Abstract
Context
Transsphenoidal surgery (TSS) is the primary treatment of choice in acromegaly. It is important to identify patients in whom surgical cure is not attainable at an early stage, both to inform patients on expected treatment outcome and to select those who are more likely to need additional therapy.
Objective
To identify predictors for remission after TSS in acromegaly.
Methods
Large multicenter study with retrospective data collection from 3 tertiary neurosurgical referral centers in The Netherlands. We analyzed clinical data since 2000 from 3 cohorts (Groningen, Nijmegen, and Rotterdam, total n = 282). Multivariate regression models were used to identify predictors of early biochemical remission (12 weeks to 1 year postoperatively) according to the 2010 consensus criteria, long-term remission (age- and sex-normalized insulin-like growth factor 1 [IGF-1] and the absence of postoperative treatment until last follow-up), and relative IGF-1 and growth hormone [GH] reduction.
Results
A larger maximum tumor diameter (odds ratio [OR] 0.91, 95% CI 0.87-0.96, P ≤ .0001) was associated with a lower chance of early biochemical remission. A larger maximum tumor diameter (OR 0.93, 95% CI 0.89-0.97, P = .0022) and a higher random GH concentration at diagnosis (OR 0.98, 95% CI 0.96-0.99, P = .0053) were associated with a lower chance of long-term remission.
Conclusion
Maximum tumor diameter and random GH concentration at diagnosis are the best predictors for remission after TSS in acromegaly.
Keywords: acromegaly, transsphenoidal surgery, remission
Acromegaly is a rare condition with a prevalence of 2.8 to 13.7/100 000 and an incidence of 0.2 to 1.1/100 000 (1). The disease is characterized by growth hormone (GH) excess resulting in the overproduction of insulin-like growth factor 1 (IGF-1). As a consequence, patients with uncontrolled acromegaly have a 10-15 years reduced life expectancy (2) and suffer from multimorbidity (3). The main complications of acromegaly include cardiovascular disease, hyperglycemia, dyslipidemia, sleep apnea, neoplasms, osteoarthritis, and vertebral fractures (4). The disease is almost always caused by a pituitary tumor. The main treatment goal is to decrease mortality and morbidity through control of GH and IGF-1 hypersecretion, and reduction or stabilization of pituitary tumor size. Transsphenoidal surgery (TSS) is the primary treatment of choice, as it is the only treatment that can provide cure and results in lower lifetime treatment costs (5). In a 2016 meta-analysis, remission rates were found to be 78% in microadenomas and 53% in macroadenomas (6). Overall, approximately 75% of patients achieve long-term remission, which sometimes takes several years after initial surgery (7, 8). It is important to identify patients in whom surgical remission is not attainable at an early stage, both to inform patients on expected treatment outcome and to select those who are more likely to need additional therapy.
The presence of cavernous sinus invasion, larger tumor size, and higher preoperative GH levels are consistently associated with lower surgical remission rates in the literature (9). However, results are discrepant between studies, and cohorts are often small, monocentric, and heterogeneous to draw robust conclusions. We performed a retrospective study in 3 tertiary neurosurgical referral centers in The Netherlands, aiming to identify clinical predictors for remission and relapse after TSS in acromegaly by utilizing the stricter criteria for cure according to the 2010 consensus criteria in the largest multicenter study available in the literature.
Patients and Methods
Study population
Patients were included from 3 retrospective cohorts: (1) the Rotterdam cohort, (2) the Groningen cohort, and (3) the Nijmegen cohort. These cohorts contain acromegaly patients who received TSS as primary treatment for acromegaly between the January 1, 2000, and the July 1, 2019, in the Erasmus University Medical Center of Rotterdam, University Medical Center of Groningen, and Radboud University Medical Center of Nijmegen, which are large tertiary referral centers in The Netherlands for patients with pituitary pathology. Study enrolment procedures are shown elsewhere (Fig. S1 (10)).
Data were collected on
-
•
baseline demographic characteristics (sex, age at diagnosis, and surgery);
-
•
tumor size at diagnosis based on maximum diameter in millimeters (both as a dichotomous variable in terms of micro <10 mm vs macro ≥10 mm and as a continuous variable);
-
•
cavernous sinus invasion according to Knosp’s classification (11);
-
•
biochemistry (IGF-1, nadir GH during oral glucose tolerance test [OGTT], random GH) at diagnosis and 12 weeks to 1 year postoperatively;
-
•
preoperative medication (categorized as a dopamine agonist [DA], first-generation somatostatin analog (SSA-1, ie, lanreotide or octreotide), second-generation somatostatin analog (SSA-2, ie, pasireotide), and GH receptor antagonist (GHRA, ie, pegvisomant);
-
•
applied postoperative treatments, categorized as repeated surgery, radiotherapy and medical therapy (subcategorized as DA, SSA-1 and -2 and GHRA);
-
•
presence of hypopituitarism at last visit;
-
•
long-term remission, defined as an age- and sex-normalized IGF-1 at last visit and the absence of postoperative treatment (repeated surgery, radiotherapy and/or medical therapy) until last follow-up.
The diagnosis of acromegaly was based on biochemical criteria valid at the first measurement at the time of diagnosis, namely failure to achieve GH suppression to <1 μg/L during an OGTT and an elevated IGF-1 concentration (corrected for sex and age) (12, 13).
