Abstract
Context
Psychiatric symptoms are common in Cushing’s disease (CD) and seem only partly reversible following treatment.
Objective
To investigate drug dispenses associated to psychiatric morbidity in CD patients before treatment and during long-term follow-up.
Design
Nationwide longitudinal register-based study.
Setting
University Hospitals in Sweden.
Subjects
CD patients diagnosed between 1990 and 2018 (N = 372) were identified in the Swedish Pituitary Register. Longitudinal data was collected from 5 years before, at diagnosis, and during follow-up. Four matched controls per patient were included. Cross-sectional subgroup analysis of 76 patients in sustained remission was also performed.
Main outcome measures
Data from the Swedish Prescribed Drug Register and the Patient Register.
Results
In the 5-year period before and at diagnosis, use of antidepressants (odds ratio [OR] 2.2 [95% confidence interval (CI) 1.3-3.7]) and 2.3 [1.6-3.5]), anxiolytics [2.9 (1.6-5.3) and 3.9 (2.3-6.6)], and sleeping pills [2.1 (1.2-3.7) and 3.8 (2.4-5.9)] was more common in CD than controls. ORs remained elevated at 5-year follow-up for antidepressants [2.4 (1.5-3.9)] and sleeping pills [3.1 (1.9-5.3)]. Proportions of CD patients using antidepressants (26%) and sleeping pills (22%) were unchanged at diagnosis and 5-year follow-up, whereas drugs for hypertension and diabetes decreased. Patients in sustained remission for median 9.3 years (interquartile range 8.1-10.4) had higher use of antidepressants [OR 2.0 (1.1-3.8)] and sleeping pills [2.4 (1.3-4.7)], but not of drugs for hypertension.
Conclusions
Increased use of psychotropic drugs in CD was observed before diagnosis and remained elevated regardless of remission status, suggesting persisting negative effects on mental health. The study highlights the importance of early diagnosis of CD, and the need for long-term monitoring of mental health.
Keywords: Cushing’s syndrome, hypercortisolism, neuropsychiatry, depression, sleeping disorder
Introduction
Cushing’s disease (CD), ie Cushing’s syndrome (CS) caused by adrenocorticotropin (ACTH)-producing pituitary adenomas, and other causes of CS, like cortisol producing adrenal tumors, are associated with significantly increased morbidity and mortality (1-4). Emotional disturbances were identified as a feature of CD in the very first description of the syndrome (5), and psychiatric symptoms have later been shown to be frequent (6-10). Major depression is present in 54% to 65% of patients with active CS with no significant difference between pituitary or adrenal causes (11-13). Other neuropsychiatric symptoms include anxiety, sleep disturbances, fatigue, cognitive impairment, and, less commonly, manic behavior (11-14). Suicidal thoughts have been described in 17% of patients with CS (7), and a recent study found suicide to contribute to the increased mortality in CD (4). Few prospective studies, mostly small, have described reversibility or partial improvement of neuropsychiatric symptoms after successful treatment (6-9,15). In other studies, impaired cognitive function and quality of life seemed to persist for a long time after biochemical remission had been achieved (16-19).
Here, we have examined a large cohort of CD patients with the aim to evaluate neuropsychiatric morbidity as reflected by dispenses of psychotropic drugs from 5 years before diagnosis, during the period of active disease, and during long-term follow-up. Dispenses of drugs commonly used for other comorbidities in CD, such as opioids, antihypertensive and antidiabetic medications were also examined, as well as visits to specialized psychiatry.
Methods
Study design
This was a register-based longitudinal study. All patients diagnosed with CD in Sweden, who have since 1991 been prospectively registered in the Swedish Pituitary Register (SPR), were included in the study.
By using a personal identification number, unique for all citizens in Sweden, a linkage to Statistics Sweden’s Total Population Register, the Swedish National Patient Register, and the Swedish Prescribed Drug Register was performed.
The study was approved by the Ethics Review board, Linköping, Sweden (approval No. 2017/60-31 and No. 2018/130-32). The Swedish Pituitary Registry is approved by the Ethics Review Board, Karolinska Institute, Stockholm, Sweden, (No. 2003/515/03 and No. 2012/915-32).
