Abstract
Introduction
Over 35% of all adults in the world are currently obese and risk of obesity in racial or ethnic minority groups exist in the US, but the causes of these differences are not all known. As obesity is a leading cause of cardiovascular disease, an improved understanding of risk factors across racial and ethnic groups may improve outcomes.
Objective
The objective of this study was to determine if susceptibility to obesity is associated with genetic variation in candidate single nucleotide polymorphisms (SNPs) in African Americans and Hispanic/Latinos.
Materials and methods
We examined data from 534 African Americans and 557 Hispanic/Latinos participants from the UIC Cohort of Patients, Family and Friends. Participants were genotyped for the top 26 obesity-associated SNPs within FTO, MC4R, TUB, APOA2, APOA5, ADIPOQ, ARL15, CDH13, KNG1, LEPR, leptin, and SCG3 genes.
Results
The mean (SD) age of participants was 49±13 years, 55% were female, and mean body mass index (BMI) was 31±7.5 kg/m2. After adjusting for age and sex, we found that rs8050136 in FTO (odds ratio [OR] 1.40, 95% confidence interval [CI] 1.1–1.8; P = 0.01) among African Americans and rs2272383 in TUB (OR 1.34, 95% CI 1.04–1.71; P = 0.02) among Hispanic/Latinos were associated with obesity. However, none of the SNPs in multivariable analysis of either AA or H/L cohorts were significant when adjusted for multiple correction.
Conclusions
We show that candidate SNPs in the FTO and TUB genes are associated with obesity in African Americans and Hispanic/Latinos individuals respectively. While the underlying pathophysiological mechanisms by which common genetic variants cause obesity remain unclear, we have identified novel therapeutic targets across racial and ethnic groups.
Introduction
It is estimated that over 35% of all adults in the world are currently obese with a greater prevalence in ethnic minority groups in the US, but the causes of these differences are not all known [1]. According to national data, the prevalence of obesity among the non-Hispanic black population was 47% versus 38% in the non-Hispanic white population [2]. As obesity is a leading cause of cardiovascular disease, an improved understanding of risk factors across racial and ethnic groups may improve outcomes. Although many traditional risk factors for cardiovascular disease have been identified in whites of European descent, ethnicity-specific risk factors are not well defined, especially among H/L individuals in the United States.
The phenotype for obesity is complex and is known to be influenced by socioeconomic, environmental and genetic determinants. Genome-wide association studies (GWAS) have identified common genetic variants associated with obesity [3–6]. Many single nucleotide polymorphisms (SNPs) reside near genes whose role in obesity remains unclear [7]. Despite some evidence in transethnic populations, the genetic architecture of obesity in minority populations largely remains poorly understood. Furthermore, the effects of combining these at-risk SNPs have yet to be fully explored. The aim of our study was to explore the association of candidate obesity SNPs among African American (AA) and Hispanic/Latino (H/L) individuals to better understand how these common genetic variations contribute to the risk of obesity across multi-ethnic populations.
Materials and methods
Ethics statement
We confirm that the University of Illinois at Chicago (UIC) Institutional Review Board specifically approved this study.
Study population
We used a random subset of AA and H/L participants from the UIC Cohort of Patients, Family and Friends (UIC Cohort). Selected participants self-identified as being AA or H/L and were aged 18 years or older. The sample for the present analysis consisted of 1,091 participants, of which ~30% were obese (body mass index [BMI] ≥30 kg/m2). Information on demographic characteristics, risk factors, and medical history, and blood for DNA extraction were obtained at the enrollment visit using a detailed questionnaire. All participants had height and weight measured by a trained study interviewer at the enrollment visit, from which BMI was derived and categorized into obese (BMI≥30 kg/m2) or non-obese (BMI<30 kg/m2) groups for the purposes of these analyses.
