent on ascorbate concentrations (33). Independent of the specific mechanism of cell death, the use of ascorbate as an anticancer therapy represents a way to leverage the elevated levels of iron in cancer cells to selectively promote cell death.
6.4. Ferroptosis
The appreciation of the role of iron in cell death has expanded dramatically with the recent discovery of ferroptosis, an iron-dependent programmed cell death(48,193).Ferroptotic cell death is independent from other known mechanisms of cell death, including apoptosis and necroptosis (115). Instead, cell death is executed by the iron-dependent generation of lethal lipid peroxides.
Ferroptosis was discovered during a search for novel anticancer agents (193). Many ferroptosis sensitizers and inhibitors have been identified, with actions that are iron dependent or iron independent (151). In initial studies, ferroptosis was suppressed by the depletion of intracellular iron by the iron chelators deferoxamine and compound 311 (193). Conversely, increasing intracellular iron with either ferric ammonium citrate, iron chloride hexahydrate, or iron-bound TF increased sensitivity to ferroptosis (48, 68). Short hairpin RNA (shRNA) knockdown of IRP2, the master regulator of cellular iron homeostasis, protected cells from ferroptosis, while shRNA knockdown of IRP2’s negative regulator FBXL5 sensitized cells to ferroptosis (48). Increases in the LIP, mediated by ferritinophagy, occur in a time-dependent manner during ferroptosis and contribute to lipid peroxidation and eventual cell death (67).
The exact function of iron in facilitating ferroptosis has not been established. It has been suggested that iron contributes to lipid peroxidation through the generation of ROS (Figure 3; Equation 4). While this is likely to occur, increased ROS generation by other mechanisms, such as hydrogen peroxide treatment, does not induce ferroptosis (48). Therefore, it is likely that iron plays additional parts in promoting ferroptosis.
The observation that cobalt chloride treatment can inhibit ferroptosis (184) suggests that iron-containing proteins may be integral to ferroptosis, as cobalt is known to displace iron from some active sites (10). Indeed, some iron-containing proteins have been shown to promote ferroptosis. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) are heme-containing proteins that catalyze the production of ROS in a regulated manner (104). GKT137831, an inhibitor specific to NOX1 and NOX4, partially inhibited the induction of ferroptosis (48). Lipoxygenases (LOX) are a family of nonheme, iron-containing enzymes that catalyze the conversion of polyunsaturated fatty acids into conjugated hydroperoxides. 15-LOX has recently been shown to generate ferroptotic lipid death signals [doubly and triply oxygenated (15-hydroperoxy)-diacylated PE species]. It is unclear whether these proteins—NOX1, NOX4, and 15-LOX—are solely responsible for the role of iron in ferroptosis or if additional iron-containing proteins are involved. Whatever the mechanism, cells with increased intracellular iron levels become more sensitive to ferroptosis. Given that cancer cells subvert normal iron metabolism to increase intracellular iron (Figure 1), ferroptosis may be a promising new therapeutic target. In this regard, it is of particular interest that ferroptosis inducers target tumor-initiating cells (18).
7. CLINICAL IMPORTANCE OF IRON
7.1. Iron in Cancer Prognosis
Because iron initiates, fosters growth, and alters the survival of cancer cells, the prognostic value of iron and iron regulatory genes has been investigated. Serum FT levels have demonstrated prognostic value in several cancers, including oral squamous cell carcinoma (100), cervical cancer (92), Hodgkin’s lymphoma (82), metastatic colorectal cancer (106), breast cancer (117), lung cancer (61, 140), renal cell carcinoma (101), stage III and IV neuroblastomas (81), and peripheral T cell lymphoma (103). Each of these studies associates increased serum FT levels with poor prognosis. This association is surprising considering the prevalence of anemia in cancer (see Section 4.1.2); anemia itself is an adverse prognostic factor for some cancers (28). It is important to note that increased serum FT does not necessarily correlate with increased body iron levels, and serum FT may be altered in response to other stimuli, such as inflammation (98). Therefore, alternative markers of iron status, such as TF saturation (37), may have better discriminative value as prognostic indicators.
Given the complex interplay between systemic iron and intratumoral iron, identifying iron regulatory genes in cancer cells and tissues may present a better opportunity to identify iron-dependent prognostic factors. In breast cancer patients, the expression of iron regulatory genes was evaluated for its prognostic ability (121). Almost half of 61 iron regulatory genes analyzed were found to be associated with survival for breast cancer patients. A subset of 16 genes contained most of the prognostic information, and this subset was termed the iron regulatory gene signature (IRGS). In a multivariate analysis, the IRGS added prognostic value even when the patient’s age, tumor size, histological grade, and nodal and estrogen receptor status were taken into account. The IRGS was also able to stratify homogeneously treated lymph node–positive patients into groups with significantly different prognoses. Interestingly, an independent group using the same 61 iron regulatory genes identified an IRGS in diffuse infiltrating gliomas (179). This study identified eight genes that predicted the median survival of patients with grade II and III gliomas; however, only two of these genes overlapped with the breast cancer IRGS, suggesting that the specific iron regulatory genes that are altered may be different in different cancer types.
Further analysis of iron gene expression in breast cancer revealed that an iron import dyad [TFRC and HFE (transferrin receptor 1 and hemochromatosis)] and an iron exportd yad [SLC40A1 and HAMP (FPN and hepcidin)] were complementary prognostic factors in predicting distant metastasis-free survival in a cohort of estrogen receptor–positive patients treated with tamoxifen (121). Poor prognosis was conferred by gene expression favoring increased cellular iron levels by both dyads (import dyad: high TFRC, low HFE; export dyad: low SLC40A1, high HAMP), consistent with the role of iron as a tumor and metastasis promoter. The prognostic value of TFRC in breast cancer was confirmed in an independent study (79). The prognostic value of the export dyad, FPN and hepcidin (SLC40A1 and HAMP), had previously been demonstrated (135). Importantly, this study provided evidence to suggest that measuring the expression of FPN and hepcidin may be useful in planning treatment for patients with estrogen receptor–positive breast cancer.
7.2. Iron in Cancer Therapy
Numerous approaches have been taken to capitalize on the iron dependence of cancer cells (which we have termed iron addiction) for therapeutic benefit. These range from designing strategies to deprive cancer cells of iron to the polar opposite: deliberately utilizing the excess iron in cancer cells to selectively deliver cytotoxic levels of ROS.