Participants
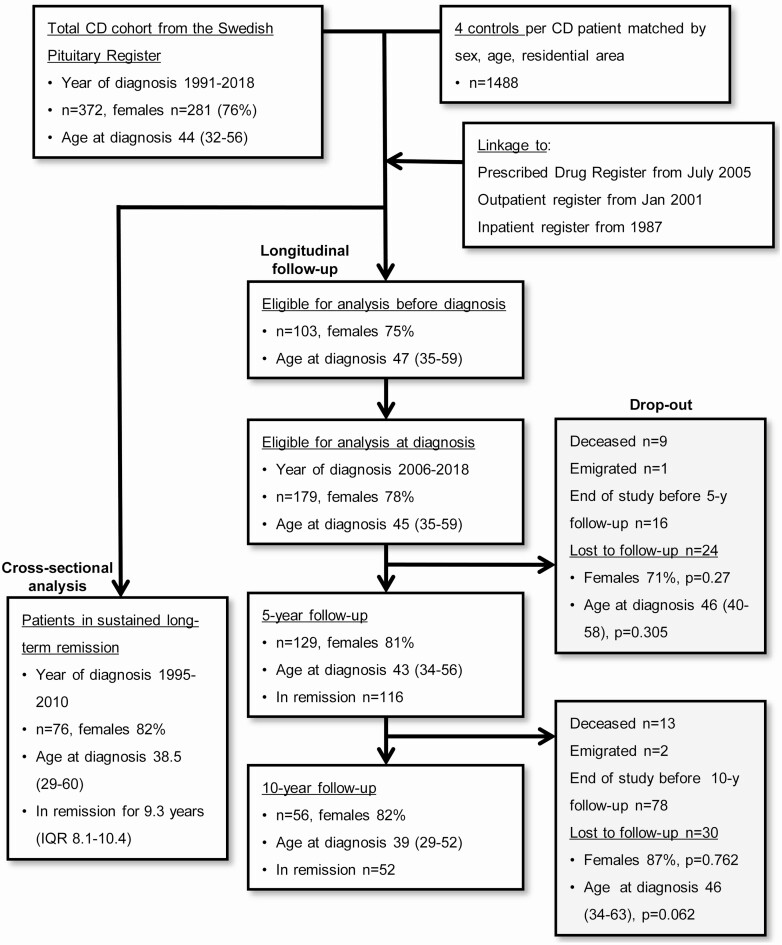
The SPR is a nationwide register based on the national Information Network for Cancer Treatment IT-platform and located at the Regional Cancer Center, Stockholm-Gotland, Sweden. CD patients are included upon diagnosis; the coverage is around 95% based on the comparison of annual incidences between the SPR, and a recent nationwide Swedish study that found an annual incidence of 1.6 cases per million in Sweden between 1987 and 1995 (20). Data collected include date of diagnosis, radiological description of the tumor size, information about treatment (surgery, radiotherapy, pharmacological), and evaluation of biochemical remission. Besides at diagnosis, data are entered at 1, 5, 10, 15, and 20 years of follow-up and when surgery or radiotherapy is performed. Since the dates of clinical follow-up do not occur at exactly the same intervals in every patient, the following intervals are used in the register: 5-year follow-up, 2.5 to 7.5 years, and 10-year follow-up, 7.5 to 12.5 years. The end of follow-up was either set as date of death, date of emigration, or October 31, 2018 (last available data on drug dispenses). Since the registers used to assess psychiatric morbidity (see following discussion) were not started the same year as the SPR, the study population was adapted depending on type of analysis (Fig. 1).
Figure 1.
Outline of the study and eligible patients for each analysis. Age is median (IQR) at diagnosis.
For each patient with CD, 4 controls matched for sex, age, and residential area, obtained from the Statistics Sweden’s Total Population Register, were included. Matching was performed on the date of diagnosis of CD. The controls were given “dummy dates” corresponding to the respective case to enable comparisons at each time point of follow-up. The Total Population Register was also used to identify any migration of the participants. At follow-up, a control corresponding to a deceased or emigrated CD case was removed.
CD diagnosis and evaluation of remission status
CD diagnosis were made according to local routines at each university hospital and included typical signs/symptoms and the conventional biochemical tests of CD (21). The treating physician evaluated disease activity at each clinical visit, and remission status was noted in the SPR. Biochemical remission was defined as normal midnight salivary cortisol levels, and/or s-cortisol < 50 nmol/L at an overnight dexamethasone suppression test, and/or 24-h urinary free cortisol levels (UFC) below the upper reference limit, or hypocortisolism after pituitary surgery or radiotherapy, or bilateral adrenalectomy. Patients who achieved normalization of urinary free cortisol levels following medical treatment with steroid inhibitors or pituitary tumor-directed therapy were not considered to be in remission.
To evaluate CD patients in long-term remission, we applied an algorithm based on the following criteria: being in remission at all consecutive follow-up and not subjected to surgery or radiotherapy in between the follow-up visits. Time in remission was calculated as the time elapsed from last pituitary surgery or radiotherapy to date of follow-up.
Registers and methods used to assess psychiatric morbidity
Swedish Prescribed Drug Register
The Swedish Prescribed Drug Register (PDR) was initiated July 1, 2005 and has full coverage of all prescribed drug dispenses in Sweden, including prescriptions made in the primary healthcare system (22,23). Date of the prescription, date of dispense, classification of the drug according to the Anatomic Therapeutic Chemical classification system and type of care unit prescribing the drug are recorded. Information on drugs associated with neuropsychiatric morbidity [ie, antidepressants (N06A), anxiolytics (N05B), and hypnotics and sedatives (in the paper referred to as sleeping pills) (N05C)] was retrieved from 5 years before CD diagnosis until October 31, 2018 in each patient. Also, dispenses of drugs used for hormone replacement were obtained. For comparison, prespecified drugs used for common somatic comorbidities were collected; antidiabetics including insulin (A10), calcium-channel inhibitors (C08), RAS-blockers (C09), and opioids (N02A). The PDR was sought for dispenses from 1 year before to 1 year after the dates of follow-up visits in the pituitary register. Two or more dispenses of a drug were considered as evidence of drug use. In the 5- year period prior to date of diagnosis, 2 or more dispenses of each drug in that period were chosen to compare CD patients with their controls.
Swedish National Patient Register
The Swedish National Patient Register collects information on diagnostic codes provided at every hospital admission, as well as outpatient visits to hospitals in Sweden. The inpatient register has from 1987 almost complete coverage (99%). Since 2001, outpatient visits are also recorded, including day care surgery and psychiatric care from both private and public caregivers (24,25). Date of visit/admission, type of care provider, and diagnostic codes according to the International Statistical Classification of Diseases are recorded. In the present study, visits to specialized psychiatry, and International Statistical Classification of Diseases codes consistent with mood/affective disorders and schizophrenia/delusional disorders were sought for.
