Abstract
An FDA-approved iron oxide nanoparticle used for the treatment of anaemia can be repurposed for leukaemia therapy.
Iron is both essential and potentially toxic. In humans, iron is required for oxygen transport by haemoglobin and the activity of proteins that catalyse reactions involved in respiration, DNA repair, DNA synthesis, free-radical detoxification and many other essential processes1. However, owing to its ability to redox-cycle, iron can also foster the formation of toxic radical species through participation in Fenton chemistry and other chemical reactions that generate reactive oxygen and nitrogen species harmful to DNA, proteins and lipids2. These dual properties of iron require that it be tightly regulated, both at the systemic and cellular level: that is, that cells have neither too much nor too little iron, and that iron is presented in a non-reactive chemical form. Among the proteins that regulate systemic and cellular iron, three play pivotal roles in maintaining this critical iron balance: transferrin, a circulating glycoprotein that binds two atoms of iron and serves as an iron delivery vehicle; transferrin receptor 1 (TFR1), which is displayed on the cell surface and binds to transferrin to enable iron import; and ferroportin (FPN), an iron efflux pump that delivers iron from cells to the circulation.
Many cancer cells exhibit a greater demand for iron relative to their non-malignant counterparts. Although the biological rationale underpinning this enhanced requirement is incompletely understood and likely to be multifactorial, it has been known for decades that cancer cells tend to up-regulate expression of TFR1, enabling increased iron uptake3. More recently, it was shown that cancer cells also frequently down-regulate expression of FPN, thereby decreasing iron efflux and consequently increasing iron retention4. Modulation of iron content through expression of FPN has important consequences, affecting tumour growth in vitro and in vivo; ferroportin expression also has striking effects on patient prognosis4,5. This metabolic shift in cancer cells to a higher baseline iron content may also represent a therapeutic opportunity: cancer cells are potentially more vulnerable than non-cancer cells to accumulation of toxic radical species in the face of iron excess.
Writing in Nature Nanotechnology, Trujillo-Alonso and co-workers now test this proposition by examining whether iron provided as ferumoxytol (Feraheme) can exert an anti-leukaemic effect6. Ferumoxytol is a colloidal, carbohydrate-coated superparamagnetic iron oxide nanoparticle with an approximate diameter of 6 nmand mass of 731 kDa (ref. 7). Intravenous ferumoxytol has been approved by the US Food and Drug Administration (FDA) as a safe and effective treatment for patients with anaemia who cannot tolerate or who fail to respond to oral iron supplements, such as patients with chronic kidney disease8.
The authors first demonstrate that leukaemic cells generally exhibit a similar iron phenotype to that observed in other cancers: when compared with normal bone marrow, FPN expression is reduced in most of the tested leukaemic cell lines and of the samples derived from patients with acute myeloid leukaemia (AML). They further observe that in leukaemic cells with low FPN, administration of ferumoxytol increases intracellular iron and decreases viability. They then test the efficacy of ferumoxytol treatment in an acute leukaemia mouse model. They observe a reduction in tumour burden in peripheral blood, bone marrow and spleen. Perhaps surprisingly, the effect that they observe is superior to that observed in mice treated with cytarabine (AraC), a standard of care for AML. The reduction in tumour burden achieved with ferumoxytol is enough to prolong the survival of tumour-bearing mice significantly. Impressively, the authors find that ferumoxytol similarly reduces tumour burden in three separate AML models derived from patient tissues expressing low FPN. These results are observed in immunocompromised mice and are accompanied by up-regulation of haem oxygenase, supporting a direct effect of ferumoxytol on oxidative stress rather than an indirect effect mediated by modulation of the immune system, a mechanism that has been suggested to explain the anti-tumour effect of ferumoxytol in mammary, liver and lung tumours9.
This study contributes substantially to the growing effort to test whether the iron dependency of cancer cells can be converted into a therapeutic point of vulnerability. For example, high-dose ascorbate, which induces oxidative stress in a manner dependent on redox-active cellular iron, is being tested clinically as a means of selectively targeting non-small-cell lung cancer and glioblastoma10. The use of small-molecule iron chelators that bind iron and foster its ability to redox cycle is also being investigated as an anti-cancer strategy11.
The approach described by Trujillo-Alonso and co-workers is promising in several ways. First, given the relative lack of toxicity of ferumoxytol (in contrast to most anti-cancer therapies), combining it with other anti-leukaemic agents is a real possibility. Second, because ferumoxytol has received FDA clearance, a considerable barrier to the rapid onset of meaningful clinical trials has already been removed. Third, treatment options for patients with AML are limited and poorly effective; thus, a positive therapeutic outcome has the potential to exert meaningful change in the lives of patients. Finally, the potential of ferumoxytol in tumour imaging12 may add yet another dimension to its utility. The observations in this paper also argue for a more nuanced approach to treatment (‘precision’ medicine), as responses to ferumoxytol were limited to leukaemias that expressed low FPN.