Primary TSS was followed by a second or third surgical procedure when a large tumor remnant accessible for surgery persisted or in case of recurrent disease. Radiotherapy was only given postoperatively to patients with evidence of persistent or recurrent disease. DA, SSA-1 and -2, and GHRA were prescribed for the purpose of preoperative disease control and postoperatively in case of persistent or recurrent disease, in accordance with international guidelines for acromegaly management (14).
For the Rotterdam cohort, written informed consent was obtained from all patients prior to inclusion, and the study was approved by the Medical Ethics Review Board of the Erasmus MC. For the Groningen and Nijmegen cohorts, the study was approved by the Medical Ethics Review Board of the UMCG and Radboudumc. The study fulfilled all requirements for patient anonymity and was in agreement with regulations of both university hospitals for publication of patient data as well as with the Dutch Civil Code (Article 458 on use of data for scientific research).
Laboratory Assays
In the Rotterdam cohort, IGF-1 concentrations were measured with the following assays: Immulite 2000 assay, a solid-phase, validated enzyme-labelled chemiluminescent immunometric assay from 2000 to February 2013 (DPC Biermann GmbH/Siemens, Fernwald, Germany; intra-assay variability of 2-5%, interassay variability of 3-7%). GH concentrations were initially measured with an immunoradiometric assay from 2000 to February 2013 (IRMA; CIS Bio International, Gif-sur-Yvette, France, intra-assay coefficients of variation [CVs] 2.8%, interassay CVs 4.4%). Since February 2013, IGF-1 and GH concentrations were measured with the immunometric IDS-iSYS assay (Boldon, UK). Interassay CVs for GH and IGF-1 were <5% (GH; n = 190) and (IGF-1; n = 190) in serum-based internal quality control measurements over a period of 1 year. All GH assays used during the study period were calibrated to the World Health Organization 98/574 standard.
In the Groningen cohort, IGF-1 concentrations were measured with a radioimmunoassay (RIA) of the Nichols Institute of Diagnostics, San-Juan Capistrano, CA, USA, before 2002 (9), the Nichols Advantage assay from 2002 to February 2006, the Immulite 2500 (Siemens) assay from 2006 to November 2011, and the Immulite 2000 (Siemens) assay from 2011 to April 2013. Since April 2013, IGF-1 concentrations have been measured with the immunometric IDS-iSYS assay (Boldon, UK).
GH concentrations were initially measured with the Delfia RIA of Perkin Elmer LifeSciences (Turku, Finland). Since January 2014, GH concentrations were measured with the immunometric IDS-iSYS assay (Boldon, UK).
In the Nijmegen cohort, IGF-1 concentrations were measured with an in-house RIA traceable to the NIBSC 91/554 standard before December 2009, the Immulite 2500 (Siemens) assay from December 2009 to March 2012, and the Immulite 2000XPI (Siemens) assay from March 2012 to January 2013. Since January 2013, a chemiluminescence IGF-1 immunoassay on a Liaison analyzer by Diasorin has been used.
GH concentrations were measured with an in-house RIA before December 2009, the Immulite 2500 (Siemens) assay from December 2009 to April 2012, the Immulite 2000XPI (Siemens) assay from April 2012 to April 2015, and the Modular E170 (Roche) assay from April 2015 to May 2017. Since May 2017, GH concentrations have been measured with the Cobas E801 assay (Roche). All GH assays used during the study period were calibrated to the World Health Organization 98/574 standard.
For the purpose of uniform reporting, IGF-1 concentrations were expressed as a fraction of the upper limit of normal (ULN) of the reference ranges used in each center (the measured IGF-1 concentration in nmol/L divided by the age- and sex-specific ULN). GH concentrations (μg/L) were measured as a single random sample and expressed as absolute value. IGF-1, nadir GH during OGTT, and random GH were only reported if measured in 1 of the 3 study centers. Values measured in other centers before referral of patients to 1 of the 3 participating centers were not included in the analysis in order to reduce assay-related analytic bias.
Outcomes
Primary endpoints were
-
•
early biochemical remission, defined based on the first available biochemistry 12 weeks to 1 year postoperatively according to the latest Consensus Statement on acromegaly therapeutic outcomes (14);
-
•
an age- and sex-normalized IGF-1;
-
•
a random GH <1 µg/L or a nadir GH after OGTT <0.4 µg/L;
-
•
long-term remission, defined as an age- and sex-normalized IGF-1 at last visit and the absence of postoperative treatment (radiation therapy, repeat surgery, or medical therapy) until last follow-up. Due to lack of data on random GH and nadir GH during OGTT at last follow-up, it was decided not to use these parameters to define long-term remission;
-
•
relapse, defined as the achievement of early biochemical remission without long-term remission.
Secondary endpoints were relative IGF and GH reduction, defined as a percentual decrease in serum IGF-1 and GH at 12 weeks to 1 year postoperatively compared with IGF-1 and GH at diagnosis.