Statistics
Quantitative data are described in terms of median and interquartile range (IQR), and nonparametric test (Mann-Whitney U-test) was used to compare continuous variables. Proportions were compared with Fisher’s 2-sided exact test. Changes of proportions within a group (ie, at diagnosis and 5-year follow-up) were also analyzed with the related-samples McNemar change test. Univariable logistic regression was performed to calculate odds ratios (OR) for each drug/diagnostic code/visit between groups (CD vs control), age at diagnosis, and sex. Results were presented as OR with corresponding 95% confidence interval (CI). Kaplan-Meier survival analysis was used to illustrate the cumulative incidence of first-time dispense of a drug from study start (ie, 5 years before diagnosis). Calculations were performed with IBM SPSS Statistics, version 26.0.0. P-values < 0.05 were considered as significant.
Results
Study cohort
The total cohort of CD patients consisted of 372 subjects diagnosed between 1991 and 2018, 281 (76%) were females. Median age at diagnosis was 44 (IQR 35-56) years.
Eligible for analysis at diagnosis were 179 patients (139 females) diagnosed from July 1, 2006 and onward (1 year after start of PDR). Median age at diagnosis was 45 (IQR 35-59) years, median follow-up after time of diagnosis was 7.0 (IQR 4.0-9.8) years. Pituitary surgery was performed in 141 (79%) patients, repeat pituitary surgery in 25 (14%), and radiotherapy in 16 (9%) patients. Information on tumor size at diagnosis was available in 157 patients; 116 (74%) had microadenomas (largest diameter < 10 mm or not visible), and 41 (26%) had macroadenomas (diameter ≥ 10 mm). The patients were followed longitudinally; at 5 and 10 years, 129 and 56 patients, respectively, had a follow-up in the SPR.
For analyses of drug use before diagnosis, patients diagnosed from July 1, 2010 and onward (5 years after start of PDR) were included, n = 103 (Fig. 1).
A cross-sectional analysis included all patients who fulfilled the criteria of sustained remission and had a valid 10-year follow-up from 1 year after start of the PDR (n = 76). These patients were diagnosed between 1995 and 2010 and median time in remission was 9.3 years (IQR 8.1-10.4) (Fig. 1). Seventy-three (96%) had undergone pituitary surgery, 20% had repeat pituitary surgery, and 7% radiotherapy. Tumor size at diagnosis was available in 65 patients; 78% had microadenomas, and 22% had macroadenomas.
Drug dispenses and visits to specialized psychiatry before diagnosis of CD
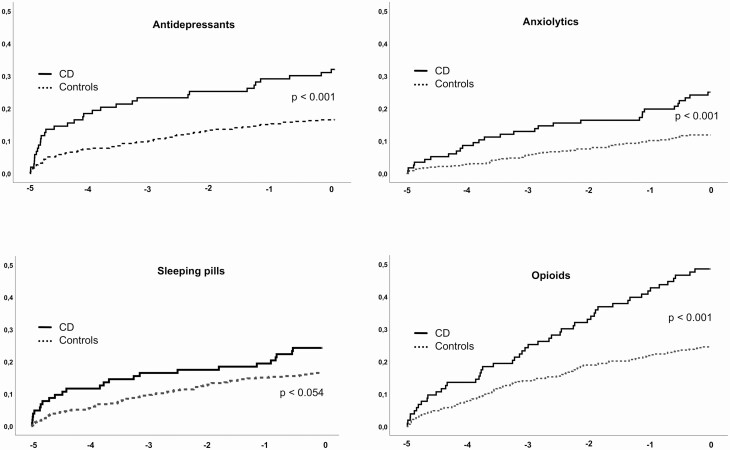
During the 5-year period before diagnosis, use of antidepressants was more common in CD patients (28%) compared to controls (15%). Patients with CD also had higher use of anxiolytics, sleeping pills, opioids, RAS-blockers, Ca-inhibitors, and antidiabetics (Table 1). Figure 2 illustrates the cumulative proportion of CD patients and controls with at least 1 dispense of antidepressants, anxiolytics, sleeping pills, and opioids from the start of observation (5 years before diagnosis) to time of diagnosis.
Table 1.
Drug dispenses during the 5 years prior to diagnosis of CD
| CD, n (%) | Controls, n (%) | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Total n | 103 | 412 | ||
| Antidepressants | 29 (28) | 62 (15) | 2.2 (1.3-3.7) | 0.002 |
| Anxiolytics | 22 (21) | 35 (8.5) | 2.9 (1.6-5.3) | <0.001 |
| Sleeping pills | 21 (20) | 45 (11) | 2.1 (1.2-3.7) | 0.011 |
| ≥1 psychotropic drug | 43 (42) | 94 (23) | 2.4 (1.5-3.8) | <0.001 |
| ≥2 psychotropic drugs | 19 (18) | 37 (9) | 2.3 (1.3-4.2) | 0.007 |
| Opioids | 42 (41) | 55 (13) | 4.5 (2.8-7.3) | <0.001 |
| Ca-inhibitors | 37 (36) | 25 (6.1) | 8.7 (4.9-15.4) | <0.001 |
| RAS-blockade | 57 (55) | 48 (12) | 9.4 (5.8-15.4) | <0.001 |
| Both Ca-inhibitors and RAS-blockade | 33 (32) | 18 (4) | 10.3 (5.5-19.3) | <0.001 |
| Antidiabetics including insulin | 20 (19) | 18 (4.4) | 5.3 (2.7-10.4) | <0.001 |
Psychotropic drugs refer to antidepressants, anxiolytics, and sleeping pills in combination. Drug dispense defined as ≥2 dispenses during the 5-year period. OR, univariable logistic regression (CD vs control) for each drug with corresponding 95% CI and P-values.