Of course, despite this optimistic outlook, it should be remembered that no patients were treated in this study; thus, the jury is out on whether ferumoxytol can be an effective addition to the anti-cancer armamentarium. Nevertheless, it may soon be time for the jury to be convened.Fig. 1
Fig. 1 |. Proposed mechanism by which Ferumoxytol induces selective cell death in leukaemic cells that express low levels of FPN.
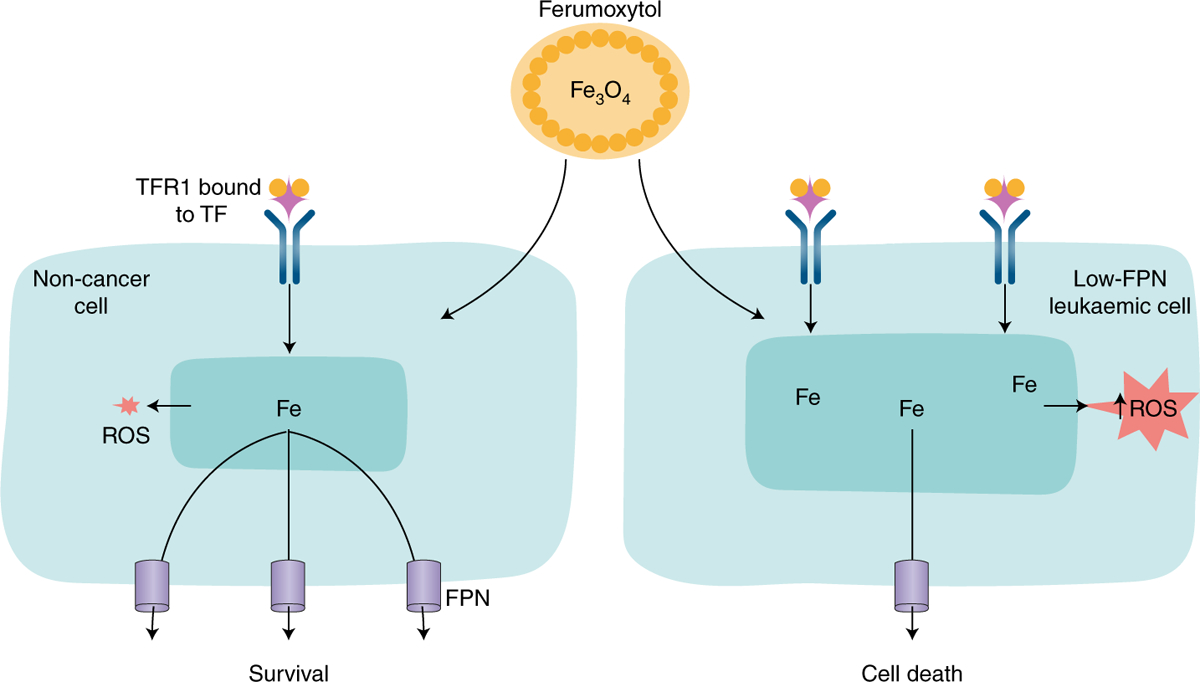
Left image depicts a non-cancer cell with moderate levels of TFR1 and high levels of ferroportin; right image depicts a leukaemic cell with high levels of TFR1 and low levels of FPN. When both cell types are exposed to the iron oxide nanoparticle ferumoxytol, non-cancer cells survive, presumably by exporting the excess iron through FPN. In contrast, owing to their low levels of FPN, leukaemic cells are unable to export excess iron, which remains in the cell, catalyses the production of excess reactive oxygen species and leads to cell death. ROS, reactive oxygen species; TF, transferrin.
References
- 1.Crichton R in Iron Metabolism: From Molecular Mechanisms to Clinical Consequences (ed. Crichton R) 22–70 (Wiley, 2016). [Google Scholar]
- 2.Torti SV, Manz DH, Paul BT, Blanchette-Farra N & Torti FM Annu. Rev. Nutr 38, 97–125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels TR et al. Biochim. Biophys. Acta 1820, 291–317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinnix ZK et al. Sci. Transl. Med 2, 43ra56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Z et al. Cancer Res. 75, 2211–2221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trujillo-Alonso V et al. Nat. Nanotechnol 10.1038/s41565-019-0406-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakrishnan VS et al. Eur. J. Clin. Invest 39, 489–496 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Vadhan-Raj S et al. Am. J. Hematol 89, 7–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanganeh S et al. Nat. Nanotechnol 11, 986–994 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenfeld JD et al. Cancer Cell 32, 268 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Kalinowski DS et al. Biochim. Biophys. Acta 1863, 727–748 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Toth GB et al. Kidney Int. 92, 47–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


