Abstract
Background
Lactation is associated with lower risks for cardiovascular disease in women. Organ-related adiposity, which plays significant roles in the development of cardiometabolic diseases, could help explain this observation. We evaluated the association of lactation duration with visceral (VAT) and pericardial (PAT) fat volumes in women.
Methods
Data were obtained from 910 women enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study (1985-1986) without diabetes prior to pregnancy who had ≥1 birth during 25 years of follow-up and had VAT and PAT measured from computed tomographic scans in 2010-2011. Cumulative lactation duration across all births since baseline was calculated from self-reports collected at periodic exams.
Results
At baseline, the average age of women (48% black, 52% white) was 24 ± 3.7 years. After controlling for baseline age, race, smoking status, body mass index, fasting glucose, family history of diabetes, fat intake, total cholesterol, physical activity, and follow-up covariates (parity, gestational diabetes), the mean fat volumes across categories of lactation [none (n = 221), 1-5 months (n = 306), 6-11 months (n = 210), and ≥12 months (n = 173)] were 122.0, 113.7 105.0, and 110.1 cm3 for VAT and 52.2, 46.7, 44.5, and 43.4 cm3 for PAT, respectively. Changes in body weight from the first post-baseline birth to the end of follow-up mediated 21% and 18% of the associations of lactation with VAT and PAT, respectively.
Conclusions
In this prospective study, longer cumulative lactation duration was associated with lower VAT and PAT volumes, with weight gain partially mediating these associations.
Keywords: lactation, visceral fat, pericardial fat, adiposity, epidemiology
Reproductive factors are known to be associated with several physiologic and endocrine processes that impact the long-term health of women. A growing number of reports suggest that lactation and longer lactation duration is associated with lower risk for cardiovascular diseases (CVD) as well as cardiometabolic conditions such as subsequent onset of the metabolic syndrome and type 2 diabetes (1-4). However, the underlying mechanisms for these associations are not well understood.
Profound metabolic changes including regional fat distribution and accumulation of ectopic fat stores occur during pregnancy (5-9). Some studies have reported as much as a 30% increase in visceral adipose tissue (VAT) mass from the first trimester to 1 year postpartum (9). VAT and pericardial adipose tissue (PAT), an ectopic fat depot contained within and surrounding the pericardium, both are known to produce adipocytokines that adversely affect several metabolic processes (10). These include diminished glucose tolerance, hypertriglyceridemia, hyperinsulinemia, and hypertension (9,11). After pregnancy, lactation increases metabolic expenditure by an estimated 480 kcal/day (12), and has been reported to increase mobilization of accumulated fat stores (6,13). This contributes to improved glucose uptake (14,15), insulin sensitivity (14,15), and lipid metabolism (16,17), thereby reversing several of the adverse metabolic changes brought on by pregnancy (6).
The influence of lactation on VAT and PAT accumulation is a potential mechanism to explain the epidemiologic evidence for the inverse association of breastfeeding with metabolic conditions leading to CVD in women (18). The long-term role of lactation on regional fat accumulation and distribution is unclear. While no studies have investigated the relation of lactation with PAT, results for the relation of lactation with VAT accumulation are equivocal (13,19,20). Very few of these studies controlled for preconception adiposity and cardiometabolic factors, which are strong predictors of postpartum weight retention (21).
Therefore, the aim of this study was to examine the association of lactation duration with PAT, VAT, and other fat depots in the abdominal region measured by computed tomography (CT), while accounting for pre- and postpregnancy biochemical, anthropometric, and behavioral risk factors measured throughout 25 years of the childbearing period. We hypothesized that longer cumulative duration of lactation would be associated with lower volumes of VAT and PAT and that these relationships would be mediated by variations in cardiometabolic factors such changes in body weight occurring after the first post-baseline birth to the end of follow-up.
Materials and Methods
Study population
The Coronary Artery Risk Development in Young Adults (CARDIA) study is an ongoing multicenter longitudinal observational study conducted in 4 US communities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) to study the development of cardiovascular risk and events beginning in young adults. At baseline (1985-1986), 5115 healthy adults were recruited from the general population to be balanced on sex, race (white or black), age (18-24 or 25-30 years), and education (high school or less or more than high school). Since then, 8 follow-up examinations have occurred at years 2, 5, 7, 10, 15, 20, 25, and 30 with 72% of the surviving cohort attending the year 25 exam (2020-2011). Data collection and follow-up protocols were approved by the institutional review boards of each field center, with all participants providing written informed consent. Details of the study design and methods are described elsewhere (22). Detailed procedures for accessing CARDIA data can be found at https://www.cardia.dopm.uab.edu/scientific-resources-landing-page2. Most CARDIA data can be also obtained from BioLINCC, a repository maintained by the National Heart, Lung, and Blood Institute at https://biolincc.nhlbi.nih.gov/.
Sample selection
Participants were considered for inclusion in this analytic sample if they had 1 or more births during follow-up. Of the 2787 women enrolled at baseline, 1339 had births post-baseline that were not preceded by diabetes, with 1238 of them having information on lactation duration. After excluding 228 women who did not attend the year 25 exam, 95 women with missing CT scans at year 25, and 5 women with outlying values for fat measures, 910 women were included in the analytic sample. Women excluded had lower educational attainment and higher body mass index (BMI) and were more likely to be of black race.
Exposure assessment
At each CARDIA exam, women were asked if they were currently pregnant or breastfeeding, the number and outcomes of pregnancies occurring since the previous visit (ie, abortions, miscarriages, stillbirths, or live births), as well as delivery dates, gestational ages, infant birth weights, pregnancy complications [ie, hypertension with or without proteinuria, gestational diabetes (GDM), preterm births less than 37 weeks gestation], and mode of delivery. Live births were defined as delivery of a live infant of ≥20 weeks of gestation that occurred between years 0 (baseline) and 25. For each birth, women reported whether they breastfed, and if they responded “yes,” then they were asked to indicate 1 of the following categories of lactation duration: <6 weeks, 6 to 11 weeks, 3 to 6 months, or >6 months. To calculate cumulative duration across all births, we assigned the midpoint of each lactation category for each birth: 21 days for <6 weeks, 66 days for 6 to 11 weeks, 135 days for 3 to 6 months, and 210 days as the upper limit for >6 months. We summed the number of days across all births to obtain the overall lactation duration for each woman. Time-dependent lactation categories were updated at each examination year as births occurred. The overall duration of lactation was divided into 4 categories representing clinically relevant periods: 0 months, 1 to <6 months, 6 to <12 months, and ≥12 months.