7.2.1. Iron chelators.
In preclinical studies, several iron chelators, differing in structure and pharmacology, have demonstrated antitumor activity through the depletion of iron (Section 5.2). Iron chelators that bind iron and permit redox cycling, thus acting through a mechanism separate from iron depletion, have also shown promise as antitumor agents (96). As a result, the safety and efficacy of iron chelators as cancer therapeutics have been tested in clinical trials. Safety concerns were found to be mild to moderate for most iron chelators [ciclopirox (122), deferasirox (69), deferiprone (38), deferitazole (126), desferoxamine (DFO) (192), and triapine (102, 128)]; gastroin testinal symptoms(diarrhea, nausea, vomiting)and fatigue appear to be the most prevalent toxicities. Trials assessing the safety of additional iron chelators, such as DpC (Dp4cycH4mT) (https://ClinicalTrials.gov/show/NCT02688101) and VLX600 (https://ClinicalTrials.gov/show/NCT02222363), are ongoing.
Initial studies in cancer patients focused on DFO due to its safety record in the clinical treatment of iron overload. DFO treatment had a 20% overall response rate (ORR; the percentage of patients with a predefined reduction in tumor size for a certain duration) in a cohort of 10 patients with advanced hepatocellular carcinoma (192). DFO was also effective in reducing bone marrow infiltrate in seven of nine patients with neuroblastoma (51). A second study found that DFO administration was ineffective in treating patients with recurrent neuroblastoma (21). DFO was also ineffective in reducing the progression of hormone-refractory prostate cancer (53). More recently, other chelators have been investigated. In 70% of patients with advanced leukemia, triapine reduced white blood cell counts by more than 50% (70). In a follow-up study, 76% of patients with advanced hematological malignancies demonstrated a 50% reduction in white blood cell counts following triapine treatment. Hematological parameters were also improved in patients with advanced hematological malignancies treated with ciclopirox (122) and deferasirox (110). However, triapine showed only a minimal ORR in metastatic renal cell carcinoma (7%) (102) and in recurrent and metastatic head and neck squamous cell carcinoma (128).
Iron chelators have also been investigated as part of combination therapies. DFO was effective in combination with cyclophosphamide, etoposide, carboplatin, and thiotepa (D-CECaT) in a cohort of patients with advanced neuroblastoma and primitive neuroectodermal tumors (50). Triapine used in combination with fludarabine, a DNA synthesis inhibitor, demonstrated an ORR of 49% and a complete remission rate of 24% in a cohort of 37 patients with accelerated myeloproliferative neoplasms and secondary acute myeloid leukemia (196). However, in a cohort of 12 patients with advanced non-small-cell lung cancer, triapine was ineffective in initial combination with (162), or resensitization (112) to, gemcitabine, a nucleoside analog inhibitor of DNA synthesis.
Thus far, the clinical data suggest that hematological malignancies may be more responsive to iron chelator therapy than other malignancies. This observation may relate to limited pharmacokinetic access of iron chelators to solid tumors, or, alternatively, may indicate an increased dependence of hematological malignancies on iron.
7.2.2. Targeting elevated cancer iron levels.
As an alternative to iron chelator therapy, several approaches attempt to leverage increased iron levels to treat cancer. Pharmacological ascorbate therapy is currently the most clinically relevant example of this approach. Ascorbate therapy specifically targets cancer cells via an iron-dependent mechanism (see Section 6.3) and is clinically effective as a single agent (27) and in combination therapy (113, 177). Alternatively, several ferroptosis inducers have been identified and offer the opportunity to selectively target cancer via increased tumor iron levels (151). The best-studied ferroptosis inducers, erastin and RSL3, are poor candidates for therapeutic use due to their pharmacological properties, and efforts to identify or synthesize new ferroptosis inducers with better pharmacokinetics are ongoing. Currently, several US Food and Drug Administration (FDA)-approved drugs have been demonstrated in preclinical models to induce ferroptosis in cancer cells. The first is sorafenib, which is used for cancer therapy and has been shown to induce multiple forms of cell death (49). The second is sulfasalazine (143), which in limited studies has not proven to be an effective therapy (139). A third is artesunate (54), an antimalarial therapy, whose efficacy as an anticancer agent is being tested (170). Further, altretamine, the FDA-approved agent for ovarian cancer, likely induces ferroptosis, based on the observation that it inhibits the activity of the ferroptotic suppressor glutathione peroxidase 4 (185). Haloperidol, a σ-receptor 1 agonist, was also shown to sensitize cells to erastin-induced ferroptosis (13). The clinical utility of these FDA-approved ferroptosis-inducing agents requires further examination.
Therapies targeting the TF receptor have also been investigated. The most straightforward approach, using TF-targeted antibodies, was effective in reducing tumor growth in preclinical models (163). An alternative approach has been the development of TF-conjugated chemotherapeutic agents to increase the specificity of drug delivery (160). TF is an excellent conjugation partner for chemotherapies due to its enhanced expression in cancer cells and the high efficiency of binding to its receptor. Further, TF can efficiently traverse the blood–brain barrier, enhancing the ability to target neurological malignancies. These factors have led to a number of different classes of TF-conjugation partners, including chemotherapeutic drugs (56), nucleotides (144), and others, that have shown varying levels of clinical efficacy. An expanded understanding of the mechanisms of iron metabolism has allowed the identification of new pathways that may represent additional opportunities for exploiting iron metabolism for drug delivery in the future. These include CD163, LRP1, HRG1, SCARA5, and FLVCR2 (40).
8. PERSPECTIVES AND FUTURE DIRECTIONS
The understanding of the role of iron in cancer has advanced considerably in recent years. It has become apparent that iron contributes not only to carcinogenesis but also to tumor progression and metastasis. Through interfaces with oncogenes and tumor suppressors, iron metabolism is intimately knit into the fabric of cancer biology.
The profound role of iron in cancer renders the expression of iron regulatory genes and gene sets of prognostic value, and it has the potential to contribute to clinical decision-making. Interestingly, the prognostic value of iron regulatory genes may vary among cancer types, possibly due to underlying variations in iron dysregulation among cancers. Considering recent findings linking iron to metastasis, it will be particularly interesting to evaluate the prognostic value of iron regulatory genes in metastatic disease.
The dysregulation of iron is observed early in the transition to malignant growth, and it is already evident in tumor-initiating cancer stem cells. These cells are believed to contribute to metastatic spread and treatment resistance. Thus, an exciting possibility is that the dependence of cancer cells on iron represents a metabolic vulnerability that can be used not only to target bulk cancer, but, by targeting tumor-initiating cells, to prevent or reduce disease recurrence.