Figure 2.
Cumulative proportion of subjects (CD n = 103, controls n = 412) with first drug dispense from start of observation (5 years before) to date of diagnosis. x-axis: years before diagnosis; y-axis: proportion of patients. P-values refer to log rank (Mantel-Cox).
There was no difference in outpatient visits to specialized psychiatry between CD and controls (8 vs 4%, ns) in this cohort. However, in an analysis including all patients diagnosed 5 years after the initiation of the outpatient register [ie, after January 1, 2006; n = 189; females n = 147, median age at diagnosis 44 years (IQR 35-59)], visits to a psychiatrist 5 years prior to diagnosis were more common in CD patients (10%) compared to controls (5%), OR 2.0 (95% CI 1.1-3.4, P = 0.022).
The inpatient register has good coverage from 1987, and all patients were included in this analysis. Of 372 patients with CD, 4 (1.1%) had been admitted to hospital for treatment of psychotic disorders, and 3 (0.8 %) for depression. In comparison, 3 (0.2%) and 9 (0.6%) of 1488 (P = 0.033 and ns, respectively) of the controls had been admitted for the same reasons.
Drug dispenses at diagnosis and 5- and 10-year follow-up
Drug dispenses in CD patients
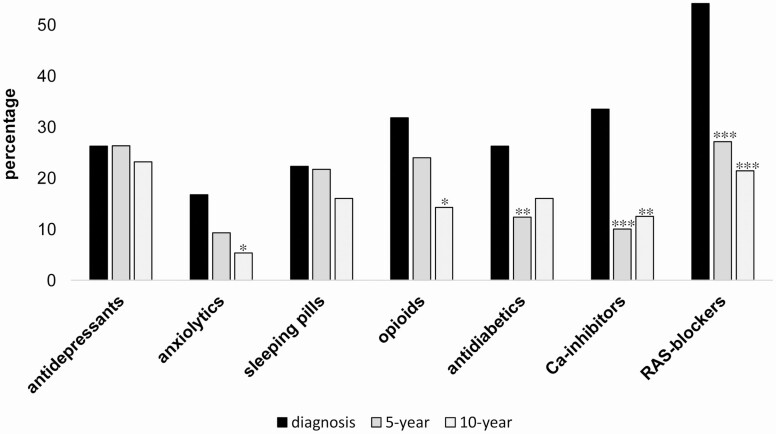
The use of antidepressants remained the same at the 3 time points: 26% at diagnosis, 26% at 5 years, and 23% at 10 years. There were no changes of dispenses for sleeping pills, anxiolytics, and opioids at diagnosis and at 5-year follow-up, whereas the use of RAS-blockers, Ca-inhibitors, and antidiabetics decreased (2-sided Fisher’s exact test, Fig. 3; Table 2). The same results were found when the McNemar change test was employed to compare the paired samples of CD patients included at both diagnosis and 5-year follow-up, with the exception of anxiolytics, which decreased (P = 0.049).
Figure 3.
Proportions of CD patients with drug dispenses at diagnosis and 5- and 10-year follow-up. Difference of proportions were tested with Fisher exact 2-sided test at 2 levels: diagnosis vs 5-year and diagnosis vs 10-year, respectively. Only significant changes are indicated: *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
Table 2.
Drug dispenses at diagnosis, and 5- and 10-year follow-up
| CD all n (%) | Controls n (%) | OR (95%CI) | P | CD in remission n (%) | Controls n (%) | OR (95%CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| At diagnosis | ||||||||
| Total n | 179 | 716 | ||||||
| Antidepressants | 47 (26) | 95 (13) | 2.3 (1.6-3.5) | <0.001 | ||||
| Anxiolytics | 30 (17) | 35 (4.9) | 3.9 (2.3-6.6) | <0.001 | ||||
| Sleeping pills | 40 (22) | 51 (7.1) | 3.8 (2.4-5.9) | <0.001 | ||||
| ≥1 psychotropic drug | 74 (41) | 130 (18) | 3.2 (2.2-4.5) | <0.001 | ||||
| ≥2 psychotropic drugs | 29 (16) | 40 (6) | 3.3 (2.0-5.4) | <0.001 | ||||
| Opioids | 57 (32) | 49 (6.8) | 6.4 (4.1-9.8) | <0.001 | ||||
| Ca-inhibitors | 60 (34) | 40 (5.6) | 8.5 (5.5-13.3) | <0.001 | ||||
| RAS-blockade | 97 (54) | 72 (10) | 10.6 (7.2-15.5) | <0.001 | ||||
| Both Ca-inhibitors and RAS-blockade | 47 (26) | 22 (3) | 11.2 (6.6-19.3) | <0.001 | ||||
| Antidiabetics including insulin | 47 (26) | 30 (4.2) | 8.1 (5.0-13.4) | <0.001 | ||||
| 5-year follow-up | ||||||||
| Total n | 129 | 503 | 110 | 429 | ||||
| Antidepressants | 34 (26) | 65 (13) | 2.4 (1.5-3.9) | <0.001 | 28 (26) | 53 (12) | 2.4 (1.5-4.1) | 0.001 |