Outcome assessment
All CT scans were performed using 64 channel multi-detector CT scanners [GE Healthcare, Milwaukee, WI, USA (Birmingham and Oakland centers) or Siemens, Erlangen, Germany (Chicago and Minneapolis centers)]. For chest scans, a low radiation exposure, prospectively electrocardiogram-gated scan sequence using a 120 kVp voltage was used to image from the level of the carina through the inferior aspect of the heart based on the scout image. Reading protocols have been validated in other cohorts (23). Images were read by experienced image analysts who were blinded to participants information. Detailed procedures on the measurement of PAT and VAT have been described elsewhere (24,25). Briefly, CT scans were used to measure abdominal fat deposited just below the skin’s surface (subcutaneous fat), around organs including the intestines (visceral fat) and abdominal muscles (intermuscular fat), and inside the muscles (intramuscular fat). These were analyzed using the 50-cm display field-of-view to include the whole abdomen (26). Tissues with attenuation of −190 through −30 Hounsfield units were defined as adipose tissue. A dedicated software for the Medical Image Processing, Analysis, and Visualization application developed by the Center for Information Technology, National Institutes of Health, was used to quantify subcutaneous and VAT volume. VAT volume was calculated as the sum of voxels across a 10-mm slice centered at the L4-L5 disks (24). To measure PAT, CT slices within 15 mm above and 30 mm below the superior extent of the left main coronary artery were analyzed using an image processing workstation (OsiriX, Pixmeo, Geneva, Switzerland). This 45-mm block of images (18 slices of 2.5 mm or 15 slices of 3.0 mm thickness) was selected for analysis as it covers pericardial fat located around the proximal coronary arteries (25). The anterior boundary of the volume is the chest wall and the posterior boundary is the aorta and the bronchus. Experienced analysts manually segmented the interface between the lungs and the paracardial space. PAT volume was determined by summing the adipose tissue containing pixels and accounting for the slice thickness. These methods quantify the combined epicardial (adipose tissue within the pericardium in direct contact with the coronary arteries) plus paracardial (adipose superficial to the pericardium) adipose tissues providing total PAT volume within the 45-mm thick block of images analyzed (25). Previous studies in CARDIA have shown that epicardial adipose is highly correlated with PAT (r = 0.92) (25). Reader reproducibility of CT measures was estimated in 158 randomly selected scan pairs rereads. The intra-reader and inter-reader variability were 2.0% and 4.2%, respectively, for PAT and 2.4% and 6.7%, respectively, for VAT (24,25).
Measures
At every CARDIA exam, standardized protocols were used to collect information on demographics, anthropometrics, lifestyle and behavioral factors, medical history, family history of medical conditions, biomarkers, and medication use from participants. Trained and certified staff assessed anthropometry measures (weight, height, waist circumference), took blood pressure readings, and drew blood samples at in-person exams scheduled at least 4 months after pregnancies using standardized methodologies and calibrated equipment. BMI was computed as weight in kilograms divided by squared height in meters. Physical activity was assessed using a modified version of the Minnesota Leisure Time Physical Activity Questionnaire with total scores representing total moderate-to-vigorous activity expressed in exercise units. Dietary intake was assessed using the CARDIA Diet History questionnaire (27).
Procedures for venipuncture, laboratory quality control, and biochemical assays are detailed elsewhere (22). We measured glucose, insulin, and lipids in fasting samples. We utilized only biochemical measurements obtained in the nonpregnant and nonlactating states. Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: fasting glucose (mmol/L) × fasting insulin (mU/L)/22.5. Diabetes was defined by 1 or more of the following: elevated fasting plasma glucose concentration ≥ 126 mg/dL, oral glucose tolerance test ≥ 200 mg/dL, hemoglobin A1c ≥ 6.5%, or self-reported use of diabetes medications (insulin or oral hypoglycemics). Total adiponectin, leptin, and tumor necrosis factor α were all measured using enzyme linked immunosorbent assays (R&D Systems Inc. Minneapolis, MN, USA). Parity was defined as the number of live births since baseline and updated at each CARDIA exam. GDM status was classified for each pregnancy. We validated pregnancy complications by medical record abstraction of discharge diagnoses, gestational age, and laboratory tests for 165 women (200 pregnancies). Self-report of GDM had high sensitivity (100%) and specificity (92%) confirmed by oral glucose tolerance tests using the Carpenter and Coustan criteria (28). Preterm birth had high sensitivity and specificity of 84% and 89%, respectively. By contrast, hypertensive disorders of pregnancy were overreported by women, with low sensitivity (40%), but high specificity (90%) to classify gestational hypertensive disorders (29).