Candidate Predictors
Variables considered as possible predictors for remission and relapse after TSS were selected based on previous studies (8, 15-18), biological plausibility, and availability of robust data ascertainment in the 3 cohorts and included age at diagnosis and surgery, sex, serum (nadir) GH and IGF-1 concentration at diagnosis, tumor size (micro- or macroadenoma at diagnosis), cavernous sinus invasion (Knosp 3 or 4) (11), and use of preoperative medical therapy (DA, SSA-1, SSA-2, GHRA). In analysis of serum IGF-1 and GH postoperatively, only those values that were measured at least 12 weeks after surgery were analyzed. In patients who used SSA-1 postoperatively, serum IGF-1 and GH that were measured at least 12 weeks after initiation of therapy were used. In patients in whom SSA-1 had been successfully withdrawn, serum IGF-1 and GH that were measured at least 12 weeks after withdrawal of the medication were used, consistent with clinical guidelines regarding the reliability of IGF-1 measurements (13, 19).
Statistical Analysis
Data are expressed as mean ± SD, median (interquartile range), or percentages when appropriate. Differences were assessed with Fisher’s exact test for categorical variables and unpaired t-tests for continuous variables. When continuous variables were not normally distributed, a Mann–Whitney U test was performed. To reduce bias due to missing values in covariates, we performed multiple imputation (10 imputed datasets). Imputation models included most candidate predictor variables and the outcome variables. There was no difference between the original or any of the imputed datasets. Analyses were performed separately in all datasets and results pooled using Rubin’s rules.
We fitted univariate linear regression models to assess the shape of the association between continuous candidate predictor variables and relative IGF-1 and GH reduction. This was done by modeling the predictor variable utilizing a natural cubic spline with 3 degrees of freedom and performing likelihood ratio tests to assess if the nonlinear effect improved model fit. Variables for which this seemed to be the case were included in a multivariate linear regression model using the spline specification; for all categorical candidate predictors linear effects were included. To take into account potential differences between centers, the model also included cohort as a factor. To keep predictors in the model liberally, we excluded predictors with P > .20.
To investigate the association between the above-named candidate predictors and early biochemical remission, we fitted univariate and multivariate logistic regression models, following the same procedure as described above. In a secondary analysis, this was repeated for long-term remission and relapse. Predictive discriminative ability was assessed using receiver operating characteristics analysis.
Calibration of all predictive models (linear and logistic) was assessed using plots. Unless specified otherwise, P < .05 (2-tailed) was considered statistically significant. All statistical analyses were performed using SPSS, version 25.0 for Windows, or R statistical software, version 3.5.2 (packages foreign, mice, pROC, and rms).
Results
Study Population and Comparison among Centers
A total of 282 patients (96 from Groningen, 82 from Nijmegen, and 104 from Rotterdam) was included in the analysis. Characteristics of the study population are shown in Table 1.
Table 1.
Patient characteristics of the total group, Groningen, Nijmegen, and Rotterdam cohort
| Total | Groningen | Nijmegen | Rotterdam | |
|---|---|---|---|---|
| Number | 282 | 96 (34) | 82 (29) | 104 (37) |
| Female | 152 (54) | 54 (56) | 43 (52) | 55 (53) |
| Age at diagnosis (years) | 48.0 (38.9; 57.1) | 51.1 (40; 61.1) | 48.5 (40; 56.25) | 44.6 (36.9; 54.9) |
| Age at surgery (years) | 48.8 (40.0; 58.8) | 51.3 (40.4; 61.79) | 49.5 (41; 57.3) | 45.2 (38.7; 56.7) |
| Follow-up from diagnosis (years) | 8.7 (3.8; 13.0) | 10.1 (4.8; 15.0) | 5.1 (2.7; 9.8) | 9.8 (5.6; 13.1) |
| Follow-up from surgery (years) | 7.5 (2.7; 12.2) | 9.4 (4.2; 14.2) | 4.2 (2.0; 9.0) | 8.5 (4.7; 12.6) |
| Macroadenoma | 223 (79) | 77 (80) | 65 (79) | 81 (78) |
| Maximum tumor diameter (mm) | 15 (10, 23) | 15 (11, 21) | 15 (10, 24) | 18 (10, 28) |