| Anxiolytics | 12 (9.3) | 31 (6.2) | 1.6 (0.8-3.1) | 0.21 | 10 (9.1) | 27 (6.3) | 1.5 (0.7-3.2) | 0.303 |
| Sleeping pills | 28 (22) | 41 (8.2) | 3.1 (1.9-5.3) | <0.001 | 22 (20) | 37 (8.6) | 2.3 (1.5-4.7) | 0.001 |
| ≥ 1 psychotropic drug | 44 (34) | 91 (18) | 2.3 (1.5-3.6) | <0.001 | 35 (32) | 76 (18) | 2.2 (1.4-3.5) | 0.001 |
| ≥ 2 psychotropic drugs | 23 (18) | 36 (7) | 2.8 (1.6-5.0) | <0.001 | 19 (17) | 31 (7) | 2.7 (1.5-5.0) | 0.002 |
| Opioids | 31 (24) | 41 (8.2) | 3.6 (2.1-6.0) | <0.001 | 25 (23) | 31 (7.2) | 3.8 (2.1-6.7) | <0.001 |
| Ca-inhibitors | 13 (10) | 35 (7.0) | 1.5 (0.8-2.9) | 0.236 | 6 (5.5) | 28 (6.5) | 0.8 (0.3-2.1) | 0.68 |
| RAS-blockade | 35 (27) | 80 (16) | 2.0 (1.3-3.1) | 0.004 | 27 (25) | 65 (15) | 1.8 (1.1-3.0) | 0.021 |
| Both Ca-inhibitors and RAS-blockade | 7 (5) | 21 (4) | 1.3 (0.5-3.2) | 0.539 | 3 (3) | 18 (4) | 0.6 (0.2-2.2) | 0.481 |
| Antidiabetics including insulin | 16 (12) | 22 (4.4) | 3.1 (1.6-6.1) | 0.001 | 14 (13) | 18 (4.2) | 3.3 (1.6-6.9) | 0.001 |
| 10-year follow-up | ||||||||
| Total n | 56 | 214 | 52 | 198 | ||||
| Antidepressants | 13 (23) | 32 (15) | 1.7 (0.8-3.6) | 0.143 | 12 (23) | 30 (15) | 1.7 (0.8-3.6) | 0.177 |
| Anxiolytics | 3 (5.4) | 12 (5.6) | 1.0 (0.3-3.5) | 0.942 | 3 (5.8) | 11 (5.6) | 1.0 (0.3-3.9) | 0.952 |
| Sleeping pills | 9 (16) | 22 (10) | 1.7 (0.7-3.9) | 0.23 | 9 (17) | 20 (10) | 1.9 (0.8-4.4) | 0.154 |
| ≥1 psychotropic drug | 17 (30) | 44 (21) | 1.7 (0.9-3.3) | 0.121 | 16 (31) | 40 (20) | 1.8 (0.9-3.5) | 0.107 |
| ≥2 psychotropic drugs | 7 (13) | 19 (9) | 1.5 (0.6-3.7) | 0.416 | 7 (14) | 18 (9) | 1.6 (0.6-4.0) | 0.353 |
| Opioids | 8 (14) | 20 (9.3) | 1.6 (0.7-3.9) | 0.284 | 8 (15) | 18 (9.1) | 1.8 (0.7-4.5) | 0.191 |
| Ca-inhibitors | 7 (13) | 12 (5.6) | 2.4 (0.9-6.4) | 0.08 | 6 (12) | 12 (6.1) | 2.0 (0.7-5.7) | 0.181 |
| RAS-blockade | 12 (21) | 32 (15) | 1.6 (0.7-3.3) | 0.245 | 9 (17) | 30 (15) | 1.2 (0.5-2.7) | 0.703 |
| Both Ca-inhibitors and RAS-blockade | 4 (7) | 10 (5) | 1.6 (0.5-5.2) | 0.461 | 3 (6) | 10 (5) | 1.2 (0.3-4.3) | 0.836 |
| Antidiabetics including insulin | 9 (16) | 4 (1.9) | 10.1 (3.0-34.0) | <0.001 | 8 (15) | 4 (2.0) | 8.8 (2.5-30.6) | 0.001 |
Psychotropic drugs refer to antidepressants, anxiolytics, and sleeping pills in combination. Drug dispense defined as ≥ 2 dispenses ± 1 year in relation to each date of follow-up. OR, univariable logistic regression (CD vs control) for each drug with corresponding 95% CI and P-values.
At 5-year follow-up, 34/129 (26%) CD patients were on antidepressants. Eighteen of these (15 in remission) had been on treatment since diagnosis whereas 16 (13 in remission) had started treatment at median 2.4 (minimum-maximum 1.1-4.5) years after diagnosis. Nine out of 27 patients who were on antidepressants at diagnosis were able to discontinue treatment before the 5-year follow-up (2 were not in remission).
Influence of remission status
Biochemical remission at 5-year follow-up was achieved in 110/129 (85%), not achieved in 15 (12%), and remission status was unknown in 4 cases. CD patients not in remission, compared to those in remission, showed no differences in use of antidepressants, anxiolytics, sleeping pills, opioids, RAS-blockers, and antidiabetics, while use of Ca-inhibitors was higher in patients not in remission (27 vs 6%, P = 0.019). At 10-year follow-up 52/56 (93%) were in biochemical remission, 3 (5%) were not in remission, and in 1 patient remission status was unknown.