Statistical analysis
Characteristics of women at baseline and during follow-up were presented according to categories of cumulative lactation duration estimated at every post-baseline visit. Differences in categorical variables were assessed using chi-squared tests, and for continuous variables, analysis of variance was used for normally distributed variables and Kruskal Wallis test for variables with skewed distributions. General linear models were used to calculate to determine the relation of lactation duration with regional fat depot. Adjusted means of VAT and PAT are reported for all models. Adjustment were made for baseline (prepregnancy) covariates, namely age, race, education, smoking status, family history of diabetes, fasting glucose, BMI, total fat intake, total blood cholesterol, and race-specific physical activity score quartiles as well as time-dependent covariates of parity and births complicated by GDM. To control for potential residual confounding, we included all GDM births and parity occurring before baseline in the time-dependent counts. These potential confounders were selected a priori due to their known associations with either lactation duration or regional fat accumulation. Potential nonlinear relations between lactation duration and VAT or PAT were evaluated using restricted cubic splines. Due to the reported differences in duration of lactation by race, we tested for interaction between lactation duration and race. This interaction, as well as that of lactation duration and baseline parity, did not approach statistical significance (P > 0.05). However, due to their intrinsic interests, we provided additional analyses stratified by race as well as those limited to women who were nulliparous at baseline. To examine whether the associations between lactation duration and VAT or PAT were mediated by changes in body weight and other potential cardiometabolic pathways such as HOMA-IR, high-density lipoprotein (HDL) cholesterol, and triglycerides from the first post-baseline birth to the end of follow-up, multiple (parallel) mediation models were conducted (30). Multiple mediation models were used since they provided means to assess multiple mechanisms simultaneously conditioned on the presence of other mediators in the model (30). Nonparametric bootstrap methods with 10 000 samples were used to estimate 95% confidence intervals with the statistical significance of indirect effects attained if the confidence intervals did not contain zero (30). All statistical tests were 2-sided and performed at the 0.05 level of significance using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics of women included in this study are presented in Table 1. The average age (SD) of women was 24.2 (3.7) years, and 48% were black. About 69% of women were nulliparous at baseline, 23% were current smokers, and 13% reported a family history of diabetes. Of the 285 (31.3%) women who had giving birth prior to baseline, 180 (63.2%) had given birth once, 79 (27.7%) had given birth twice, and 26 (9.1%) had given birth 3 or more times. Before the first birth after baseline (1985-1986), women who did not lactate or lactated for a short duration had more adverse cardiometabolic and health behavior lifestyle profiles compared to women who went on to lactate for a longer duration. Follow-up characteristics of the cohort at the time of CT scanning (2010-2011) are shown in Table 2. The median (interquartile range) time from baseline to the first post-baseline birth was 5.4 (2.9-9.0) years. The mean age (SD) at the last birth was 33.5 (5.0) years with the average time (SD) from the last birth to the assessment of regional fat depots being 15.8 (5.2) years. During the 25 years of follow-up, 359 (39.5%) women gave birth once, 366 (40.2%) gave birth twice, and 185 (20.3%) gave birth 3 or more times. With regard to lactation, 24.3%, 33.6%, 23.1%, and 19% of women reported none, 1 to 6 months, 7 to 11 months, and 12 or more months of lactation duration across all births, respectively. A greater proportion of women with longer durations of lactation were of white race, had 2 or more post-baseline births, and had higher educational attainment than women with no history of lactation. Furthermore, a greater proportion of women with no history of lactation were current smokers, used anti-hypertensive medications, and developed metabolic syndrome and diabetes than women with longer lactation durations. There were no significant differences across lactation duration categories on the occurrence of cesarean section, preterm births, gestational diabetes, and hypertensive disorders of pregnancy. Women who had longer lactation durations had lower BMI, waist circumference, systolic blood pressure, HOMA-IR, fasting glucose, leptin, tumor necrosis factor α, and triglycerides, but higher physical activity and levels of adiponectin and HDL cholesterol. Furthermore, compared to women with no history of lactation, women with longer lactation durations had significantly lower gains in body weight and HOMA-IR, but had greater gains in HDL cholesterol from the first post-baseline birth to the end of follow-up. PAT was significantly correlated with VAT (0.71) and other cardiometabolic factors (Supplemental Table 1 (31)).
Table 1.
Baseline characteristics of women stratified by future lactation duration categories, CARDIA study, 1985-1986
| Lactation duration | |||||
|---|---|---|---|---|---|
| None (n = 221) | >0 to 6 months (n = 306) | >6 to <12 months (n = 210) | ≥ 12 months (n = 173) | P-value | |
| Age, years | 23.5 (3.6) | 24.5 (3.7) | 24.6 (3.5) | 24.3 (3.8) | 0.010 |
| Race, % | <0.001 | ||||
| Black | 78.7 | 48.4 | 34.3 | 24.3 | |
| White | 21.3 | 51.6 | 65.7 | 75.7 | |
| > High school education, % | 43.9 | 69.3 | 77.1 | 80.9 | <0.001 |
| Current smoker, % | 35.3 | 21.6 | 19.5 | 15.6 | <0.001 |
| Parity, % | <0.001 | ||||
| None | 46.6 | 70.3 | 76.2 | 85.0 | |
| 1 | 35.3 | 19.6 | 11.9 | 9.80 | |
| 2 | 16.3 | 7.2 | 7.1 | 3.50 | |
| ≥ | 1.8 | 2.9 | 4.8 | 1.70 | |
| BMI, kg/m2 | 25.1 (5.9) | 23.7 (4.7) | 23.5 (4.6) | 22.2 (3.2) | <0.001 |
| Waist girth, cm | 75.6 (11.7) | 72.5 (9.6) | 71.5 (9.1) | 69.7 (6.9) | <0.001 |
| Systolic blood pressure, mmHg | 106.5 (10.0) | 105.5 (8.5) | 105.6 (9.6) | 104.8 (8.8) | 0.305 |
| Total energy intake per day,a Kcal | 2387 (1704-3469) | 2017 (1594-2681) | 1965 (1542-2547) | 2050 (1621-2731) | <0.001 |
| Total fat intake per day,a gm | 100 (67-151) | 86 (60-116) | 79 (58-112) | 82.1 (61-117) | <0.001 |
| Physical activity,a exercise units | 220 (93-408) | 288 (147-474) | 351 (216-554) | 366 (214-543) | <0.001 |
| Family history of diabetes | 19.0 | 11.8 | 10.5 | 11.6 | 0.039 |
| HOMA-IRa | 1.5 (1.2-2.0) | 1.4 (1.2-1.8) | 1.4 (1.1-1.8) | 1.3 (1.1-1.7) | 0.004 |
| Fasting glucose, mg/dL | 79.0 (8.6) | 79.5 (8.0) | 80.3 (7.2) | 79.0 (7.3) | 0.305 |
| Adiponectin,a,b ug/mL | 6.0 (4.4-9.6) | 8.6 (6.1-12.3) | 9.0 (5.5-20.9) | 9.5 (7.2-12.2) | <0.001 |
| Leptin,a,b ng/mL | 15.4 (7.7-28.1) | 12.2 (5.5-27.0) | 9.0 (5.5-20.9) | 7.2 (4.4-15.2) | <0.001 |
| Tumor necrosis factor α,a,b pg/mL | 0.95 (0.75-1.28) | 0.91 (0.73-1.16) | 0.85 (0.67-1.16) | 0.89 (0.70-1.16) | 0.089 |
| Triglycerides,a mg/dL | 61.0 (44.0-77.5) | 57.0 (42.0-80.0) | 55.0 (44.0-72.0) | 54.0 (41.0-67.0) | 0.059 |
| HDL-cholesterol, mg/dL | 53.7 (12.3) | 56.1 (11.5) | 58.6 (13.3) | 57.4 (12.5) | <0.001 |
| Total cholesterol, mg/dL | 178.5 (32.6) | 176.7 (34.2) | 181.3 (33.1) | 172.6 (29.3) | 0.067 |
Values are mean (SD) or median (interquartile range) for continuous variables and percentages for categorical variables.
a P-value obtained from Kruskal-Wallis test.
b Measured 2 years after baseline among 649 women.