The iron-seeking phenotype that characterizes most tumors can be exploited in two ways: One is to restrict iron availability, thus starving these cells of a critical nutrient on which they exhibit enhanced dependence, and the second is to use the redox properties of excess iron to preferentially foster cytotoxic oxidative stress in cancer cells. Both strategies are being actively explored. In addition, strategies designed to use pathways of iron uptake to preferentially deliver anticancer drugs into cells are being developed.
In the same light, the recent discovery of ferroptosis provides a new avenue for designing iron-targeted cancer therapies. The selective killing of cancer cells, including tumor-initiating cells, by ferroptosis may emerge as an attractive anticancer strategy. Further understanding of the exact role of iron in ferroptosis will aid in the development of clinically useful ferroptotic agents.
ACKNOWLEDGMENTS
This work was supported in part by grants NCI R01 CA188025 (S.V.T), NCI R01 CA171101 (F.M.T), and F30 DE026380 and T90 DE021989 from the National Institute of Dental and Craniofacial Research (D.H.M.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abbaspour N, Hurrell R, Kelishadi R. 2014. Review on iron and its importance for human health. J. Res. Med. Sci. 19:164–74 [PMC free article] [PubMed] [Google Scholar]
- 2.Abeysinghe RD, Greene BT, Haynes R, Willingham MC, Turner J, et al. 2001. p53-independent apoptosis mediated by tachpyridine, an anti-cancer iron chelator. Carcinogenesis 22:1607–14 [DOI] [PubMed] [Google Scholar]
- 3.Adams PC. 2015. Epidemiology and diagnostic testing for hemochromatosis and iron overload. Int. J. Lab. Hematol. 37(Suppl. 1):25–30 [DOI] [PubMed] [Google Scholar]
- 4.Aisen P, Enns C, Wessling-Resnick M. 2001. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 33:940–59 [DOI] [PubMed] [Google Scholar]
- 5.Akatsuka S, Yamashita Y, Ohara H, Liu YT, Izumiya M, et al. 2012. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLOS ONE 7:e43403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander DD, Mink PJ, Cushing CA, Sceurman B. 2010. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr. J. 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander DD, Morimoto LM, Mink PJ, Cushing CA. 2010. A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr. Res. Rev. 23:349–65 [DOI] [PubMed] [Google Scholar]
- 8.Alexander DD, Weed DL, Cushing CA, Lowe KA. 2011. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur. J. Cancer Prev. 20:293–307 [DOI] [PubMed] [Google Scholar]
- 9.Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL. 2013. Sirtuin-3 (SIRT3) and the hallmarks of cancer. Genes Cancer 4:164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anjem A, Imlay JA. 2012. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J. Biol. Chem. 287:15544–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, et al. 2013. Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control 24:611–27 [DOI] [PubMed] [Google Scholar]
- 12.Azuma A, Matsuo A, Suzuki T, Kurosawa T, Zhang X, Aida Y. 2006. Human immunodeficiency virustype1Vpr induces cell cycle arrest at theG1 phase and apoptosis via disruption of mitochondrial function in rodent cells. Microbes Infect. 8:670–79 [DOI] [PubMed] [Google Scholar]
- 13.Bai T, Wang S, Zhao Y, Zhu R, Wang W, Sun Y. 2017. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 491:919–25 [DOI] [PubMed] [Google Scholar]
- 14.Bao G, Clifton M, Hoette TM, Mori K, Deng SX, et al. 2010. Iron traffics in circulation bound to a siderocalin (Ngal)–catechol complex. Nat. Chem. Biol. 6:602–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basit F, van Oppen LM, Schockel L, Bossen broek HM, van Emst-de-Vries SE, et al. 2017.Mitochondrial¨ complex Iinhibition triggers amitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 8:e2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastide NM, Chenni F, Audebert M, Santarelli RL, Tache S, et al. 2015. A central role for heme iron incolon carcinogenesis associated with red meat intake. Cancer Res. 75:870–79 [DOI] [PubMed] [Google Scholar]
- 17.Bastide NM, Pierre FH, Corpet DE. 2011. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev. Res. 4:177–84 [DOI] [PubMed] [Google Scholar]
- 18.Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, et al. 2017. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 36:4089–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein AM, Song M, Zhang X, Pan A, Wang M, et al. 2015. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLOS ONE 10:e0135959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingham SA, Hughes R, Cross AJ. 2002. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J. Nutr. 132:3522S–25S [DOI] [PubMed] [Google Scholar]
- 21.Blatt J. 1994. Deferoxamine in children with recurrent neuroblastoma. Anticancer Res. 14:2109–12 22. [PubMed] [Google Scholar]
- 22.Brookes MJ, Boult J, Roberts K, Cooper BT, Hotchin NA, et al. 2008. A role for iron in Wnt signalling. Oncogene 27:966–75 [DOI] [PubMed] [Google Scholar]
- 23.Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, et al. 2006. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut 55:1449–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buck I, Morceau F, Grigorakaki C, Dicato M, Diederich M. 2009. Linking anemia to inflammation and cancer: the crucial role of TNFα. Biochem. Pharmacol. 77:1572–79 [DOI] [PubMed] [Google Scholar]
- 25.Bylsma LC, Alexander DD. 2015. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr. J. 14:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cairns RA, Harris IS, Mak TW. 2011. Regulation of cancer cell metabolism. Nat. Rev. Cancer 11:85–95 [DOI] [PubMed] [Google Scholar]
- 27.Cameron E, Pauling L. 1978. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. PNAS 75:4538–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caro JJ, Salas M, Ward A, Goss G. 2001. Anemia as an independent prognostic factor for survival inpatients with cancer: a systemic, quantitative review. Cancer 91:2214–21 [PubMed] [Google Scholar]
- 29.Carosio R, Zuccari G, Orienti I, Mangraviti S, Montaldo PG. 2007. Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol. Cancer 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carr PR, Walter V, Brenner H, Hoffmeister M. 2016. Meat subtypes and their association with colorectal cancer: systematic review and meta-analysis. Int. J. Cancer 138:293–302 [DOI] [PubMed] [Google Scholar]