Influence of age and sex in CD patients
At 5-year follow-up, but not at diagnosis, older age correlated to use of antidepressants (OR 1.03; 95% CI 1.01-1.06, P = 0.02), but not to anxiolytics or sleeping pills.
At 5-year follow-up, but not at diagnosis, women had higher use of antidepressants compared to men (32/105 vs 2/24, P = 0.038).
Drop-out analysis
There were no significant differences in age or sex in CD patients who were lost to follow-up at 5 and 10 years after diagnosis (Fig. 1). Out of 24 CD patients who were lost at 5-year follow-up, 10 (42%) used antidepressants at diagnosis, compared to 27/129 (25%) of those who completed a 5-year follow-up (P = 0.039).
Comparison between CD and controls
When compared to controls, CD patients had a higher use of antidepressants, anxiolytics, and sleeping pills at diagnosis. At 5-year follow-up, the use of antidepressants and sleeping pills remained higher among patients, whereas at 10-year follow-up, the use of these drugs was not different from the control group (Table 2).
CD patients in remission also had higher use of antidepressants and sleeping pills at 5-year follow-up compared to controls, but not at 10-year follow-up (Table 2).
Cross-sectional analysis of patients in sustained long-term remission
Seventy-six patients in remission for median 9.3 (IQR 8.1-10.4) years were analyzed. In comparison to controls, CD patients had a higher use of antidepressants (25 vs 14%) and sleeping pills (22 vs 11%). Use of antidiabetics was significantly increased but use of anxiolytics, opioids, RAS-blockers, and Ca-inhibitors was not (Table 3).
Table 3.
Drug dispenses in CD patients in sustained long-term remission for median 9.3 years
| CD n (%) | Controls n (%) | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Total n | 76 | 292 | ||
| Antidepressants | 19 (25) | 41 (14) | 2.0 (1.1-3.8) | 0.023 |
| Anxiolytics | 9 (12) | 18 (6) | 2.1 (0.9-4.8) | 0.097 |
| Sleeping pills | 17 (22) | 31 (11) | 2.4 (1.3-4.7) | 0.008 |
| ≥1 psychotropic drug | 27 (36) | 60 (21) | 2.1 (1.2-3.7) | 0.007 |
| ≥2 psychotropic drugs | 15 (20) | 23 (8) | 2.9 (1.4-5.8) | 0.003 |
| Opioids | 9 (12) | 29 (10) | 1.2 (0.6-2.7) | 0.626 |
| Ca-inhibitors | 7 (9) | 24 (8) | 1.1 (0.5-2.7) | 0.782 |
| RAS-blockade | 15 (20) | 40 (14) | 1.6 (0.8-3.0) | 0.191 |
| Both Ca-inhibitors and RAS-blockade | 7 (9) | 14 (5) | 2.0 (0.8-5.2) | 0.146 |
| Antidiabetics including insulin | 11 (15) | 11 (4) | 4.3 (1.8-10.4) | 0.001 |
Psychotropic drugs refer to antidepressants, anxiolytics and sleeping pills in combination. Drug dispense defined as ≥ 2 dispenses ± 1 year in relation to date of follow-up. OR, univariable logistic regression (CD vs control) for each drug with corresponding 95% CI and P-values.
Replacement therapy for pituitary hormones in patients with sustained long-term remission
At the follow-up of patients in sustained long-term remission 29/76 (38%) were on cortisol replacement therapy, 26 (34%) on thyroid hormone replacement, 8 (11%) on growth hormone replacement, and 4 (5%) were taking desmopressin. Six of 14 men (43%) had dispenses of androgens. Of patients with cortisol replacement therapy, 9/29 (31%) vs 10/47 (21%) without cortisol replacement used antidepressants (ns), and 10/29 (35%) vs 7/47 (15%) used sleeping pills (ns). Replacement with thyroid hormones, growth hormone, and androgens did not correlate to use of psychotropic drugs.
Discussion
Glucocorticoid excess has a major impact on the human brain with accompanying neuropsychiatric consequences (26). In patients with CD, the mental consequences of hypercortisolism have not been fully addressed, and the reversibility after cure is debated. Prior longitudinal studies may have underestimated the true burden of the neuropsychiatric consequences after remission (8,9,15). We hypothesized that most CD patients with depression, insomnia, and anxiety would primarily consult with their general practitioner with a resulting psychiatric diagnosis only in the severe cases. In the present longitudinal study, we investigated psychiatric morbidity, as mirrored in the use of psychotropic drugs, in a large cohort of CD patients. The results showed a doubled or higher rate of dispenses of antidepressants, anxiolytics, and sleeping pills before diagnosis and in active disease, compared to matched controls. Regardless of remission status dispenses of antidepressants in CD patients remained at nearly the same rate, of about 25%, during the study. In accordance with previous prospective studies (7-9,15,27), some patients seemed to improve; however, at 5-year follow-up, 66% of the patients who were on treatment with antidepressant drugs at diagnosis had continued therapy, and 16 of 129 (12%) had started antidepressant therapy after diagnosis. In contrast to psychotropic drugs, dispenses of drugs for hypertension and diabetes dropped markedly from diagnosis to the 5-year follow-up. At the 10-year follow-up, use of antidepressants in CD patients was unchanged compared to at diagnosis and 5 years thereafter and was not significantly higher than in controls. However, in the cross-sectional analysis of the larger group of patients with sustained remission, use of antidepressants was higher in CD patients [OR 2.0 (95% CI 1.1-3.8)]. The larger number of patients in the cross-sectional analysis most likely explains the difference in significance levels; the earlier years of diagnosis may possibly contribute. Hence, our results underscore that although somatic comorbidities improve after remission, many patients with CD will have persistent mental health problems. The present findings support 2 previous cross-sectional studies in which increased prevalence of psychopathology and cognitive impairment was found after remission for 11 (range 1-32), and 13 (range 5-19) years, respectively (16,18). The underlying mechanisms of incomplete recovery are not fully understood. The brain is rich in glucocorticoid and mineralocorticoid receptors, both activated by cortisol (28), and neurotoxic effects of chronic hypercortisolism have been demonstrated in animal models (29). Brain imaging studies in patients with hypercortisolism suggest persisting effects following treatment (30,31).