| – 81 missing | – 23 missing | – 12 missing | – 46 missing | |
| Cavernous sinus invasion (Knosp 3 or 4) | 67 (26) | 16 (21) | 12 (15) | 39 (38) |
| – 21 missing | – 21 missing | |||
| Disease activity at diagnosis | ||||
| IGF-1 (× ULN) | 3.25 (2.48; 4.20) | 3.68 (2.9; 4.71) | 3.21 (2.38; 4.13) | 2.78 (2.27; 3.68) |
| – 4 missing | – 2 missing | – 2 missing | ||
| Nadir GH during OGTT (µg/L) | 10.40 (4.53; 26.14) | 18.75 (6.16; 34.98) | 7.95 (2.9; 19.96) | 7.5 (4.4; 22.1) |
| – 123 missing | – 36 missing | – 40 missing | – 47 missing | |
| Random GH (µg/L) | 13.91 (5.72; 33.08) | 23.4 (9.49; 51.28) | 12 (4.56; 23.6) | 11.4 (4.8; 29.9) |
| – 38 missing | – 15 missing | – 18 missing | – 5 missing | |
| Preoperative medical treatment | 204 (72) | 72 (75) | 79 (96) | 53 (51) |
| DA | 3 (1) | 2 (2) | 1 (1) | 0 |
| SSA-1 | 180 (64) | 69 (72) | 78 (95) | 33 (32) |
| SSA-2 | 3 (1) | 0 | 0 | 3 (3) |
| GHRA | 18 (6) | 1 (1) | 0 | 17 (16) |
| Postoperative disease activity | ||||
| IGF-1 (× ULN) | 1.21 (0.88; 1.86) | 1.40 (1.07; 2.29) | 1.07 (0.86; 1.4) | 1.20 (0.8; 2.03) |
| – 11 missing | – 3 missing | – 8 missing | ||
| Absolute IGF-1 reduction (× ULN) | 1.79 (0.98; 2.62) | 2.01 (1.46; 2.95) | 2.03 (1.2; 2.69) | 1.47 (0.73; 2.15) |
| – 14 missing | – 5 missing | – 9 missing | ||
| Relative IGF-1 reduction (%) | 61.09 (38.86; 73.34) | 60.77 (42.65; 73.09) | 66.04 (51.34; 75.55) | 53.25 (27.19; 71.46) |
| – 14 missing | – 5 missing | – 9 missing | ||
| Nadir GH during OGTT (µg/L) | 0.57 (0.15; 1.2) | 0.47 (0.13; 1.45) | 0.57 (0.15; 0.73) | 3.2 (0.3; 4.48) |
| – 163 missing | – 46 missing | – 21 missing | – 96 missing | |
| Absolute nadir GH reduction (µg/L) | 2.68 (9.38; 22.74) | 11.04 (3.07; 24.67) | 7.33 (2.67; 19) | 2.1 |
| – 218 missing | – 66 missing | – 51 missing | – 101 missing | |
| Relative nadir GH reduction (%) | 93.86 (81.96; 98.43) | 95.40 (76.51; 99.28) | 93.21 (87.4; 97.01)– 51 missing | 88.35– 101 missing |
| – 218 missing | – 66 missing | |||
| Random GH (µg/L) | 1.6 (0.6; 4.2) | 1.74 (0.43; 4.93) | 1.51 (0.67; 3.48) | 1.45 (0.6; 4.48) |
| – 9 missing | – 7 missing | – 2 missing | ||
| Absolute random GH reduction (µg/L) | 11.84 (3.78; 30.17) | 17.95 (6.05; 45.29) | 10.3 (3.57; 18.73) | 7 (2.3; 25.7) |
| – 44 missing | – 20 missing | – 19 missing | – 5 missing | |
| Relative random GH reduction (%) | 87.23 (72.16; 95.50) | 89.93 (79.46; 97.62) | 87.86 (74.07; 93.61) | 84 (59.38; 94.13) |
| – 44 missing | – 20 missing | – 19 missing | –5 missing | |
| Remission | ||||
| Early biochemical remission (2010 criteria) | 81 (34) | 22 (25) | 32 (44) | 27 (34) |
| – 43 missing | – 9 missing | – 10 missing | – 24 missing | |
| Long-term remission | 109 (39) | 35 (37) | 46 (56) | 28 (27) |
| Relapse | 9 (3) | 0 | 1 (1) | 8 (8) |
Data are given as absolute numbers (%) or as median (interquartile range).
Abbreviations: DA, dopamine agonist; GH, growth hormone; GHRA, growth hormone receptor antagonist; IGF-1, insulin-like growth factor 1; OGTT, oral glucose tolerance test; SSA-1, first-generation somatostatin analog (octreotide or lanreotide); SSA-2, second-generation somatostatin analog (pasireotide); TSS, transsphenoidal surgery; ULN, upper limit of normal;.
Patients from the Rotterdam cohort were younger at diagnosis (45 vs 51 and 48 years) and more often presented with cavernous sinus invasion (38% vs 21% and 15%). Patients from the Groningen cohort presented with the most biochemically active disease (IGF-1 3.68 vs 3.21 and 2.78 × ULN). The highest early biochemical remission percentages were attained in the Nijmegen cohort (44% vs 25% and 34%). Distinct differences were found in the use of preoperative medication; GHRA and SSA-2 were applied nearly exclusively in the Erasmus MC. In the total cohort, early biochemical remission occurred in 34% of patients and long-term remission and relapse in 39% and 3%, respectively. Data on applied postoperative treatment and presence of hypopituitarism at last visit are shown elsewhere (Table S1 (10)).
Univariate Analyses for Selection of Predictors
All univariate analyses of the candidate predictors for the primary and secondary endpoints are shown in Tables 2 and 3 respectively. Since relapse only occurred in 9 patients, we were unable to perform multivariate analyses for this endpoint.
Table 2.