- 31.Cascino I, Fiucci G, Papoff G, Ruberti G. 1995. Three functional soluble forms of the human apoptosis inducing Fas molecule are produced by alternative splicing. J. Immunol. 154:2706–13 [PubMed] [Google Scholar]
- 32.Chang X, Xu X, Xue X, Ma J, Li Z, et al. 2016. NDRG1 controls gastric cancer migration and invasion through regulating MMP-9. Pathol. Oncol. Res. 22:789–96 [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, et al. 2005. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. PNAS 102:13604–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zhang Z, Yang K, Du J, Xu Y, Liu S. 2015. Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor growth through enforcing ferroportin-conducted iron egress. Oncogene 34:3839–47 [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Zhang D, Yue F, Zheng M, Kovacevic Z, Richardson DR. 2012. The iron chelators Dp44mTand DFO inhibit TGF-β-induced epithelial–mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J. Biol. Chem. 287:17016–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, et al. 1994. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263:1759–62 [DOI] [PubMed] [Google Scholar]
- 37.Chua AC, Knuiman MW, Trinder D, Divitini ML, Olynyk JK. 2016. Higher concentrations of serumiron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am. J. Clin. Nutr. 104:736–42 [DOI] [PubMed] [Google Scholar]
- 38.Cohen AR, Galanello R, Piga A, Dipalma A, Vullo C, Tricta F. 2000. Safety profile of the oral iron chelator deferiprone: a multicentre study. Br. J. Haematol. 108:305–12 [DOI] [PubMed] [Google Scholar]
- 39.Crichton R. 2016. The essential role of iron in biology. In Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, ed. Crichton R, pp. 22–70. Chichester, UK: Wiley. 4th ed. [Google Scholar]
- 40.Crielaard BJ, Lammers T, Rivella S. 2017. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 16:400–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, et al. 2010. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 70:2406–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. 2007. A prospective study of red and processed meat intake in relation to cancer risk. PLOS Med. 4:e325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross AJ, Pollock JR, Bingham SA. 2003. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 63:2358–60 [PubMed] [Google Scholar]
- 44.Dai MC, Zhong ZH, Sun YH, Sun QF, Wang YT, et al. 2013. Curcumin protects against iron induced neurotoxicity in primary cortical neurons by attenuating necroptosis. Neurosci. Lett. 536:41–46 [DOI] [PubMed] [Google Scholar]
- 45.Deng Z, Manz DH, Torti SV, Torti FM. 2017. Effects of ferroportin-mediated iron depletion in cells representative of different histological subtypes of prostate cancer. Antioxid. Redox Signal. In press. 10.1089/ars.2017.7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng Z, Manz DH, Torti SV, Torti FM. 2017. Iron-responsive element-binding protein 2 plays an essential role in regulating prostate cancer cell growth. Oncotarget 8:82231–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devireddy LR, Gazin C, Zhu X, Green MR. 2005. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123:1293–305 [DOI] [PubMed] [Google Scholar]
- 48.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, et al. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, et al. 2014. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3:e02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donfrancesco A, Deb G, Angioni A, Maurizio C, Cozza R, et al. 1993. D-CECaT: a breakthrough for patients with neuroblastoma. Anticancer Drugs 4:317–21 [PubMed] [Google Scholar]
- 51.Donfrancesco A, Deb G, Dominici C, Pileggi D, Castello MA, Helson L. 1990. Effects of a single course of deferoxamine in neuroblastoma patients. Cancer Res. 50:4929–30 [PubMed] [Google Scholar]
- 52.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, et al. 2014. Selective VPS 34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16:1069–79 [DOI] [PubMed] [Google Scholar]
- 53.Dreicer R, Kemp JD, Stegink LD, Cardillo T, Davis CS, et al. 1997. A phase II trial of deferoxamine inpatients with hormone-refractory metastatic prostate cancer. Cancer Investig. 15:311–17 [DOI] [PubMed] [Google Scholar]
- 54.Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. 2015. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2:517–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elledge SJ, Zhou Z, Allen JB. 1992. Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem. Sci. 17:119–23 [DOI] [PubMed] [Google Scholar]
- 56.Elliott RL, Stjernholm R, Elliott MC. 1988. Preliminary evaluation of platinum transferrin (MPTC-63) as a potential nontoxic treatment for breast cancer. Cancer Detect. Prev. 12:469–80 [PubMed] [Google Scholar]
- 57.Elmberg M, Hultcrantz R, Ekbom A, Brandt L, Olsson S, et al. 2003. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology 125:1733–41 [DOI] [PubMed] [Google Scholar]
- 58.Eshhar Z, Order SE, Katz DH. 1974. Ferritin, a Hodgkin’s disease associated antigen. PNAS 71:3956–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fargion S, Valenti L, Fracanzani AL. 2010. Hemochromatosis gene (HFE) mutations and cancer risk: expanding the clinical manifestations of hereditary iron overload. Hepatology 51:1119–21 [DOI] [PubMed] [Google Scholar]
- 60.Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. 2005. The matrix metalloproteinase9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin. Cancer Res. 11:5390–95 [DOI] [PubMed] [Google Scholar]
- 61.Ferrigno D, Buccheri G. 1992. Serum ferritin levels in lung cancer patients. Eur. J. Cancer 28:241. [DOI] [PubMed] [Google Scholar]
- 62.Ferrucci LM, Cross AJ, Graubard BI, Brinton LA, McCarty CA, et al. 2009. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Br. J. Cancer 101:178–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, et al. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–21 [DOI] [PubMed] [Google Scholar]
- 64.Fonseca-Nunes A, Agudo A, Aranda N, Arija V, Cross AJ, et al. 2015. Body iron status and gastric cancer risk in the EURGAST study. Int. J. Cancer 137:2904–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fonseca-Nunes A, Jakszyn P, Agudo A. 2014. Iron and cancer risk—a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol. Biomark. Prev. 23:12–31 [DOI] [PubMed] [Google Scholar]