Table 2.
Follow-up characteristics of women by history of lactation duration categories, CARDIA study, 2010-2011
| Lactation duration | |||||
|---|---|---|---|---|---|
| None (n = 221) | >0 to 6 months (n = 306) | >6 to <12 months (n = 210) | ≥12 months (n = 173) | P-value | |
| Age, years | 48.6 (3.7) | 49.6 (3.7) | 49.7 (3.6) | 49.4 (3.9) | 0.009 |
| Race, % | <0.001 | ||||
| Black | 78.7 | 48.4 | 34.3 | 24.3 | |
| White | 21.3 | 51.6 | 65.7 | 75.7 | |
| Parity, % | <0.001 | ||||
| 1 | 49.3 | 54.9 | 34.3 | 5.8 | |
| 2 | 32.1 | 33.0 | 50.5 | 50.9 | |
| ≥3 | 18.6 | 12.1 | 15.2 | 43.4 | |
| Hypertensive disorders of pregnancy, % | 30.8 | 26.1 | 27.1 | 26.6 | 0.672 |
| Gestational diabetes, % | 12.2 | 11.4 | 13.3 | 14.5 | 0.791 |
| Preterm birth (<37 weeks), % | 35.3 | 30.4 | 25.2 | 24.9 | 0.062 |
| Cesarean section, % | 33.5 | 30.4 | 28.6 | 25.4 | 0.360 |
| >High school education, % | 61.1 | 81.7 | 90.5 | 94.2 | <0.001 |
| Current smoker, % | 24.0 | 13.1 | 9.5 | 5.2 | <0.001 |
| BMI, kg/m2 | 32.5 (7.8) | 30.4 (7.6) | 29.1 (7.3) | 27.5 (6.3) | <0.001 |
| Waist girth, cm | 95.4 (15.9) | 90.1 (15.8) | 87.5 (15.7) | 84.5 (12.9) | <0.001 |
| Systolic blood pressure, mmHg | 122.5 (19.3) | 118.0 (16.4) | 114.5 (15.4) | 111.6 (15.8) | <0.001 |
| Anti-hypertensive medications, % | 36.7 | 22.9 | 13.3 | 13.9 | <0.001 |
| Physical activity,a exercise units | 144 (48-301) | 256 (112-466) | 281 (148-489) | 268 (144-441) | <0.001 |
| HOMA-IRa | 2.4 (1.4-3.9) | 1.9 (1.2-3.3) | 1.6 (1.0-2.7) | 1.6 (1.0-2.5) | <0.001 |
| Fasting glucose, mg/dL | 100.8 (36.6) | 95.0 (21.4) | 91.8 (13.9) | 92.7 (15.8) | 0.001 |
| Adiponectin,a,b ug/mL | 6.6 (4.1-12.4) | 7.6 (5.2-11.5) | 8.2 (5.1-13.3) | 10.2 (6.0-14.6) | 0.025 |
| Leptin,a,b ng/mL | 22.4 (10.9-47.1) | 21.8 (9.6-43.6) | 20.0 (6.8-38.7) | 14.4 (7.3-24.2) | 0.006 |
| Tumor necrosis factor α a,b pg/mL | 0.88 (0.74-1.20) | 0.83 (0.58-1.12) | 0.69 (0.53-0.98) | 0.74 (0.57-0.93) | 0.018 |
| Lipid-lowering medications, % | 14.9 | 9.5 | 7.1 | 9.8 | 0.054 |
| Triglycerides,a mg/dL | 89.0 (67.0-124.0) | 83.0 (65.0-118.0) | 80.0 (62.0-112.0) | 76.0 (60.0-107.) | 0.041 |
| HDL-cholesterol, mg/dL | 59.1 (17.4) | 62.7 (19.2) | 66.5 (18.6) | 65.3 (15.1) | <0.001 |
| Total cholesterol, mg/dL | 190.4 (38.2) | 193.2 (36.2) | 200.4 (33.8) | 189.8 (32.2) | 0.009 |
| Incident metabolic syndrome, % | 27.6 | 19.9 | 13.3 | 11.6 | <0.001 |
| Incident diabetes (postdelivery), % | 15.8 | 6.9 | 3.8 | 4.6 | <0.001 |
| Change from first exam post birth to the end of follow-up | |||||
| Body weight, kga | 25.0 (9.0-46.8) | 17.5 (3.0-41.8) | 12.0 (0.5-30.5) | 13.3 (2.8-29.0) | <0.001 |
| HDL cholesterol,a mg/dL | 5.0 (−3.0-12.0) | 6.0 (−2.0-16.0) | 7.0 (1.0-17.0) | 7.0 (−2.0-14.0) | 0.024 |
| Triglycerides,a mg/dL | 24.0 (−4.0, 54.0) | 19.5 (−4.0-44.0) | 17.0 (−6.0-37.0) | 21.0 (−3.0-42.0) | 0.439 |
| HOMA-IRa | 0.47 (−0.48-1.62) | 0.33 (−0.38-1.25) | 0.01 (−0.57-0.84) | 0.17 (−0.27-0.81) | 0.015 |
Values are mean (standard deviation) or median (interquartile range) for continuous variables and percentages for categorical variables
a P-value obtained from Kruskal-Wallis test.
b Measured 5 years before end of follow-up among 322 women.
In unadjusted models, longer cumulative lactation duration was associated with lower volumes of VAT, subcutaneous fat, total abdominal fat, and PAT, but not with volumes of intermuscular fat (Table 3, Supplemental Table 2 (31)). After adjusting for baseline prepregnancy covariates (model 1) and follow up covariates (model 2), only the inverse associations of lactation duration with VAT and PAT persisted (Table 3). These associations were consistent across both races (Supplemental Table 3 (31)). Of note, the association of lactation duration and PAT did not change significantly upon additional adjustment for VAT (Table 3, model 3). When analyses were limited to the 625 women who were nulliparous at baseline, we observed associations that were similar to those seen for the entire cohort (Supplemental Tables 4-6 (31)). Also, among women who were nulliparous at baseline and had only 1 birth during follow-up, the results were in the expected direction (Supplemental Table 7 (31)). However, all these results did not meet statistical significance. Restricted cubic spline models (Supplemental Figure 1 (31)) showed that the association between lactation duration and PAT was monotonically decreasing, with the greatest reductions in PAT observed after 12 months of lactation. An apparent threshold effect of lactation on VAT volume was observed at 12 months of lactation, with monotonically decreasing VAT volumes before 12 months.