Univariate analysis for selection of predictors of early biochemical remission, long-term remission and relapse
| Early biochemical remission | Long-term remission | Relapse | |
|---|---|---|---|
| Sex | — | — | — |
| Age at diagnosis (years) | Older* | Older** | Younger* |
| Age at surgery (years) | Older* | Older** | Younger* |
| Nadir GH at diagnosis (µg/L) | ↓ nadir GH* | ↓ nadir GH** | ↑ nadir GHa |
| GH at diagnosis (µg/L) | ↓ GH** | ↓ GH** | ↑ GH* |
| IGF-1 at diagnosis (× ULN) | ↓ IGF-1** | ↓ IGF-1a | — |
| Tumor size (micro/macro) | Micro*** | Micro*** | Macro* |
| Maximum tumor diameter (mm) | Smaller *** | Smaller *** | Larger *** |
| Cavernous sinus invasion | No CSI** | No CSI*** | — |
| Preoperative medical treatment | |||
| DA | — | — | — |
| SSA-1 | Yesa | Yesa | — |
| SSA-2 | — | — | — |
| GHRA | — | — | — |
Abbreviations: CSI, cavernous sinus invasion; DA, dopamine agonist; GH, growth hormone; GHRA, growth hormone receptor antagonist (ie, pegvisomant); IGF-1, insulin-like growth factor 1; SSA-1, first-generation somatostatin analog (ie, lanreotide or octreotide); SSA-2, second-generation somatostatin analog (ie, pasireotide); ULN, upper limit of normal.
*P < .05; **P < .01; *** P < .0001.
a Trend towards significance at P < .20.
Table 3.
Univariate analysis for selection of predictors of relative IGF-1 and GH reduction
| Relative IGF-1 reduction | Relative GH reduction | |
|---|---|---|
| Sex | — | — |
| Age at diagnosis (years) | Older** | — |
| Age at surgery (years) | Older** | — |
| Nadir GH at diagnosis (µg/L) | — | ↑ nadir GH* |
| GH at diagnosis (µg/L) | ↓ GH** | ↑ GH** |
| IGF-1 at diagnosis (× ULN) | ↑ IGF-1*** | ↑ IGF-1** |
| Tumor size (micro/macro) | Micro** | — |
| Maximum tumor diameter (mm) | Smaller *** | Smaller * |
| Cavernous sinus invasion | No CSI** | — |
| Preoperative medical treatment DA SSA-1 SSA-2 GHRA |
— — — No** |
— — — — |
Abbreviations: CSI, cavernous sinus invasion; DA, dopamine agonist; GH, growth hormone; GHRA, growth hormone receptor antagonist (pegvisomant); IGF-1, insulin-like growth factor 1; SSA-1, first-generation somatostatin analog (octreotide or lanreotide); SSA-2, second-generation somatostatin analog (pasireotide); ULN, upper limit of normal.
*P < .05, **P < .01, *** P < .0001.
Multivariate Analyses for Primary Endpoints
Early biochemical remission
Maximum tumor diameter at diagnosis was the only determinant of early biochemical remission (Table 4). A larger tumor was associated with a lower chance of early biochemical remission (odd ratio [OR] 0.91, 95% CI 0.87-0.96, P ≤ .0001), in other words with every millimeter increase in tumor diameter, the odds of achieving early biochemical remission decreases by 9%. The discriminative ability of tumor size diagnosis to predict early biochemical remission was adequate (area under the curve 0.76, 95% CI 0.69-0.84; Fig. S2 (10)).
Table 4.
Multivariate analysis for primary endpoints
| OR | 95% CI | P value | |
|---|---|---|---|
| Early biochemical remission | |||
| Maximum tumor diameter (mm) | 0.91 | 0.87-0.96 | ≤.0001 |
| Long-term remission | |||
| Maximum tumor diameter (mm) | 0.93 | 0.89-0.97 | .0022 |
| Random GH at diagnosis (µg/L) | 0.98 | 0.96-0.99 | .0053 |
Abbreviations: GH, growth hormone; OR, odds ratio.
Long-term remission
Maximum tumor diameter and random GH concentration at diagnosis were determinants of long-term remission (Table 4). A larger tumor (OR 0.93, 95% CI 0.89-0.97, P = .0022) and a higher random GH concentration at diagnosis (OR 0.98, 95% CI 0.96-0.99, P = .0053) were associated with a lower chance of long-term remission. The combined discriminative ability of maximum tumor diameter and random GH concentration at diagnosis to predict long-term remission was adequate (area under the curve of 0.80, 95% CI 0.73-0.86; Fig. S3 (10)).
Multivariate Analyses for Secondary Endpoints
Relative IGF-1 reduction
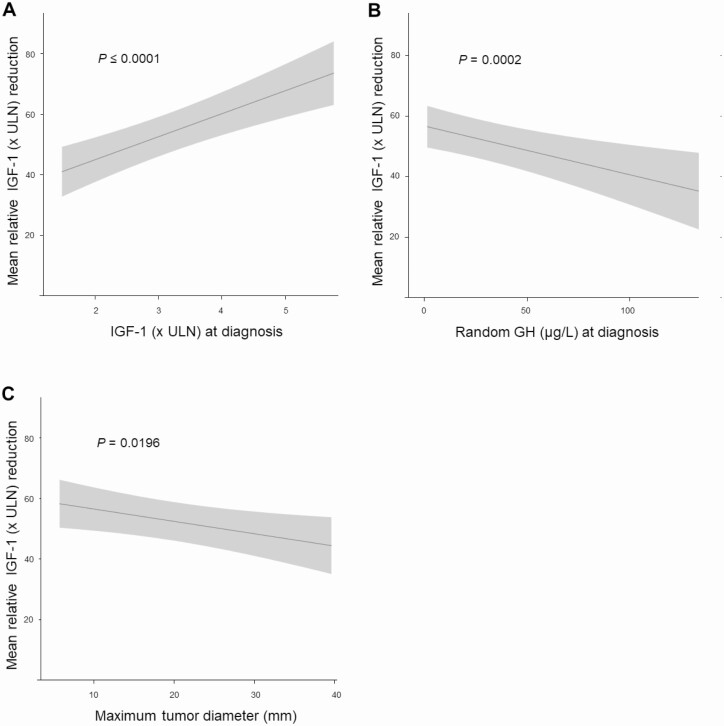
IGF-1, random GH concentration, and maximum tumor diameter at diagnosis were determinants of relative IGF-1 reduction (Table 5). A positive association was found for IGF-1 concentration (β 7.60, SE 1.56, P ≤ .0001; Fig. 1A). An inverse association was found for random GH concentration (β –0.16, SE 0.05, P = .0002; Fig. 1B) and for maximum tumor diameter at diagnosis (β –0.41, SE 0.17, P = .0196; Fig. 1C).