- 66.Gangaidzo IT, Gordeuk VR. 1995. Hepatocellular carcinoma and African iron overload. Gut 37:727–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. 2016. Ferroptosis is an autophagic cell death process. Cell Res. 26:1021–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. 2015. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59:298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gattermann N, Finelli C, Porta MD, Fenaux P, Ganser A, et al. 2010. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk. Res. 34:1143–50 [DOI] [PubMed] [Google Scholar]
- 70.Giles FJ, Fracasso PM, Kantarjian HM, Cortes JE, Brown RA, et al. 2003. Phase I and pharmacodynamic study of Triapine®, a novel ribonucleotide reductase inhibitor, inpatients with advanced leukemia. Leuk. Res. 27:1077–83 [DOI] [PubMed] [Google Scholar]
- 71.Gnagnarella P, Caini S, Maisonneuve P, Gandini S. 2018. Carcinogenicity of high consumption of meat and lung cancer risk among non-smokers: a comprehensive meta-analysis. Nutr. Cancer 70:1–13 [DOI] [PubMed] [Google Scholar]
- 72.Gomes IM, Maia CJ, Santos CR. 2012. STEAP proteins: from structure to applications in cancer therapy. Mol. Cancer Res. 10:573–87 [DOI] [PubMed] [Google Scholar]
- 73.Gordeuk VR, Caleffi A, Corradini E, Ferrara F, Jones RA, et al. 2003. Iron overload in Africans and African-Americans and a common mutation in the SCL40A1 (ferroportin 1) gene. Blood Cells Mol. Dis. 31:299–304 [DOI] [PubMed] [Google Scholar]
- 74.Graf E, Eaton JW. 1985. Dietary suppression of colonic cancer. Fiber or phytate? Cancer 56:717–18 [DOI] [PubMed] [Google Scholar]
- 75.Greene BT, Thorburn J, Willingham MC, Thorburn A, Planalp RP, et al. 2002. Activation of caspase pathways during iron chelator–mediated apoptosis. J. Biol. Chem. 277:25568–75 [DOI] [PubMed] [Google Scholar]
- 76.Groopman JE, Itri LM. 1999. Chemotherapy-induced anemia in adults: incidence and treatment. J. Natl. Cancer Inst. 91:1616–34 [DOI] [PubMed] [Google Scholar]
- 77.Guo J, Wei W, Zhan L. 2015. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res. Treatment 151:191–98 [DOI] [PubMed] [Google Scholar]
- 78.Guo W, Zhang S, Chen Y, Zhang D, Yuan L, et al. 2015. An important role of the hepcidin–ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim. Biophys. Sin. 47:703–15 [DOI] [PubMed] [Google Scholar]
- 79.Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, et al. 2010. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res. Treat. 119:283–93 [DOI] [PubMed] [Google Scholar]
- 80.Hambidge M. 2003. Biomarkers of trace mineral intake and status. J. Nutr. 133(Suppl. 3):948S–55S [DOI] [PubMed] [Google Scholar]
- 81.Hann HW, Evans AE, Siegel SE, Wong KY, Sather H, et al. 1985. Prognostic importance of serum ferritin in patients with stages III and IV neuroblastoma: the Children’s Cancer Study Group experience. Cancer Res. 45:2843–48 [PubMed] [Google Scholar]
- 82.Hann HW, Lange B, Stahlhut MW, McGlynn KA. 1990. Prognostic importance of serum transferrin and ferritin in childhood Hodgkin’s disease. Cancer 66:313–16 [DOI] [PubMed] [Google Scholar]
- 83.Hann HW, Stahlhut MW, Blumberg BS. 1988. Iron nutrition and tumor growth: decreased tumor growth in iron-deficient mice. Cancer Res. 48:4168–70 [PubMed] [Google Scholar]
- 84.Hann HW, Stahlhut MW, Menduke H. 1991. Iron enhances tumor growth: observation on spontaneous mammary tumors in mice. Cancer 68:2407–10 [DOI] [PubMed] [Google Scholar]
- 85.Hjalgrim H, Edgren G, Rostgaard K, Reilly M, Tran TN, et al. 2007. Cancer incidence in blood transfusion recipients. J. Natl. Cancer Inst. 99:1864–74 [DOI] [PubMed] [Google Scholar]
- 86.Hogemann-Savellano D, Bos E, Blondet C, Sato F, Abe T, et al. 2003. The transferrin receptor: a potential molecular imaging marker for human cancer. Neoplasia 5:495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holler N, Zaru R, Micheau O, Thome M, Attinger A, et al. 2000. Fas triggers an alternative, caspase8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489–95 [DOI] [PubMed] [Google Scholar]
- 88.Horniblow RD, Bedford M, Hollingworth R, Evans S, Sutton E, et al. 2017. BRAF mutations are associated with increased iron regulatory protein-2 expression in colorectal tumorigenesis. Cancer Sci. 108:1135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang X. 2003. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat. Res. 533:153–71 [DOI] [PubMed] [Google Scholar]
- 90.Ichim G, Tait SW. 2016. A fate worse than death: apoptosis as an oncogenic process. Nat. Rev. Cancer 16:539–48 [DOI] [PubMed] [Google Scholar]
- 91.Inoue-Choi M, Sinha R, Gierach GL, Ward MH. 2016. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH—ARP Diet and Health Study. Int. J. Cancer 138:1609–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ito H, Takagi Y, Ando Y, Kubo A, Hashimoto S, et al. 1980. Serum ferritin levels in patients with cervical cancer. Obstet. Gynecol. 55:358–62 [DOI] [PubMed] [Google Scholar]
- 93.Jakszyn P, Agudo A, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, et al. 2012. Dietary intake of heme iron and risk of gastric cancer in the European Prospective Investigation into Cancer and nutrition study. Int. J. Cancer 130:2654–63 [DOI] [PubMed] [Google Scholar]
- 94.Jeong SM, Lee J, Finley LW, Schmidt PJ, Fleming MD, Haigis MC. 2015. SIRT3 regulates cellular iron metabolism and cancer growth by repressing iron regulatory protein 1. Oncogene 34:2115–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, et al. 2009. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic. Biol. Med. 46:1439–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalinowski DS, Stefani C, Toyokuni S, Ganz T, Anderson GJ, et al. 2016. Redox cycling metals: pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim. Biophys. Acta 1863:727–48 [DOI] [PubMed] [Google Scholar]
- 97.Kasvosve I, Gangaidzo IT, Gomo ZA, Gordeuk VR. 2000. African iron overload. ActaClin.Belg.55:88–93 [DOI] [PubMed] [Google Scholar]
- 98.Kell DB, Pretorius E. 2014. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 6:748–73 [DOI] [PubMed] [Google Scholar]
- 99.Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. 2012. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann. Oncol. 23:2319–26 [DOI] [PubMed] [Google Scholar]
- 100.Khanna V, Karjodkar F, Robbins S, Behl M, Arya S, Tripathi A. 2017. Estimation of serum ferritin levelin potentially malignant disorders, oral squamous cell carcinoma, and treated cases of oral squamous cell carcinoma. J. Cancer Res. Ther. 13:550–55 [DOI] [PubMed] [Google Scholar]
- 101.Kırkalı Z, Güzelsoy M, Mungan MU, Kırkalı G, Yorukoglu K. 1999. Serum ferritin as a clinical marker for renal cell carcinoma: influence of tumor size and volume. Urol. Int. 62:21–25 [DOI] [PubMed] [Google Scholar]