Table 3.
Lactation duration and measures of adiposity, CARDIA study, 1985-2011
| Lactation duration groups | |||||
|---|---|---|---|---|---|
| Adiposity measures | None (n = 221) | >0 to 6 months (n = 306) | >6 to <12 months (n = 210) | ≥12 months (n = 173) | P-value |
| Abdomen, mean (95% CI) | |||||
| Visceral fat volume, cm3 | |||||
| Unadjusted | 123.7 (115.9, 131.5) | 110.6 (104.0, 117.3) | 102.2 (94.2, 110.2) | 99.2 (90.3,108.0) | <0.001 |
| Model 1 | 121.7 (113.0, 130.5) | 113.9 (105.9, 121.9) | 104.8 (95.4, 114.2) | 108.8 (98.6, 119.0) | 0.034 |
| Model 2 | 122.0 (112.5, 131.5) | 113.7 (105.0, 122.4) | 105.1 (95.3, 114.9) | 110.1 (99.5, 120.8) | 0.026 |
| Subcutaneous fat volume (cm3) | |||||
| Unadjusted | 423.1 (400.4, 445.9) | 376.5 (357.1, 395.8) | 346.6 (323.2, 370.0) | 309.0 (283.2, 334.7) | <0.001 |
| Model 1 | 361.1 (339.0, 383.2) | 365.7 (345.6, 385.8) | 351.7 (328.0, 375.3) | 343.4 (317.6, 369.1) | 0.394 |
| Model 2 | 363.8 (339.9, 387.8) | 366.1 (344.2, 388.0) | 355.2 (330.5, 379.8) | 352.6 (325.8, 379.4) | 0.750 |
| Intermuscular fat volume, cm3 | |||||
| Unadjusted | 18.1 (16.8, 19.4) | 17.7 (16.6, 18.8) | 17.4 (16.1, 18.7) | 16.6 (15.1, 18.0) | 0.453 |
| Model 1 | 17.9 (16.6, 19.3) | 18.1 (16.9, 19.4) | 17.3 (15.9, 18.8) | 17.6 (16.0, 19.2) | 0.747 |
| Model 2 | 17.0 (15.5, 18.5) | 17.2 (15.8, 18.5) | 16.4 (14.8, 17.9) | 16.6 (14.9, 18.3) | 0.769 |
| Total abdominal fat volume, cm3 | |||||
| Unadjusted | 564.9 (536.1, 593.7) | 504.8 (480.3, 529.3) | 466.2 (436.6, 495.7) | 424.7 (392.2, 457.3) | <0.001 |
| Model 1 | 500.8 (472.2, 529.4) | 497.8 (471.8, 523.8) | 473.8 (443.1, 504.4) | 469.7 (436.4, 503.0) | 0.244 |
| Model 2 | 502.8 (471.8, 533.8) | 497.0 (468.6, 525.3) | 476.6 (444.7, 508.5) | 479.3 (444.6, 514.1) | 0.458 |
| Heart | |||||
| Pericardial fat volume (cm3) | |||||
| Unadjusted | 50.9 (47.8, 54.1) | 46.3 (43.6, 49.0) | 44.7 (41.5, 48.0) | 42.0 (38.4, 45.5) | 0.002 |
| Model 1 | 52.7 (49.1, 56.2) | 47.4 (44.2, 50.6) | 44.9 (41.1, 48.6) | 43.2 (39.1, 47.2) | 0.001 |
| Model 2 | 52.2 (48.4, 56.0) | 46.7 (43.2, 50.2) | 44.5 (40.6, 48.4) | 43.4 (39.1, 47.7) | 0.002 |
| Model 3 | 48.7 (45.8, 51.6) | 45.2 (42.6, 47.9) | 45.3 (42.3, 48.3) | 42.8 (39.6, 46.1) | 0.025 |
Model 1 is adjusted for baseline (prepregnancy) covariates: age, race, smoking status, body mass index, fasting blood glucose, family history of diabetes, total fat intake, total cholesterol, and race-specific physical activity score quartiles. Model 2 is adjusted for baseline (prepregnancy) covariates: age, race, smoking status, body mass index, fasting blood glucose, family history of diabetes, total fat intake, total cholesterol, and race-specific physical activity score quartiles as well as and follow-up covariates (gestational diabetes, parity). Model 3 is the same as Model 2 with additional adjustment for visceral fat.