Definitions
Obesity was defined as a BMI ≥ 30 kg/m2 and non-obese participants had a BMI < 30 kg/m2, based on measured height and weight at the time of enrollment. Arterial hypertension (HTN) was defined by a history of HTN and/or the presence of antihypertensive therapy. Criteria for coronary artery disease (CAD) included a history of myocardial infarction (MI), previous coronary bypass surgery or percutaneous coronary intervention (PCI). Congestive heart failure (CHF) was defined by a history of CHF. A history of obstructive sleep apnea (OSA) was considered positive if the participant had a positive sleep study or was receiving continuous positive airways pressure therapy. Left atria (LA) and left ventricular (LV) measurements from the M-mode echocardiograms were done by an experienced cardiologist.
Sample processing
DNA extraction from the buffy coat layer containing the white blood cells was conducted from the collected serum. DNA was extracted using a commercially available kit (Qiagen Puregene, Valencia, CA), and samples were stored in -80°C until genotyping. Samples were processed according to the Agena iPLEX Gold Protocol. DNA concentrations were between 5–200 ng/μl and aliquots of 2 μl were transferred to a 384 well reaction plate for the target specific multiplex PCR amplification. This was followed by shrimp alkaline phosphatase (SAP) treatment, and then a single base extension which was carried out for each multiplex plate. The reaction products were transferred to SpectroCHIP arrays using the RS1000 Nanodispenser. The spectral analysis for single bases when incorporated during extension steps was completed using Matrix Assisted Laser Desorption Ionization—Time of Flight (MALDI-TOF) mass spectrometer and MassARRAY Analyzer software.
Genetic variants
We selected SNPs in the following candidate genes: FTO, MC4R, TUB, APOA2, APOA5, ADIPOQ, ARL15, CDH13, KNG1, LEPR, leptin, and SCG3 that have been significantly associated with obesity in whites of European descent identified in genome wide association studies (GWAS) [3–11]. Common genetic variants in the fat mass and obesity-associated (FTO) locus have consistently been associated with obesity in mostly whites of European descent with the strongest signal residing in the first and second intronic regions of FTO [12–14]. RPGRIP1L, the closest neighboring gene to the FTO locus, is postulated to regulate trafficking of leptin receptors in cilia to maintain the ciliary function in the body with defects in this process causing obesity [15]. Melanocortin-4 receptor (MC4R) is a key regulator of body weight and genetic mutations in MC4R gain weight from early childhood [16]. TUB (TUB bipartite transcription factor) is a protein coding gene and has been associated with obesity [6]. A functional SNP in apolipoprotein A-II (APOA5) gene promoter has been associated with food intake and obesity risk in non-Hispanic white Americans [3, 10, 11]. The APOA5 gene encodes apolipoprotein A-V protein which determines plasma triglyceride levels, a major risk factor for obesity. ADIPOQ encodes adiponectin, a protein hormone important in regulating glucose levels as well as fatty acid breakdown. ADP-ribosylation factor-like 15 (ARL15) is a protein in humans that is encoded by the ARL15 gene with a GWAS reporting that variants in ARL15 not only influenced adiponectin levels but were associated with obesity [17]. A GWAS not only showed that plasma adiponectin levels correlated with the CDH13 locus, which encodes a receptor for high molecular weight forms of adiponectin, but also identified a novel KNG1-ADIPOQ haplotype that was strongly associated with adiponectin levels in Filipinos [18]. LEP and LEPR genes encode leptin and leptin receptor respectively, which are intimately involved in body weight regulation and SNPs in both have been associated with obesity. A SNP in secretogranin III (SCG3) was significantly associated with obesity in a Japanese population [11].
Multiplex assay design for genotyping at the 26 SNPs was carried out by Agena Assay Design Suite v2.0. Genotyping calls were made by TyperAnalyzer software using the Autocluster method. Calls were manually reviewed with quality control (QC) and genotype reports in a tabular formation. All SNP calls were >95% with an average call rate of 99%. Furthermore, a polygenic risk score (PRS), consisting of the 26 candidate SNPs for obesity, was constructed by summing the risk alleles weighted by the effect sizes from the GWAS results. Patients were then stratified into quartiles of low: 0–20%; intermediate: 20–80%; and high: 80–100% genetic risk, and the risk of obesity was estimated by a logistic regression model with adjusted sex, age and clinical risk factors (HTN, DM, MI, CHF).