- 102.Knox JJ, Hotte SJ, Kollmanns berger C, Winquist E, Fisher B, Eisenhauer EA. 2007. Phase II study of Triapine® in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161). Investig. New Drugs 25:471–77 [DOI] [PubMed] [Google Scholar]
- 103.Koyama S, Fujisawa S, Watanabe R, Itabashi M, Ishibashi D, et al. 2017. Serum ferritin level is a prognostic marker in patients with peripheral T-cell lymphoma. Int. J. Lab. Hematol. 39:112–17 [DOI] [PubMed] [Google Scholar]
- 104.Lambeth JD. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181–89 [DOI] [PubMed] [Google Scholar]
- 105.Larsson SC, Wolk A. 2012. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br. J. Cancer 106:603–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee S, Song A, Eo W. 2016. Serum ferritin as a prognostic biomarker for survival in relapsed or refractory metastatic colorectal cancer. J. Cancer 7:957–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leng X, Wu Y, Arlinghaus RB. 2011. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J. Cell. Physiol. 226:309–14 [DOI] [PubMed] [Google Scholar]
- 108.Liang SX, Richardson DR. 2003. The effect of potent iron chelators on the regulation of p53: examination of the expression, localization and DNA-binding activity of p53 and the transactivation of WAF1. Carcinogenesis 24:1601–14 [DOI] [PubMed] [Google Scholar]
- 109.Linkermann A, Green DR. 2014. Necroptosis. N. Engl. J. Med. 370:455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.List AF, Baer MR, Steensma DP, Raza A, Esposito J, et al. 2012. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J. Clin. Oncol. 30:2134–39 [DOI] [PubMed] [Google Scholar]
- 111.Lui GY, Obeidy P, Ford SJ, Tselepis C, Sharp DM, et al. 2013. The iron chelator, deferasirox, as a novel strategy for cancer treatment: oral activity against human lung tumor xenografts and molecular mechanism of action. Mol. Pharmacol. 83:179–90 [DOI] [PubMed] [Google Scholar]
- 112.Ma B, Goh BC, Tan EH, Lam KC, Soo R, et al. 2008. A multicenter phase II trial of 3-aminopyridine-2carboxaldehyde thiosemicarbazone (3-AP, Triapine®) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Investig. New Drugs 26:169–73 [DOI] [PubMed] [Google Scholar]
- 113.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. 2014. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 6:222ra18 [DOI] [PubMed] [Google Scholar]
- 114.Maffettone C, Chen G, Drozdov I, Ouzounis C, Pantopoulos K. 2010. Tumorigenic properties of iron regulatory protein 2 (IRP2) mediated by its specific 73-amino acids insert. PLOS ONE 5:e10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. 2016. Iron and cancer: recent insights. Ann. N. Y. Acad. Sci. 1368:149–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marcus DM, Zinberg N. 1974. Isolation of ferritin from human mammary and pancreatic carcinomas by means of antibody immunoadsorbents. Arch. Biochem. Biophys. 162:493–501 [DOI] [PubMed] [Google Scholar]
- 117.Marcus DM, Zinberg N. 1975. Measurement of serum ferritin by radioimmunoassay: results in normal individuals and patients with breast cancer. J. Natl. Cancer Inst. 55:791–95 [DOI] [PubMed] [Google Scholar]
- 118.Marsee DK, Pinkus GS, Yu H. 2010. CD71 (transferrin receptor): an effective marker for erythroid precursors in bone marrow biopsy specimens. Am. J. Clin. Pathol. 134:429–35 [DOI] [PubMed] [Google Scholar]
- 119.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, et al. 2000. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5:299–309 [DOI] [PubMed] [Google Scholar]
- 120.Merk K, Mattsson B, Mattsson A, Holm G, Gullbring B, Bjorkholm M. 1990. The incidence of cancer among blood donors. Int. J. Epidemiol. 19:505–9 [DOI] [PubMed] [Google Scholar]
- 121.Miller LD, Coffman LG, Chou JW, Black MA, Bergh J, et al. 2011. An iron regulatory gene signature predicts outcome in breast cancer. Cancer Res. 71:6728–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Minden MD, Hogge DE, Weir SJ, Kasper J, Webster DA, et al. 2014. Oral ciclopirox olamine displays biological activity in a phase I study in patients with advanced hematologic malignancies. Am. J. Hematol. 89:363–68 [DOI] [PubMed] [Google Scholar]
- 123.Muka T, Kraja B, Ruiter R, Lahousse L, de Keyser CE, et al. 2017. Dietary mineral intake and lung cancer risk: the Rotterdam Study. Eur. J. Nutr. 56:1637–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Musallam KM, Cappellini MD, Wood JC, Taher AT. 2012.Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. 26(Suppl. 1): S16–19 [DOI] [PubMed] [Google Scholar]
- 125.Nemeth E, Ganz T. 2014. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 28:671–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Neufeld EJ, Galanello R, Viprakasit V, Aydinok Y, Piga A, et al. 2012. A phase 2 study of the safety, tolerability, and pharmacodynamics of FBS0701, a novel oral iron chelator, intransfusional iron ov erload. Blood 119:3263–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, et al. 2001. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. PNAS 98:8780–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nutting CM, van Herpen CM, Miah AB, Bhide SA, Machiels JP, et al. 2009. Phase II study of 3-APTriapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 20:1275–79 [DOI] [PubMed] [Google Scholar]
- 129.Paluszkiewicz P, Smolinska K, Debinska I, Turski WA. 2012. Main dietary compounds and pancreatic cancer risk: the quantitative analysis of case–control and cohort studies. Cancer Epidemiol. 36:60–67 [DOI] [PubMed] [Google Scholar]
- 130.Papasaikas P, Tejedor JR, Vigevani L, Valcarcel J. 2015. Functional splicing network reveals extensive regulatory potential of the core spliceosomal machinery. Mol. Cell 57:7–22 [DOI] [PubMed] [Google Scholar]
- 131.Pham NM, Mizoue T, Tanaka K, Tsuji I, Tamakoshi A, et al. 2014. Meat consumption and colorectal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 44:641–50 [DOI] [PubMed] [Google Scholar]
- 132.Pietrangelo A. 2015. Genetics, genetic testing, and management of hemochromatosis: 15 years since hepcidin. Gastroenterology 149:1240–51 [DOI] [PubMed] [Google Scholar]
- 133.Pietsch EC, Chan JY, Torti FM, Torti SV. 2003. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J. Biol. Chem. 278:2361–69 [DOI] [PubMed] [Google Scholar]
- 134.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, et al. 2001. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 276:7811–19 [DOI] [PubMed] [Google Scholar]