Table 5.
Multivariate analysis for secondary endpoints
| β | SE | P value | |
|---|---|---|---|
| Relative IGF-1 reduction | |||
| IGF-1 at diagnosis (× ULN) | 7.60 | 1.56 | ≤.0001 |
| GH at diagnosis (µg/L) | –0.16 | 0.05 | .0002 |
| Maximum tumor diameter (mm) | –0.41 | 0.17 | .0196 |
| Relative GH reduction | |||
| GH at diagnosis (µg/L) | 0.25 | 0.07 | .0010 |
| IGF-1 at diagnosis (x ULN) | 5.15 | 2.21 | .0176 |
| Maximum tumor diameter (mm) | –0.52 | 0.26 | .0436 |
Abbreviations: GH, growth hormone; IGF-1, insulin-like growth factor 1; ULN, upper limit of normal.
Figure 1.
Predictors of IGF-1 reduction. Association between relative IGF-1 reduction and (A) IGF-1 (× ULN) at diagnosis, (B) GH concentration (µg/L) at diagnosis, and (C) maximum tumor diameter (mm). IGF-1, insulin-like growth factor 1; ULN, upper limit of normal.
Relative GH concentration reduction
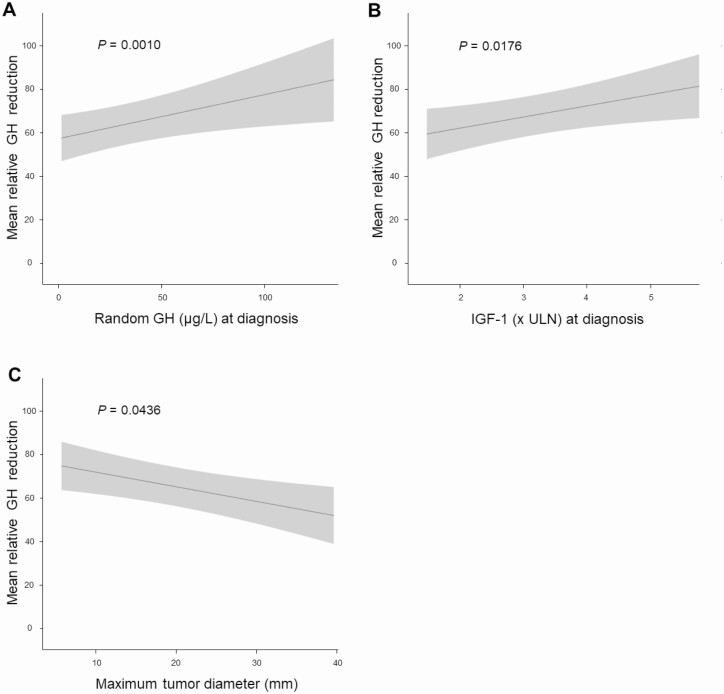
IGF-1, random GH concentration, and maximum tumor diameter at diagnosis were determinants of relative GH reduction (Table 5). A positive association was found for random GH concentration (β 0.25, SE 0.07, P = .0010; Fig. 2A) and for IGF-1 concentration (β 5.15, SE 2.21, P = .0176; Fig. 2B), while a negative association was found for maximum tumor diameter at diagnosis (β –0.52, SE 0.26, P = .0436; Fig. 2C).
Figure 2.
Predictors of GH reduction. Association between relative GH reduction and (A) GH concentration (µg/L) at diagnosis, (B) IGF-1 (× ULN) at diagnosis, and (C) maximum tumor diameter (mm). IGF-1, insulin-like growth factor 1; ULN, upper limit of normal.
Discussion
This is the largest multicenter study available in the literature to focus on identifying clinical predictors for remission after TSS in acromegaly. Its main findings are that (1) early biochemical remission is best distinguished by maximum tumor diameter at diagnosis, and (2) long-term remission occurs more frequently in patients with a lower random GH concentration at diagnosis who harbor a smaller tumor.
In this study, maximum tumor diameter and random GH at diagnosis are the best predictors for remission. Given that complete tumor resection or debulking decreases basal GH secretion and that larger tumors secrete more GH, it becomes apparent why patients harboring smaller tumors are more likely to achieve remission after TSS. Moreover, tumors invading the cavernous sinus are unlikely to be completely removed. These patients rarely achieve remission as a result of surgery alone and often require medical treatment postoperatively (9, 12-15, 18-20). We should mention that we included 204 (72%) patients who received preoperative medical treatment in our study, which could also influence both random GH and IGF-1 concentrations and tumor size (21-23). However, we did not find clear evidence for a beneficial effect of preoperative SSA-1 in enhancing surgical outcome.