Statistical methods
Descriptive statistics are presented as mean ± standard deviation (SD) for continuous variables and counts (proportions) for categorical variables. We assessed differences between non-obese controls and obese cases using a chi-square test or a Student’s t-test for variables listed in Tables 1 and 2. Fisher’s exact test was conducted in place of chi-square test when at least one of the expected sample counts was less than 5. Genotyping data, assay statistics, and QC parameters for the selected samples were derived from peak area data. All quality matrices including statistics of assay and sample performance across the entire dataset were used to exclude poor performing samples and assays from downstream analyses. Individual genotype outputs in a tabular format were combined into a large matrix format for subsequent genetic analysis using PLINK and Golden Helix genome data analyses. Using logistic regression in PLINK, association analysis was performed in both unadjusted and adjusted for age and sex assuming an additive genetic model for all SNPs that passed QC. Multiple comparisons were accounted for using the Bonferroni correction (0.05/26 = 0.002) to determine the appropriate significant threshold.
Table 1. Selected characteristics of the African American and Hispanic/Latino cohorts.
| Characteristics | African American | Hispanic/Latino | ||||
|---|---|---|---|---|---|---|
| Non-Obese (BMI<30) n = 274 | Obese (BMI≥30) n = 260 | P-value | Non-Obese (BMI<30) n = 294 | Obese (BMI≥30) n = 263 | P-value | |
| Age (SD) | 53±11 | 51±10 | 0.06 | 45±14 | 46±13 | 0.29 |
| BMI kg/m2 (SD) | 25±3 | 37±6 | <0.001 | 26±6 | 37±6 | <0.001 |
| Women (%) | 101 (36.0) | 173 (66.0) | <0.001 | 152 (52.0) | 174 (66.0) | <0.001 |
| COPD (%) | 127 (8.2) | 13 (5.0) | 0.15 | 12 (4.2) | 7 (2.7) | 0.35 |
| Hypertension (%) | 172 (46.0) | 165 (64.0) | <0.001 | 75 (25.5) | 93 (35.6) | 0.01 |
| Diabetes mellitus (%) | 35 (13.0) | 67 (26.0) | <0.001 | 2 (0.8) | 60 (20.8) | <0.001 |
| RHD (%) | 5 (2.0) | 7 (2.6) | 0.64 | 5 (0.8) | 2 (0.7) | 0.90 |
| MI (%) | 13 (4.8) | 17 (6.6) | 0.37 | 14 (4.8) | 6 (2.3) | 0.11 |
| Heart failure (%) | 21 (8.1) | 5 (1.8) | <0.001 | 4 (1.4) | 4 (1.5) | 0.89 |
| Stroke (%) | 19 (7.0) | 15 (5.8) | 0.56 | 8 (2.7) | 4 (1.5) | 0.34 |
Abbreviations: BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; MI, myocardial infarction; RHD: Rheumatic heart disease. Heart failure for Congestive heart failure.
Table 2. Multivariable analysis of BMI groups in African Americans.