- 135.Pinnix ZK, Miller LD, Wang W, D’Agostino R Jr., Kute T, et al. 2010. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2:43ra56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Radulescu S, Brookes MJ, Salgueiro P, Ridgway RA, McGhee E, et al. 2012. Luminal iron levels govern intestinal tumorigenesis after Apc loss in vivo. Cell Rep. 2:270–82. Erratum. 2016. Cell Rep. 17:2805–7 [DOI] [PubMed] [Google Scholar]
- 137.Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, et al. 2004. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117:773–86 [DOI] [PubMed] [Google Scholar]
- 138.Richmond HG. 1959. Induction of sarcoma in the rat by iron–dextran complex. Br. Med. J. 1(5127):947–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Robe PA, Martin DH, Nguyen-Khac MT, Artesi M, Deprez M, et al. 2009. Early termination ofISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer 9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sakowicz A, Kwiek S, Wiatr E. 1991. [Levels of ferritin in serum of patients with lung cancer]. Pneumonol. Alergol. Polska 59:38–42 [PubMed] [Google Scholar]
- 141.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, et al. 2017. O2– and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 32:268. [DOI] [PubMed] [Google Scholar]
- 142.Schonberg DL, Miller TE, Wu Q, Flavahan WA, Das NK, et al. 2015. Preferential iron trafficking characterizes glioblastoma stem-like cells. Cancer Cell 28:441–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sehm T, Fan Z, Ghoochani A, Rauh M, Engelhorn T, et al. 2016. Sulfasalazine impacts on ferroptotic cell death and alleviates the tumor microenvironment and glioma-induced brain edema. Oncotarget 7:36021–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, et al. 2013. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol. Ther. 21:1096–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. 1999. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 59:5704–9 [PubMed] [Google Scholar]
- 146.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. 2000. Red meat and colon cancer: Dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium. Carcinogenesis 21:1909–15 [DOI] [PubMed] [Google Scholar]
- 147.Siew SS, Kauppinen T, Kyyronen P, Heikkila P, Pukkala E. 2008. Exposure to iron and welding fumes and the risk of lung cancer. Scand. J. Work Environ. Health 34:444–50 [DOI] [PubMed] [Google Scholar]
- 148.Simonart T, Degraef C, Andrei G, Mosselmans R, Hermans P, et al. 2000. Iron chelators inhibit the growth and induce the apoptosis of Kaposi’s sarcoma cells and of their putative endothelial precursors. J. Investig. Dermatol. 115:893–900 [DOI] [PubMed] [Google Scholar]
- 149.Sinha R, Knize MG, Salmon CP, Brown ED, Rhodes D, et al. 1998. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem. Toxicol. 36:289–97 [DOI] [PubMed] [Google Scholar]
- 150.Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. 1994. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int. J. Cancer 56:364–69 [DOI] [PubMed] [Google Scholar]
- 151.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, et al. 2017. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun J, Zhang D, Zheng Y, Zhao Q, Zheng M, et al. 2013. Targeting the metastasis suppressor, NDRG1, using novel iron chelators: regulation of stress fiber-mediated tumor cell migration via modulation of the ROCK1/pMLC2 signaling pathway. Mol. Pharmacol. 83:454–69 [DOI] [PubMed] [Google Scholar]
- 153.Tasevska N, Sinha R, Kipnis V, Subar AF, Leitzmann MF, et al. 2009. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am. J. Clin. Nutr. 89:1884–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taunk P, Hecht E, Stolzenberg-Solomon R. 2016. Are meat and heme iron intake associated with pancreatic cancer? Results from the NIH–AARP diet and health cohort. Int. J. Cancer 138:2172–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taylor RC, Cullen SP, Martin SJ. 2008. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9:231–41 [DOI] [PubMed] [Google Scholar]
- 156.Tejedor JR, Papasaikas P, Valcarcel J. 2015.Genome-wide identification of Fas/CD95 alternative splicing regulators reveals links with iron homeostasis. Mol. Cell 57:23–38 [DOI] [PubMed] [Google Scholar]
- 157.Tesfay L, Clausen KA, Kim JW, Hegde P, Wang X, et al. 2015. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 75:2254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. 2005. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem. J. 391:441–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Torti SV, Torti FM. 2013. Iron and cancer: more ore to be mined. Nat. Rev. Cancer 13:342–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tortorella S, Karagiannis TC. 2014. Transferrin receptor–mediated endocytosis: a useful target for cancer therapy. J. Membr. Biol. 247:291–307 [DOI] [PubMed] [Google Scholar]
- 161.Toyokuni S, Mori T, Dizdaroglu M. 1994. DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int. J. Cancer 57:123–28 [DOI] [PubMed] [Google Scholar]
- 162.Traynor AM, Lee JW, Bayer GK, Tate JM, Thomas SP, et al. 2010. A phase II trial of triapine (NSC#663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer: Eastern Cooperative Oncology Group Study 1503. Investig. New Drugs 28:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Trowbridge IS, Lopez F. 1982. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. PNAS 79:1175–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsuji Y, Kwak E, Saika T, Torti SV, Torti FM. 1993. Preferential repression of the H subunit of ferritin by adenovirus E1A in NIH-3T3 mouse fibroblasts. J. Biol. Chem. 268:7270–75 [PubMed] [Google Scholar]
- 165.Tung MC, Hsieh SC, Yang SF, Cheng CW, Tsai RT, et al. 2013. Knockdown of lipocalin-2 suppresses the growth and invasion of prostate cancer cells. Prostate 73:1281–90 [DOI] [PubMed] [Google Scholar]
- 166.Turner ND, Lloyd SK. 2017. Association between red meat consumption and colon cancer: a systematic review of experimental results. Exp. Biol. Med. 242:813–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vanden Berghe T, van Loo G, Saelens X, Van Gurp M, Brouckaert G, et al. 2004. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. J. Biol. Chem. 279:7925–33 [DOI] [PubMed] [Google Scholar]
- 168.Velez-Pardo C, Jimenez Del Rio M, Verschueren H, Ebinger G, Vauquelin G. 1997. Dopamine andiron induce apoptosis in PC12 cells. Pharmacol. Toxicol. 80:76–84 [DOI] [PubMed] [Google Scholar]