Little is known about quality of sleep after long-term remission of CD. Starkman et al reported middle insomnia (awakening at least once during the night) in 24/35 patients with active Cushing’s syndrome of which 15/24 (62%) improved following treatment (7,27). In the present study, the use of sleeping pills was at the same rate (22%) at diagnosis, at 5-year follow-up, and in patients with sustained long-term remission.
Use of opioids was a secondary outcome variable. In 2 other studies, CD patients have reported more bodily pain (17,19). Osteoporotic vertebral and rib fractures are common in CD (2), which could contribute the higher use of opioids in the present cohort. Our data indicate that severe pain may be a substantial problem in CD and persist despite many years of biochemical remission. Yet this topic is seldom addressed in treatment guidelines (32).
Higher urinary cortisol levels at diagnosis, older age, and female sex have been associated to depression in CD patients (12). At 5-year follow-up, but not at diagnosis, older age and female sex predicted use of antidepressants in CD patients in the present study. Also, delay of diagnosis has been correlated to depression in patients with CS in remission (6,33). In a recent meta-analysis the mean time from first symptom to diagnosis in CD was 38 months (34). Our data suggest that neuropsychiatric symptoms start several years before the diagnosis is established indicating a longer time of unrecognized disease. Most patients consult several specialists prior to a correct diagnosis (35,36). Psychiatric symptoms might be the first sign of CD (15), but patients without obvious signs of glucocorticoid excess may not undergo evaluation for CD, and undiagnosed hypercortisolism is occasionally misinterpreted as major psychiatric disease, such as psychosis and severe depression (37). In the present study relatively few patients, 7 of 372 (1.9%), were admitted for in-ward psychiatric treatment before diagnosis of CD, and 10% had been in contact with the outpatient psychiatry. Thus, most psychotropic drugs had not been prescribed by psychiatrists.
The study has some limitations. For some analyses, especially the comparisons between the sexes, patients in remission and not in remission, and the influence of pituitary hormone replacement, the number of patients with dispensed drugs was small in either of the groups, and firm conclusions were not feasible. The time period during which CD patients might have been hypocortisolemic and the potential influence of adrenal insufficiency on the use of psychotropic drugs could not be evaluated in the study. Frequencies of psychotropic drug dispenses may not be equivalent to the prevalence of overt psychiatric disorders. However, in our opinion, dispenses of psychotropic drugs can be useful as an indicator of mental health and offer a more complete picture of such problems. A strength in regards of morbidity before diagnosis is that the study design diminished the risk of recall bias, a common problem in retrospective studies using interviews or inquiries.
In conclusion, this nationwide register-based study shows that use of psychotropic drugs in CD patients increases from several years before diagnosis. Regardless of remission status, psychotropic drug use remains elevated suggesting persisting negative effects on mental health. Efforts to achieve earlier diagnosis of CD is mandatory and should include an increased attention to neuropsychiatric symptoms as an important part of the disease. The study also highlights the need for long-term monitoring of mental health in CD patients.
Acknowledgments
We thank the Regional Cancer Center, Sweden (RCC) for providing data from the Swedish Pituitary Register (SPR). We are grateful for statistical help from Jacob Järås at RCC. We also want to acknowledge all colleagues and coordinating nurses working with the SPR. Finally, special thanks to all CD patients participating in the SPR.
Financial Support: DB received a grant from the Medical Research Council of Southeast Sweden. OR’s work in this study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-771841 and 936463). PB received a grant from Skåne University Hospital.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in references.