Relapse after TSS occurred more frequently in patients who were younger at diagnosis and harbored larger tumors that secrete more GH. This observation confirms and builds upon previous studies (11, 24, 25), proposing age at diagnosis to be a clinical marker of tumor size and aggressiveness. Younger patients (age <40 years) tend to have larger and more aggressive tumors, most likely due to the increased prevalence of the AIP and GPR101 genes (26). Older patients with a microadenoma and a relatively low GH concentration at diagnosis are more likely to achieve surgical cure. Therefore, the results of our study may be of added value when doubt exists about the need for and timing of follow-up pituitary magnetic resonance imaging, which may help prevent unnecessary gadolinium exposure since long-term retention of the compound has become a topic of emerging concern (27).
It is well known that IGF-1 responds linearly to GH concentration only up to a certain level and plateaus at higher GH concentrations (28, 29), which may explain why IGF-1 concentration at diagnosis was found to be less predictive for early and long-term remission in our analyses. This finding is consistent with the literature, as in studies evaluating both GH and IGF-1, the predictive value of IGF-1 was lower or similar to preoperative GH levels (9).
A strength of our study is the large number of patients in whom the biochemical response to TSS was systematically investigated compared with previous literature (11-15, 19, 20). This study provides specific advantages in that it is not limited to a single center nor does it deal with patients managed with only a single treatment modality, and, therefore, may better reflect the general population of acromegaly patients and overcome selection bias.
In this study, we utilized the stricter criteria for cure according to the 2010 consensus criteria. To overcome the limitation of a cutoff validity, we confirmed our data by using the 2000 consensus criteria (OGTT cutoff of <1.0 µg/L) in multivariate analyses, which did not affect the results (data not shown).
The main limitations of our study lie in its retrospective nature, limiting the availability of robust data on random GH levels and nadir GH during OGTT. We are also limited by the use of different GH and IGF-1 assays, both over time and between centers. In addition, our study lacks data on radiological and histological parameters such as T2-signal intensity, granulation pattern, prolactin expression, Ki-67/mitotic index, p53). However, these limitations are common in medical research and reflect the nature of daily practice, in which physicians often make therapeutic decisions based on the available data. As our study confirmed the low incidence of relapse after TSS in acromegaly, it was not possible to perform multivariate analysis for this outcome measure. A significantly larger multicenter study is needed to perform these analyses properly, stressing the need for large international multicenter collaboration. Finally, we are limited by the fact that patients were operated on by at least 13 different neurosurgeons. It has long been known that remission rates, overall clinical outcomes, and surgical complication rates in TSS are related to neurosurgeon practice volume and experience (30). Although it is clear that we should strive for harmonization of GH and IGF-1 data for future research by using a single assay, our current approach demonstrates that it is possible to generate robust and credible data in a multicenter setting involving many neurosurgeons across 2 decades of assay evolution and optimization of surgical techniques.
TSS remains the primary treatment of choice in acromegaly, but some patients have a lower chance of achieving remission. Tumor size and GH concentration at diagnosis are the best clinical predictors for remission after TSS in acromegaly.
The results of this study can be used to better inform patients on expected treatment outcome and to personalize postoperative treatment and follow-up. In addition, further optimization of surgical techniques to improve remission rates for patients who harbor large tumors extending laterally into the cavernous sinus is warranted.
Acknowledgments
Financial Support: This research did not receive any grant from any funding agency in the public, commercial, or nonprofit sector.