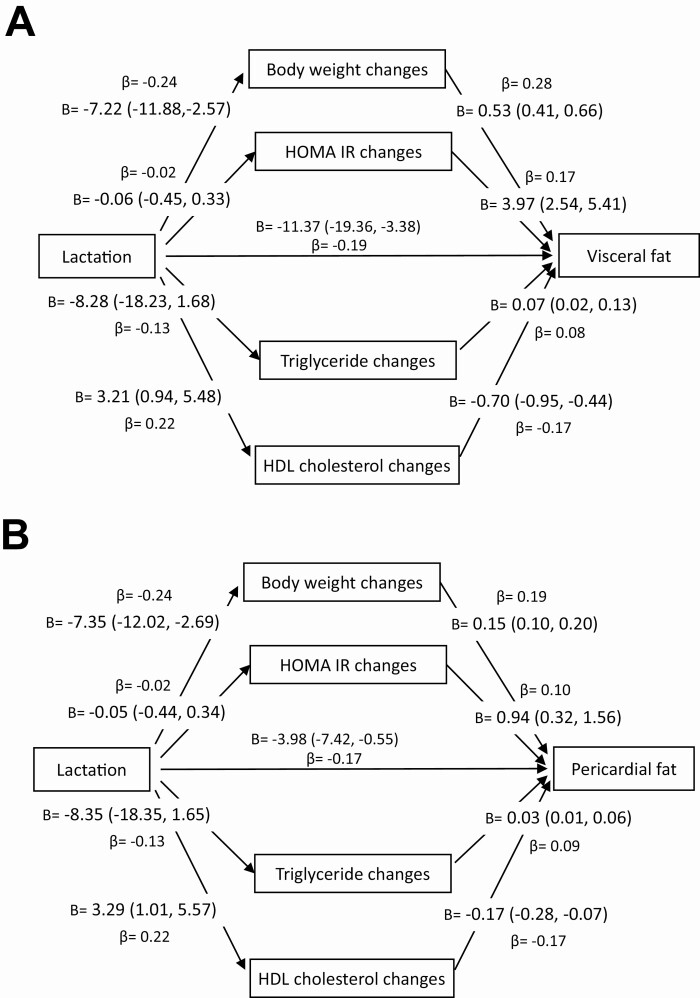
Standardized and unstandardized coefficient values for each mediator along each path of the multiple mediator model are shown in Figure 1. Changes in body weight, HOMA-IR, HDL cholesterol, and triglycerides from the first post-baseline birth to the end of follow-up mediated 37.8% and 33.3% of the associations of lactation with VAT and PAT, respectively (Supplemental Table 8 (31)). For both outcomes, changes in body weight provided the highest indirect effect and mediated 21% and 18% of the relation of lactation with VAT and PAT, respectively. The proportion of the total effects of lactation on VAT and PAT mediated by the other factors were as follows, respectively: HDL cholesterol (12% and 10%), triglycerides (3.3% and 4.7%), and HOMA-IR (1.3% and 0.74%). However, the indirect effects of triglycerides did not attain statistical significance.
Figure 1.
Mediation models of the influence of changes in body weight, HOMA-IR, HDL cholesterol and triglycerides from the first post-baseline birth to the end of follow-up on the association of lactation with (A) visceral fat and (B) pericardial fat volumes. Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance.
Discussion
In this biracial cohort, longer lactation durations was associated with lower VAT and PAT volumes approximately 15 years after the last birth. These results did not vary by race and were independent of prepregnancy factors of age, race, smoking status, BMI, fasting blood glucose, family history of diabetes, total fat intake, total blood cholesterol, and physical activity as well as time-dependent parity and GDM. The associations of lactation duration with VAT and PAT were partially mediated by changes in cardiometabolic factors, most due to weight change, from the first post-baseline birth to the end of follow-up. To our knowledge, this is the first study to observe an inverse association between lactation duration and PAT volume.
Several lines of evidence show that longer lactation duration is associated with lower risks for diabetes and CVD (1-4). However, the underlying mechanisms for these associations are not well understood. Substantial changes in regional fats deposits occur during pregnancy and the postpartum period. Some studies have reported as much as a 30% increase in VAT mass from the first trimester to 1 year postpartum (9). Few longitudinal studies have assessed the relation of lactation with regional adiposity controlling for preconception cardiometabolic risk factors. This present study, which controlled for prepregnancy factors as well as time-dependent characteristics, showed that longer cumulative duration of lactation was associated with lower volumes of VAT and PAT several years after giving birth. In accord with other studies on lactation and body fat composition (19,20), we found no significant relation between lactation and volumes of subcutaneous fat, intermuscular fat, and total abdominal fat after controlling for prepregnancy cardiometabolic factors. In some animal studies, preferential utilization of visceral over subcutaneous fat stores for lactogenesis have been observed when fats are being mobilized from the trunk and thigh regions during lactation (19,32). Similar to other studies that enrolled women of different races (19,20), we found no significant racial differences in the association of lactation duration with VAT or PAT. Our findings of a lower proportion of black women with lactation duration ≥ 12 months compared to white women is reflective of national estimates that indicate substantial differences between black and white women in breastfeeding initiation and duration. Results from the recent National Immunization Survey show that 85.9% of non-Hispanic white women and 69.4% of non-Hispanic black women initiated breastfeeding (33) while 30.8% of non-Hispanic white women and 17.1% of non-Hispanic black women were still breastfeeding at 12 months postpartum (34).
It is unclear how long the apparent benefit of lactation on regional fat accumulation and distribution persists after weaning. A report on 89 women enrolled in the Women and Infant Study of Healthy Hearts showed that after 7 years since the last birth, women who lactated for less than 3 months after each birth exhibited more metabolically active visceral fat than women who consistently lactated (13). Another study of 351 women aged 45 to 58 years enrolled in the Study of Women’s Health Across the Nation (SWAN)-Heart Study observed that among premenopausal/early peri-menopausal mothers, those who never breastfed had 28% greater VAT than mothers who breastfed all of their children for at least 3 months (19). However, among late peri-menopausal/postmenopausal women, there was no difference in VAT between women who breastfeed for at least 3 months and those who did not breastfeed any of their children (19). Results from a pooled cohort of 436 community-dwelling women aged 55 to 80 years from the Rancho Bernardo Study, the Filipino Women’s Health Study, and the Health Assessment Study of African-American Women showed that women who breastfed for more than 3 months on average had 8.8 cm3 lower VAT than women who breastfed less than 3 months across all births (20). In the present study of mostly premenopausal women aged 42 to 56 years at the time of VAT and PAT fat assessment, we observed that any duration of lactation was significantly related to lower material VAT volumes over more than 15 years after the last birth. Importantly, unlike most studies that evaluated lactation history several years after the first birth making them more prone to recall bias, this current study prospectively evaluated lactation duration at approximately every 5 years.
The association of lactation and cardiac fat deposition measured by PAT volumes found in the present study provides novel insights on the role of lactation in the pathogenesis of diabetes and CVD. The location of fat is important in influencing structural and functional changes in the cardiovascular system. Excess accumulation of fat in the pericardial space has been reported to be associated with adverse changes in cardiac morphology as well as dysfunction in cardiac physiology due to its anatomical and functional proximity to the myocardium and coronary vessels (35). These include left ventricular hypertrophy, lower stroke volume and cardiac output, impaired global longitudinal strain, higher accumulation of coronary calcium, increased carotid stiffness and intima-media thickness, poor cardiorespiratory fitness, and overt CVD (10,36). Some studies (37,38) report a stronger relation of pericardial fat with some of these adverse changes in cardiac physiology than epicardial fat: a fat depot located between the myocardium and visceral pericardium. Cardiac ectopic fats, in particular epicardial fat, are highly metabolically active and they express increased pro-inflammatory cytokines such as monocyte chemotactic protein-1, interleukin-6, interleukin 1 beta, plasminogen activator inhibitor-1, tumor necrosis factor-α, and free fatty acids (10). Additionally, PAT, which is not in local anatomical contact with the coronary arteries but constitutes about 70% of cardiac fat accumulation, is reported to be associated with insulin-resistance and metabolic syndrome, independent of BMI and waist girth (37,38). Despite the strong correlations between PAT and VAT, we observed an inverse association between lactation duration and PAT even after controlling for VAT. This suggests that lactation plays a significant role in reducing the accumulation of pathogenic fat deposits around the heart above and beyond the influence of VAT.