| CHR | Genes | SNP | Minor Allele | MAF | Adjusted OR* | SE | L95 | U95 | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | APOA2 | rs50820000 | G | 0.245388 | 1.27 | 0.15 | 0.95 | 1.70 | 0.10 |
| 1 | APOA2 | rs50850000 | G | 0.093461 | 0.89 | 0.22 | 0.58 | 1.36 | 0.59 |
| 1 | LEPR | rs11371010 | A | 0.479325 | 1.01 | 0.13 | 0.77 | 1.30 | 0.97 |
| 1 | APOA2 | rs64134530 | A | 0.024297 | 0.74 | 0.43 | 0.32 | 1.72 | 0.49 |
| 3 | KNG1 | rs11924390 | C | 0.492602 | 1.24 | 0.12 | 0.97 | 1.59 | 0.08 |
| 3 | KNG1 | rs18516650 | G | 0.438151 | 1.27 | 0.12 | 0.99 | 1.64 | 0.06 |
| 3 | ADIPOQ | rs64441750 | A | 0.314015 | 1.10 | 0.14 | 0.84 | 1.46 | 0.45 |
| 3 | CDH13 | rs86426500 | T | 0.151891 | 1.23 | 0.18 | 0.86 | 1.76 | 0.26 |
| 5 | ADIPOQ | rs43113940 | G | 0.301842 | 1.01 | 0.14 | 0.77 | 1.33 | 0.93 |
| 7 | Leptin | rs77990390 | A | 0.091181 | 1.21 | 0.30 | 0.77 | 1.91 | 0.40 |
| 11 | TUB | rs15281330 | G | 0.147654 | 1.00 | 0.18 | 0.69 | 1.44 | 1.00 |
| 11 | TUB | rs22723830 | A | 0.129245 | 1.25 | 0.19 | 0.86 | 1.80 | 0.24 |
| 11 | APOA5 | rs66279900 | G | 0.123607 | 0.81 | 0.19 | 0.56 | 1.20 | 0.30 |
| 15 | SCG3 | rs16964465 | C | 0.180158 | 1.20 | 0.16 | 0.88 | 1.67 | 0.25 |
| 15 | SCG3 | rs16964476 | G | 0.182862 | 1.17 | 0.16 | 0.84 | 1.63 | 0.35 |
| 15 | SCG3 | rs37642200 | G | 0.176916 | 1.23 | 0.17 | 0.89 | 1.71 | 0.21 |
| 16 | FTO | rs11219800 | A | 0.471949 | 1.27 | 0.13 | 0.99 | 1.64 | 0.06 |
| 16 | CDH13 | rs38651880 | T | 0.381064 | 1.01 | 0.13 | 0.79 | 1.305 | 0.91 |
| 16 | ADIPOQ | rs71937880 | G | 0.1375 | 0.69 | 0.20 | 0.47 | 1.03 | 0.07 |
| 16 | FTO | rs11076023 | A | 0.334827 | 0.90 | 0.14 | 0.69 | 1.18 | 0.45 |
| 16 | FTO | rs14210850 | C | 0.084113 | 1.452 | 0.23 | 0.91 | 2.30 | 0.11 |
| 16 | FTO | rs15589020 | A | 0.074835 | 1.25 | 0.24 | 0.77 | 2.01 | 0.37 |
| 16 | FTO | rs80501360 | A | 0.44015 | 1.40 | 0.13 | 1.08 | 1.80 | 0.01 |
| 18 | MC4R | rs22296160 | T | 0.022479 | 0.67 | 0.45 | 0.28 | 1.64 | 0.39 |
Abbreviations: CHR, chromosome; SNP, single nucleotide polymorphism; MAF, minor allele frequency; OR, odds ratio; SE, standard error.
* Adjusted for sex and age.
Results
We examined data from 534 AA and 557 H/L participants from the UIC Cohort. The mean (SD) age of participants was 49±13 years, 55% were female, and the mean BMI was 31±7.5 kg/m2. Baseline clinical characteristics and co-morbidities of AA and H/L respectively are shown in Table 1. Two-thirds of the participants were women in both obese groups. As expected the prevalence of HTN and DM were significantly different in the obese and non-obese groups in AAs and H/Ls as was CHF.