Glossary
Abbreviations
- CV
coefficient of variation
- DA
dopamine agonist
- GH
growth hormone
- GHRA
GH receptor antagonist
- IGF 1
insulin-like growth factor-1
- OGTT
oral glucose tolerance test
- OR
odds ratio
- RIA
radioimmunoassay
- SSA-1
first-generation somatostatin analog
- SSA-2
second-generation somatostatin analog
- TSS
transsphenoidal surgery
- ULN
upper limit of normal
Additional Information
Disclosures: The authors have nothing to declare.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ritvonen E, Löyttyniemi E, Jaatinen P, et al. Mortality in acromegaly: a 20-year follow-up study. Endocr Relat Cancer. 2016;23(6):469-480. [DOI] [PubMed] [Google Scholar]
- 3. Pivonello R, Auriemma RS, Grasso LF, et al. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20(1):46-62. [DOI] [PubMed] [Google Scholar]
- 4. Giustina A, Barkan A, Beckers A, et al. A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. 2020;105(4):e937-e946. [DOI] [PubMed] [Google Scholar]
- 5. Biermasz NR, Roelfsema F, Pereira AM, Romijn JA. Cost-effectiveness of lanreotide Autogel in treatment algorithms of acromegaly. Expert Rev Pharmacoecon Outcomes Res. 2009;9(3):223-234. [DOI] [PubMed] [Google Scholar]
- 6. Starnoni D, Daniel RT, Marino L, Pitteloud N, Levivier M, Messerer M. Surgical treatment of acromegaly according to the 2010 remission criteria: systematic review and meta-analysis. Acta Neurochir (Wien). 2016;158(11):2109-2121. [DOI] [PubMed] [Google Scholar]
- 7. Donegan DM, Iñiguez-Ariza N, Sharma A, et al. Necessity of multimodal treatment of acromegaly and outcomes. Endocr Pract. 2018;24(7):668-676. [DOI] [PubMed] [Google Scholar]
- 8. Babu H, Ortega A, Nuno M, et al. Long-term endocrine outcomes following endoscopic endonasal transsphenoidal surgery for acromegaly and associated prognostic factors. Neurosurgery. 2017;81(2):357-366. [DOI] [PubMed] [Google Scholar]
- 9. Agrawal N, Ioachimescu AG. Prognostic factors of biochemical remission after transsphenoidal surgery for acromegaly: a structured review. Pituitary. 2020;23(5):582-594. [DOI] [PubMed] [Google Scholar]
- 10. Coopmans EC, Postma MR, Wolters TLC, et al. Predictors for remission after transsphenoidal surgery in acromegaly: a Dutch multicenter study, revised. Figshare Repository. ProMED-mail website. Deposited January 19, 2021. 10.6084/m9.figshare.13607318.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micko AS, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015;122(4):803-811. [DOI] [PubMed] [Google Scholar]
- 12. Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85(2):526-529. [DOI] [PubMed] [Google Scholar]
- 13. Giustina A, Chanson P, Bronstein MD, et al. ; Acromegaly Consensus Group . A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95(7):3141-3148. [DOI] [PubMed] [Google Scholar]
- 14. Melmed S, Bronstein MD, Chanson P, et al. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012;15(1):71-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen CJ, Ironside N, Pomeraniec IJ, et al. Microsurgical versus endoscopic transsphenoidal resection for acromegaly: a systematic review of outcomes and complications. Acta Neurochir (Wien). 2017;159(11):2193-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunha MLVD, Borba LAB, Boguszewski CL. Random Gh and Igf-I levels after transsphenoidal surgery for acromegaly: relation with long-term remission. Endocrine. 2020;68(1):182-191. [DOI] [PubMed] [Google Scholar]
- 18. Ghajar A, Jones PS, Guarda FJ, et al. Biochemical control in acromegaly with multimodality therapies: outcomes from a pituitary center and changes over time. J Clin Endocrinol Metab. 2020;105(3):e532–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katznelson L, Laws ER Jr, Melmed S, et al. ; Endocrine Society . Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933-3951. [DOI] [PubMed] [Google Scholar]
- 20. van Beek AP, van den Bergh AC, van den Berg LM, et al. Radiotherapy is not associated with reduced quality of life and cognitive function in patients treated for nonfunctioning pituitary adenoma. Int J Radiat Oncol Biol Phys. 2007;68(4):986-991. [DOI] [PubMed] [Google Scholar]
- 21. Yang C, Li G, Jiang S, Bao X, Wang R. Preoperative somatostatin analogues in patients with newly-diagnosed acromegaly: a systematic review and meta-analysis of comparative studies. Sci Rep. 2019;9(1):14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abreu C, Guinto G, Mercado M. Surgical-pharmacological interactions in the treatment of acromegaly. Expert Rev Endocrinol Metab. 2019;14(1):35-42. [DOI] [PubMed] [Google Scholar]
- 23. Nunes VS, Correa JM, Puga ME, Silva EM, Boguszewski CL. Preoperative somatostatin analogues versus direct transsphenoidal surgery for newly-diagnosed acromegaly patients: a systematic review and meta-analysis using the GRADE system. Pituitary. 2015;18(4):500-508. [DOI] [PubMed] [Google Scholar]
- 24. Besser GM, Burman P, Daly AF. Predictors and rates of treatment-resistant tumor growth in acromegaly. Eur J Endocrinol. 2005;153(2):187-193. [DOI] [PubMed] [Google Scholar]
- 25. Petrossians P, Tichomirowa MA, Stevenaert A, Martin D, Daly AF, Beckers A. The Liege Acromegaly Survey (LAS): a new software tool for the study of acromegaly. Ann Endocrinol (Paris). 2012;73(3):190-201. [DOI] [PubMed] [Google Scholar]
- 26. Gadelha MR, Kasuki L, Korbonits M. The genetic background of acromegaly. Pituitary. 2017;20(1):10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nachtigall LB, Karavitaki N, Kiseljak-Vassiliades K, et al. Physicians’ awareness of gadolinium retention and MRI timing practices in the longitudinal management of pituitary tumors: a “Pituitary Society” survey. Pituitary. 2019;22(1):37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barkan AL, Beitins IZ, Kelch RP. Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab. 1988;67(1):69-73. [DOI] [PubMed] [Google Scholar]
- 29. Oldfield EH, Jane JA Jr, Thorner MO, Pledger CL, Sheehan JP, Vance ML. Correlation between GH and IGF-1 during treatment for acromegaly. J Neurosurg. 2017;126(6):1959-1966. [DOI] [PubMed] [Google Scholar]
- 30. Shahlaie K, McLaughlin N, Kassam AB, Kelly DF. The role of outcomes data for assessing the expertise of a pituitary surgeon. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):369-376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.