The relation of lactation with VAT and PAT was partially mediated by changes in cardiometabolic factors, namely body weight and, to a lesser extent, HOMA-IR, and HDL cholesterol. Pregnancy has been reported to result in several maternal metabolic changes to support the developing fetus and allow accumulation of energy stores in anticipation of lactation (28). These changes include increases in lipids, triglycerides, insulin resistance, and regional fat accumulation and distribution in the visceral compartment (5-9). Accumulating evidence from animal (6) and epidemiological studies suggests that longer lactation duration reverses a majority of these metabolic changes pertaining to improved glucose uptake (14,15), insulin sensitivity (14,15), and lipid metabolism (16,17). Findings of the role of lactation on changes in body weight have been equivocal (39,40). Taken together, these findings provide a potential physiologic basis for the relation of lactation with the accumulation of metabolically active regional fat deposits while suggesting that lactation could also influence VAT or PAT metabolism in women through other biologic and nonbiologic mechanisms. It is possible that the association between lactation and lower volumes of VAT and PAT may be confounded by other health behaviors. In the current study, women without a history of lactation had adverse cardiometabolic and health behavior profiles long before giving birth. Some evidence suggests that maternal metabolic health status before pregnancy affects lactation performance. Insulin plays essential roles in lactogenesis, and pregravid insulin resistance is related to low or no milk production (41). Obesity is a strong risk factor for insulin resistance, and women with pregravid obesity are less likely to initiate and sustain breastfeeding, possibly because of increased rates of adverse perinatal outcomes and much less education and lactation support (41,42). Thus, the high volumes of VAT and PAT and adverse cardiometabolic conditions observed in the current study among women with no or low durations of lactation may represent underlying maternal disease risk.
This study has several notable strengths, including the use of a large population-based biracial sample of women of reproductive age with well-characterized cardiometabolic profiles before and after birth. However, several limitations should to be acknowledged in interpreting our results. Lactation was self-reported at approximately every 5 years during 25 years of follow-up. Although participants provided information on lactation duration, information on lactation intensity was not obtained. This study did not evaluate the clinical, cultural, or social factors that may potentially influence lactation duration, especially among racial minority women. In CARDIA, the assessment of PAT included both epicardial and paracardial adipose tissue as a measure of adipose tissue around the heart. Measure of PAT, VAT, and other abdominal fats were assessed at only 1 point in time. Even though we controlled for several prepregnancy cardiometabolic factors, the presence of residual confounding influencing these findings cannot be ruled out entirely.
In summary, longer lactation duration was associated with lower volumes of VAT and PAT independent of prepregnancy and time dependent cardiometabolic factors. These results, which provide support for lactation being an important modifiable behavior that holds promise for long‐term maternal cardiovascular health benefits, also provide a potential physiologic basis for prior findings of the beneficial effects of lactation on the incidence of diabetes and CVD. The World Health Organization and the American Academy of Pediatrics recommends exclusive breastfeeding for the first 6 months after birth and continued breastfeeding supplemented with other food through at least 12 months (43). Additionally, they advise that breastfeeding may be continued for 1 or 2 more years if mutually desired by the mother and infant. With breastfeeding affording several health benefits for mothers and infants, addressing barriers to breastfeeding in the United States, such as early return to work and providing lactation support, will go a long way to enhance breastfeeding rates among American women, which will in turn improve their cardiometabolic health.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their contributions.
Financial Support: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI). The current study was supported by grants from R01 DK090047 (Gunderson, PI) and K01 DK059944 (Gunderson, PI) from the National Institute of Diabetes, Digestive and Kidney Diseases.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Detailed procedures for accessing CARDIA data can be found at https://www.cardia.dopm.uab.edu/. Most CARDIA data can be also obtained from BioLINCC, a repository maintained by the National Heart, Lung, and Blood Institute at https://biolincc.nhlbi.nih.gov/.