- 169.Verrax J, Cadrobbi J, Marques C, Taper H, Habraken Y, et al. 2004. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis 9:223–33 [DOI] [PubMed] [Google Scholar]
- 170.von Hagens C, Walter-Sack I, Goeckenjan M, Osburg J, Storch-Hagenlocher B, et al. 2017. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res. Treat. 164:359–69 [DOI] [PubMed] [Google Scholar]
- 171.Waldvogel-Abramowski S, Waeber G, Gassner C, Buser A, Frey BM, et al. 2014. Physiology of iron metabolism. Transfus. Med. Hemotherapy 41:213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang B, Zhang J, Song F, Tian M, Shi B, et al. 2016. EGFR regulates iron homeostasis to promote cancer growth through redistribution of transferrin receptor 1. Cancer Lett. 381:331–40 [DOI] [PubMed] [Google Scholar]
- 173.Wang J, Yin D, Xie C, Zheng T, Liang Y, et al. 2014. The iron chelator Dp44mT inhibits hepatocellular carcinoma metastasis via N-Myc downstream-regulated gene 2 (NDRG2)/gp130/STAT3 pathway. Oncotarget 5:8478–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang W, Deng Z, Hatcher H, Miller LD, Di X, et al. 2014. IRP2 regulates breast tumor growth. Cancer Res. 74:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang ZJ, Lam KW, Lam TT, Tso MO. 1998. Iron-induced apoptosis in the photoreceptor cells of rats. Investig. Ophthalmol. Vis. Sci. 39:631–33 [PubMed] [Google Scholar]
- 176.Weinberg ED. 1986. Iron, infection and neoplasia. Clin. Physiol. Biochem. 4:50–60 [PubMed] [Google Scholar]
- 177.Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, et al. 2013. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 71:765–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.West AR, Oates PS. 2008. Mechanisms of heme iron absorption: current questions and controversies. World J. Gastroenterol. 14:4101–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weston C, Klobusicky J, Weston J, Connor J, Toms SA, Marko NF. 2016. Aberrations in the iron regulatory gene signature are associated with decreased survival in diffuse infiltrating gliomas. PLOS ONE 11:e0166593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.White E. 2015. The role for autophagy in cancer. J. Clin. Investig. 125:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.White S, Taetle R, Seligman PA, Rutherford M, Trowbridge IS. 1990. Combinations of anti-transferrin receptor monoclonal antibodies inhibit human tumor cell growth in vitro and in vivo: evidence for synergistic antiproliferative effects. Cancer Res. 50:6295–301 [PubMed] [Google Scholar]
- 182.WHO (World Health Organ.). 1996. Guidelines for Drinking-Water Quality. Volume 2: Health Criteria and Other Supporting Information. Geneva: WHO. 2nd ed. [Google Scholar]
- 183.Wilfinger N, Austin S, Scheiber-Mojdehkar B, Berger W, Reipert S, et al. 2016. Novel p53-dependent anticancer strategy by targeting iron signaling and BNIP3L-induced mitophagy. Oncotarget 7:1242–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wolpaw AJ, Shimada K, Skouta R, Welsch ME, Akavia UD, et al. 2011. Modulatory profiling identifies mechanisms of small molecule-induced cell death. PNAS 108:E771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, et al. 2015. Elucidating compound mechanism of action by network perturbation analysis. Cell 162:441–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu J, Zeng R, Huang J, Li X, Zhang J, et al. 2016. Dietary protein sources and incidence of breast cancer: a dose–response meta-analysis of prospective studies. Nutrients 8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu KJ, Polack A, Dalla-Favera R. 1999. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science 283:676–79 [DOI] [PubMed] [Google Scholar]
- 188.Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E. 2004. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann. Epidemiol. 14:195–201 [DOI] [PubMed] [Google Scholar]
- 189.Xie C, Zhang N, Zhou H, Li J, Li Q, et al. 2005. Distinct roles of basal steady-state and induced H-ferritin in tumor necrosis factor–induced death in L929 cells. Mol. Cell. Biol. 25:6673–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xue X, Ramakrishnan SK, Weisz K, Triner D, Xie L, et al. 2016. Iron uptake via DMT1 integrates cell cycle with JAK–STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 24:447–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xue XJ, Gao Q, Qiao JH, Zhang J, Xu CP, Liu J. 2014. Red and processed meat consumption and the risk of lung cancer: a dose–response meta-analysis of 33 published studies. Int. J. Clin. Exp. Med. 7:1542–53 [PMC free article] [PubMed] [Google Scholar]
- 192.Yamasaki T, Terai S, Sakaida I. 2011. Deferoxamine for advanced hepatocellular carcinoma. N. Engl. J. Med. 365:576–78 [DOI] [PubMed] [Google Scholar]
- 193.Yang WS, Stockwell BR. 2008. Synthetic lethal screening identifies compounds activating iron dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15:234–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, et al. 2012. Meat consumption and risk of lung cancer: evidence from observational studies. Ann. Oncol. 23:3163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, et al. 2008. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J. Natl. Cancer Inst. 100:996–1002 [DOI] [PubMed] [Google Scholar]
- 196.Zeidner JF, Karp JE, Blackford AL, Smith BD, Gojo I, et al. 2014. A phase II trial of sequential ribonucleotide reductase inhibition in aggressive myeloproliferative neoplasms. Haematologica 99:672–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang F, Wang W, Tsuji Y, Torti SV, Torti FM. 2008. Post-transcriptional modulation of iron homeostasis during p53-dependent growth arrest. J. Biol. Chem. 283:33911–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang S, Chen Y, Guo W, Yuan L, Zhang D, et al. 2014. Disordered hepcidin–ferroportin signaling promotes breast cancer growth. Cell. Signal. 26:2539–50 [DOI] [PubMed] [Google Scholar]
- 199.Zhao R, Planalp RP, Ma R, Greene BT, Jones BT, et al. 2004. Role of zinc and iron chelation in apoptosis mediated by tachpyridine, an anti-cancer iron chelator. Biochem. Pharmacol. 67:1677–88 [DOI] [PubMed] [Google Scholar]
- 200.Zhu CS, Pinsky PF, Kramer BS, Prorok PC, Purdue MP, et al. 2013. The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial and its associated research resource. J. Natl. Cancer Inst. 105:1684–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhu HC, Yang X, Xu LP, Zhao LJ, Tao GZ, et al. 2014. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig. Dis. Sci. 59:664–73 [DOI] [PubMed] [Google Scholar]