References
- 1. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913-927. [DOI] [PubMed] [Google Scholar]
- 2. Sharma ST, Nieman LK, Feelders RA. Comorbidities in Cushing’s disease. Pituitary. 2015;18(2):188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611-629. [DOI] [PubMed] [Google Scholar]
- 4. Ragnarsson O, Olsson DS, Papakokkinou E, et al. Overall and disease-specific mortality in patients with Cushing disease: A Swedish Nationwide Study. J Clin Endocrinol Metab. 2019;104(6):2375-2384. [DOI] [PubMed] [Google Scholar]
- 5. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp. 1932;50:137-195. [Google Scholar]
- 6. Jeffcoate WJ, Silverstone JT, Edwards CR, Besser GM. Psychiatric manifestations of Cushing’s syndrome: response to lowering of plasma cortisol. Q J Med. 1979;48(191):465-472. [PubMed] [Google Scholar]
- 7. Starkman MN, Schteingart DE, Schork MA. Depressed mood and other psychiatric manifestations of Cushing’s syndrome: relationship to hormone levels. Psychosom Med. 1981;43(1):3-18. [DOI] [PubMed] [Google Scholar]
- 8. Sonino N, Fava GA, Belluardo P, Girelli ME, Boscaro M. Course of depression in Cushing’s syndrome: response to treatment and comparison with Graves’ disease. Horm Res. 1993;39(5-6):202-206. [DOI] [PubMed] [Google Scholar]
- 9. Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919. [DOI] [PubMed] [Google Scholar]
- 10. Sonino N, Fava GA, Raffi AR, Boscaro M, Fallo F. Clinical correlates of major depression in Cushing’s disease. Psychopathology. 1998;31(6):302-306. [DOI] [PubMed] [Google Scholar]
- 11. Pivonello R, Simeoli C, De Martino MC, et al. Neuropsychiatric disorders in Cushing’s syndrome. Front Neurosci. 2015;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos A, Resmini E, Pascual JC, Crespo I, Webb SM. Psychiatric symptoms in patients with Cushing’s Syndrome: prevalence, diagnosis and management. Drugs. 2017;77(8):829-842. [DOI] [PubMed] [Google Scholar]
- 13. Sonino N, Fava GA. Psychiatric disorders associated with Cushing’s syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15(5):361-373. [DOI] [PubMed] [Google Scholar]
- 14. Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):65-70. [DOI] [PubMed] [Google Scholar]
- 15. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol (Oxf). 1996;45(6):715-720. [DOI] [PubMed] [Google Scholar]
- 16. Tiemensma J, Biermasz NR, Middelkoop HA, van der Mast RC, Romijn JA, Pereira AM. Increased prevalence of psychopathology and maladaptive personality traits after long-term cure of Cushing’s disease. J Clin Endocrinol Metab. 2010;95(10):E129-E141. [DOI] [PubMed] [Google Scholar]
- 17. Wagenmakers MA, Netea-Maier RT, Prins JB, Dekkers T, den Heijer M, Hermus AR. Impaired quality of life in patients in long-term remission of Cushing’s syndrome of both adrenal and pituitary origin: a remaining effect of long-standing hypercortisolism? Eur J Endocrinol. 2012;167(5):687-695. [DOI] [PubMed] [Google Scholar]
- 18. Ragnarsson O, Berglund P, Eder DN, Johannsson G. Long-term cognitive impairments and attentional deficits in patients with Cushing’s disease and cortisol-producing adrenal adenoma in remission. J Clin Endocrinol Metab. 2012;97(9):E1640-E1648. [DOI] [PubMed] [Google Scholar]
- 19. Valassi E, Feelders R, Maiter D, et al. ; ERCUSYN Study Group . Worse Health-Related Quality of Life at long-term follow-up in patients with Cushing’s disease than patients with cortisol producing adenoma. Data from the ERCUSYN. Clin Endocrinol (Oxf). 2018;88(6):787-798. [DOI] [PubMed] [Google Scholar]
- 20. Ragnarsson O, Olsson DS, Chantzichristos D, et al. The incidence of Cushing’s disease: a nationwide Swedish study. Pituitary. 2019;22(2):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swedish Prescribed Drug Registry. Sweden National Board of Health and Welfare; 2019. [Google Scholar]
- 23. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register: opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726-735. [DOI] [PubMed] [Google Scholar]
- 24. Swedish National Patient Registry. Sweden National Board of Health and Welfare; 2019. [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piasecka M, Papakokkinou E, Valassi E, et al. Psychiatric and neurocognitive consequences of endogenous hypercortisolism. J Intern Med. 2020;288(2):168-182. [DOI] [PubMed] [Google Scholar]
- 27. Starkman MN. Neuropsychiatric findings in Cushing syndrome and exogenous glucocorticoid administration. Endocrinol Metab Clin North Am. 2013;42(3):477-488. [DOI] [PubMed] [Google Scholar]
- 28. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463-475. [DOI] [PubMed] [Google Scholar]
- 29. Tata DA, Anderson BJ. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav. 2010;99(2):186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andela CD, van der Werff SJ, Pannekoek JN, et al. Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing’s disease: a case-control study. Eur J Endocrinol. 2013;169(6):811-819. [DOI] [PubMed] [Google Scholar]
- 31. van der Werff SJ, Andela CD, Nienke Pannekoek J, et al. Widespread reductions of white matter integrity in patients with long-term remission of Cushing’s disease. Neuroimage Clin. 2014;4:659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valassi E, Crespo I, Keevil BG, et al. Affective alterations in patients with Cushing’s syndrome in remission are associated with decreased BDNF and cortisone levels. Eur J Endocrinol. 2017;176(2):221-231. [DOI] [PubMed] [Google Scholar]
- 34. Rubinstein G, Osswald A, Hoster E, et al. Time to diagnosis in Cushing’s syndrome: a meta-analysis based on 5367 patients. J Clin Endocrinol Metab. 2020;105(3):dgz13. [DOI] [PubMed] [Google Scholar]
- 35. Kreitschmann-Andermahr I, Psaras T, Tsiogka M, et al. From first symptoms to final diagnosis of Cushing’s disease: experiences of 176 patients. Eur J Endocrinol. 2015;172(3):285-289. [DOI] [PubMed] [Google Scholar]
- 36. Valassi E, Santos A, Yaneva M, et al. ; ERCUSYN Study Group . The European Registry on Cushing’s syndrome: 2-year experience. baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165(3):383-392. [DOI] [PubMed] [Google Scholar]
- 37. Rasmussen SA, Rosebush PI, Smyth HS, Mazurek MF. Cushing disease presenting as primary psychiatric illness: a case report and literature review. J Psychiatr Pract. 2015;21(6):449-457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in references.