Only two SNPs out of 26 failed to meet the Hardy-Weinberg equilibrium (HWE) in AAs (rs26671700, P = 0.02; rs99305060, P = 0.001). Three SNPs rs16964465 (P = 0.0004), rs16964476 (P = 0.002), and rs37642200 (P = 0.002) failed to meet the HWE in H/Ls. The SNPs which did not meet HWE were excluded from multivariable analysis. Thereby, association analysis was performed in 26 candidate SNPs. The genome-wide association permutation tests, a robust measure for those SNPs that failed to meet HWE, showed that neither of these SNPs were significant in either of the two ethnic groups. The same permutation tests resulted in one significant SNP rs8050130 (P = 0.004) in the AA cohort and one rs2272383 with P = 0.02 in the H/L cohort.
The bivariate analysis of 26 SNPs in the obese and non-obese groups in the AA cohort identified one SNP on chromosome 11: rs11219800 (P = 0.03, odds ratio [OR] 1.30, 95% confidence interval [CI] 1.02–1.66; P = 0.02) and two SNPs on chromosome 16: rs71937880 (P = 0.02, OR 0.65, 95% CI 0.0.46–0.94; P = 0.02) and rs8050136 (P = 0.003, OR = 1.44, 95% CI 1.13–1.84; P = 0.003. A similar analysis in the H/L cohort identified one rs2272383 SNP on chromosome 11 (OR = 1.34, 95% CI 1.05–1.72; P = 0.02).
Multivariable analysis in the AA cohort revealed that the rs80501360 SNP on chromosome 16 in FTO was associated with obesity (OR 1.40; 95% CI 1.08–1.86; P = 0.01; Table 2). A similar analysis in the H/L cohort identified the rs227383 SNP on chromosome 11 in TUB to be associated with obesity (OR 1.34; 95% CI: 1.04–1.71; P = 0.02; Table 3). Analysis of BMI as a quantitative trait, instead of binary non-obese and obese groups, with the 26 candidate SNPs revealed similar results. In the AA cohort, rs8050136 on chromosome 16 was the most significant SNP (P = 0.002) as with the binary analysis. Two additional SNPs rs864265 (P = 0.01) and rs11924390 (P = 0.04) were also associated with a continuous BMI outcome. In the H/L cohort, the rs22772383 remained significant (P = 0.01) as in the binary obese data analysis. One additional SNP rs1558902 was associated (P = 0.04) with the continuous BMI outcome. However, none of the SNPs in multivariable analysis of either AA or H/L cohorts were significant at the Bonferroni corrected alpha 0.05/26 (≈0.002).