References
- 1. Nguyen B, Gale J, Nassar N, Bauman A, Joshy G, Ding D. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian Cohort study. J Am Heart Assoc. 2019;8(6):e011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24(2):107-115. [DOI] [PubMed] [Google Scholar]
- 3. Stuebe AM, Schwarz EB, Grewen K, et al. . Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174(10):1147-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gunderson EP, Jacobs DR Jr, Chiang V, et al. . Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes. 2010;59(2):495-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61(2):115-118. [DOI] [PubMed] [Google Scholar]
- 6. Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Can MM, Can E, Ozveren O, Okuyan E, Ayca B, Dinckal MH. Epicardial fat tissue thickness in preeclamptic and normal pregnancies. ISRN Obstet Gynecol. 2012;2012:389539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gunderson EP, Sternfeld B, Wellons MF, et al. . Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring). 2008;16(5):1078-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janumala I, Toro-Ramos T, Widen E, et al. . Increased visceral adipose tissue without weight retention at 59 weeks postpartum. Obesity (Silver Spring). 2020;28(3):552-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosito GA, Massaro JM, Hoffmann U, et al. . Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605-613. [DOI] [PubMed] [Google Scholar]
- 11. Shah RV, Murthy VL, Abbasi SA, et al. . Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA study. JACC Cardiovasc Imaging. 2014;7(12):1221-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butte NF, Wong WW, Hopkinson JM. Energy requirements of lactating women derived from doubly labeled water and milk energy output. J Nutr. 2001;131(1):53-58. [DOI] [PubMed] [Google Scholar]
- 13. McClure CK, Catov J, Ness R, Schwarz EB. Maternal visceral adiposity by consistency of lactation. Matern Child Health J. 2012;16(2):316-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunderson EP, Hedderson MM, Chiang V, et al. . Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35(1):50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunderson EP, Crites Y, Chiang V, et al. . Influence of breastfeeding during the postpartum oral glucose tolerance test on plasma glucose and insulin. Obstet Gynecol. 2012;120(1):136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qureshi IA, Xi XR, Limbu YR, Bin HY, Chen MI. Hyperlipidaemia during normal pregnancy, parturition and lactation. Ann Acad Med Singap. 1999;28(2):217-221. [PubMed] [Google Scholar]
- 17. Knopp RH, Walden CE, Wahl PW, et al. . Effect of postpartum lactation on lipoprotein lipids and apoproteins. J Clin Endocrinol Metab. 1985;60(3):542-547. [DOI] [PubMed] [Google Scholar]
- 18. Zachou G, Armeni E, Lambrinoudaki I. Lactation and maternal cardiovascular disease risk in later life. Maturitas. 2019;122:73-79. [DOI] [PubMed] [Google Scholar]
- 19. McClure CK, Schwarz EB, Conroy MB, Tepper PG, Janssen I, Sutton-Tyrrell KC. Breastfeeding and subsequent maternal visceral adiposity. Obesity (Silver Spring). 2011;19(11):2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armenta RF, Kritz-Silverstein D, Wingard D, et al. . Association of breastfeeding with postmenopausal visceral adiposity among three racial/ethnic groups. Obesity (Silver Spring). 2015;23(2):475-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bogaerts A, De Baetselier E, Ameye L, Dilles T, Van Rompaey B, Devlieger R. Postpartum weight trajectories in overweight and lean women. Midwifery. 2017;49:134-141. [DOI] [PubMed] [Google Scholar]
- 22. Friedman GD, Cutter GR, Donahue RP, et al. . CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. [DOI] [PubMed] [Google Scholar]
- 23. Ding J, Hsu FC, Harris TB, et al. . The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90(3):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terry JG, Shay CM, Schreiner PJ, et al. . Intermuscular adipose tissue and subclinical coronary artery calcification in midlife: the CARDIA study (Coronary Artery Risk Development in Young Adults). Arterioscler Thromb Vasc Biol. 2017;37(12):2370-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alman AC, Jacobs DR Jr, Lewis CE, et al. . Higher pericardial adiposity is associated with prevalent diabetes: the Coronary Artery Risk Development in Young Adults study. Nutr Metab Cardiovasc Dis. 2016;26(4):326-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terry JG, Hartley KG, Steffen LM, et al. . Association of smoking with abdominal adipose deposition and muscle composition in Coronary Artery Risk Development in Young Adults (CARDIA) participants at mid-life: a population-based cohort study. PloS Med. 2020;17(7):e1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDonald A, Van Horn L, Slattery M, et al. . The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91(9):1104-1112. [PubMed] [Google Scholar]
- 28. Gunderson EP, Lewis CE, Tsai AL, et al. . A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetes. 2007;56(12):2990-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gunderson EP, Chiang V, Lewis CE, et al. . Long-term blood pressure changes measured from before to after pregnancy relative to nonparous women. Obstet Gynecol. 2008;112(6):1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: The Guilford Press; 2018. [Google Scholar]
- 31. Appiah D. Data from: Supplemental materials for the association of lactation duration with visceral and pericardial fat volumes in parous women: 25-Year Follow-up in the CARDIA Study. Dryad 2020. ProMED-mail website. Deposited December 6, 2020. https://datadryad.org/stash/share/oaxm6l-X0SA2i-Rk3wPM7nOrgzY3bCAqmpyKC_seL20 [Google Scholar]
- 32. Moore BJ, Olsen JL, Marks F, Brasel JA. The effects of high fat feeding during one cycle of reproduction consisting of pregnancy, lactation and recovery on body composition and fat pad cellularity in the rat. J Nutr. 1984;114(9):1566-1573. [DOI] [PubMed] [Google Scholar]
- 33. Beauregard JL, Hamner HC, Chen J, Avila-Rodriguez W, Elam-Evans LD, Perrine CG. Racial disparities in breastfeeding initiation and duration among U.S. infants born in 2015. MMWR Morb Mortal Wkly Rep. 2019;68(34):745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anstey EH, Chen J, Elam-Evans LD, Perrine CG. Racial and geographic differences in breastfeeding - United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2017;66(27):723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nafakhi H, Al-Mosawi AA, Al Esawi RW. Pericardial adiposity versus body adiposity measured by BMI in the assessment of coronary atherosclerosis burden in patients with hypertension. Clin Exp Hypertens. 2021;43(1):13-17. [DOI] [PubMed] [Google Scholar]
- 36. Brinkley TE, Ding J, Carr JJ, Nicklas BJ. Pericardial fat loss in postmenopausal women under conditions of equal energy deficit. Med Sci Sports Exerc. 2011;43(5):808-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nyman K, Granér M, Pentikäinen MO, et al. . Cardiac steatosis and left ventricular function in men with metabolic syndrome. J Cardiovasc Magn Reson. 2013;15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sironi AM, Petz R, De Marchi D, et al. . Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet Med. 2012;29(5):622-627. [DOI] [PubMed] [Google Scholar]
- 39. He X, Zhu M, Hu C, et al. . Breast-feeding and postpartum weight retention: a systematic review and meta-analysis. Public Health Nutr. 2015;18(18):3308-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change: a systematic review and critical evaluation. Int J Obes (Lond). 2014;38(4):577-590. [DOI] [PubMed] [Google Scholar]
- 41. Stuebe AM. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin Perinatol. 2015;39(4):290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kair LR, Colaizy TT. Obese mothers have lower odds of experiencing pro-breastfeeding hospital practices than mothers of normal weight: CDC Pregnancy Risk Assessment Monitoring System (PRAMS), 2004-2008. Matern Child Health J. 2016;20(3):593-601. [DOI] [PubMed] [Google Scholar]
- 43. Eunice Kennedy Shriver National Institute of Child Health and Human Development. What are the recommendations for breastfeeding? January 31, 2017. https://www.nichd.nih.gov/health/topics/breastfeeding/conditioninfo/recommendations
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Detailed procedures for accessing CARDIA data can be found at https://www.cardia.dopm.uab.edu/. Most CARDIA data can be also obtained from BioLINCC, a repository maintained by the National Heart, Lung, and Blood Institute at https://biolincc.nhlbi.nih.gov/.