Table 3. Multivariable analysis of BMI groups in Hispanics/Latinos.
| CHR | Genes | SNP | Minor Allele | MAF | Adjusted OR* | SE | L95 | U95 | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | APOA2 | rs50820000 | G | 0.225348 | 1.2879 | 0.14 | 0.97 | 1.70 | 0.08 |
| 1 | APOA2 | rs50850000 | G | 0.236071 | 0.83 | 0.15 | 0.63 | 1.12 | 0.23 |
| 1 | LEPR | rs11371010 | G | 0.446475 | 1.09 | 0.12 | 0.85 | 1.38 | 0.50 |
| 1 | APOA2 | rs64134530 | A | 0.081824 | 0.86 | 0.23 | 0.55 | 1.36 | 0.53 |
| 3 | KNG1 | rs11924390 | C | 0.320317 | 0.93 | 0.13 | 0.72 | 1.21 | 0.61 |
| 3 | KNG1 | rs18516650 | G | 0.310926 | 0.86 | 0.14 | 0.66 | 1.13 | 0.27 |
| 3 | ADIPOQ | rs26671700 | T | 0.439887 | 0.96 | 0.12 | 0.76 | 1.22 | 0.74 |
| 3 | ADIPOQ | rs64441750 | A | 0.301615 | 0.93 | 0.13 | 0.72 | 1.21 | 0.60 |
| 3 | CDH13 | rs86426500 | T | 0.136175 | 1.03 | 0.18 | 0.73 | 1.46 | 0.86 |
| 5 | ADIPOQ | rs43113940 | G | 0.224208 | 1.02 | 0.15 | 0.76 | 1.37 | 0.87 |
| 7 | Leptin | rs77990390 | A | 0.349158 | 1.03 | 0.12 | 0.80 | 1.31 | 0.83 |
| 11 | TUB | rs15281330 | G | 0.140965 | 0.85 | 0.17 | 0.60 | 1.20 | 0.35 |
| 11 | TUB | rs22723830 | A | 0.355708 | 1.34 | 0.13 | 1.04 | 1.71 | 0.02 |
| 11 | APOA5 | rs66279900 | G | 0.162497 | 0.92 | 0.16 | 0.66 | 1.27 | 0.61 |
| 16 | FTO | rs11219800 | A | 0.313276 | 1.21 | 0.13 | 0.93 | 1.57 | 0.15 |
| 16 | CDH13 | rs38651880 | T | 0.303603 | 1.21 | 0.13 | 0.94 | 1.55 | 0.13 |
| 16 | ADIPOQ | rs71937880 | G | 0.135859 | 0.85 | 0.18 | 0.59 | 1.23 | 0.40 |
| 16 | FTO | rs11076023 | T | 0.442483 | 1.05 | 0.12 | 0.83 | 1.33 | 0.66 |
| 16 | FTO | rs14210850 | C | 0.204882 | 1.17 | 0.15 | 0.87 | 1.57 | 0.29 |
| 16 | FTO | rs15589020 | A | 0.184932 | 1.22 | 0.16 | 0.89 | 1.65 | 0.21 |
| 16 | FTO | rs80501360 | A | 0.276263 | 1.15 | 0.14 | 0.88 | 1.52 | 0.30 |
| 16 | FTO | rs99305060 | G | 0.248823 | 1.23 | 0.15 | 0.92 | 1.64 | 0.16 |
| 18 | MC4R | rs22296160 | - | 0 | NA | NA | NA | NA | NA |
Abbreviations: CHR, chromosome; SNP, single nucleotide polymorphism; MAF, minor allele frequency; OR, odds ratio; SE, standard error.
* Adjusted for sex and age.
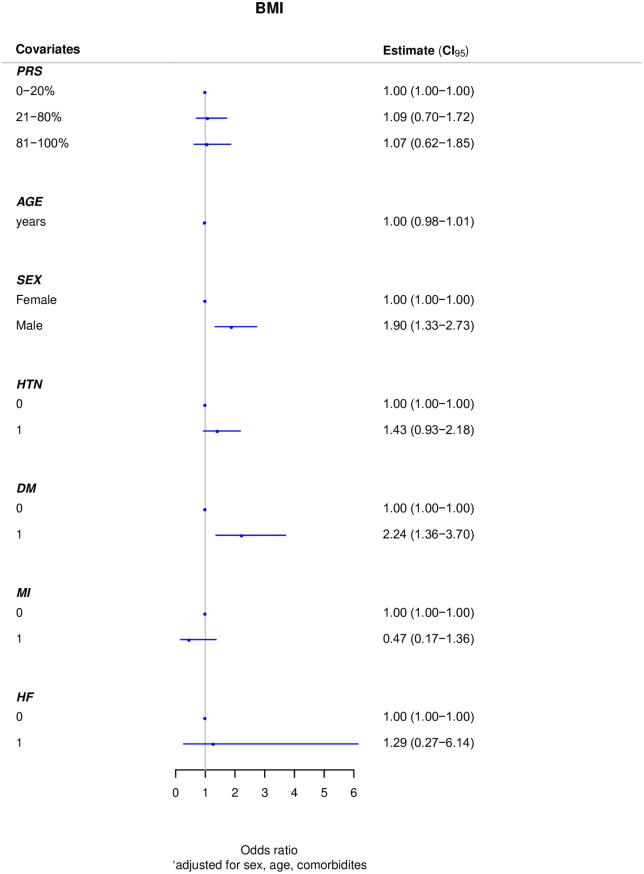
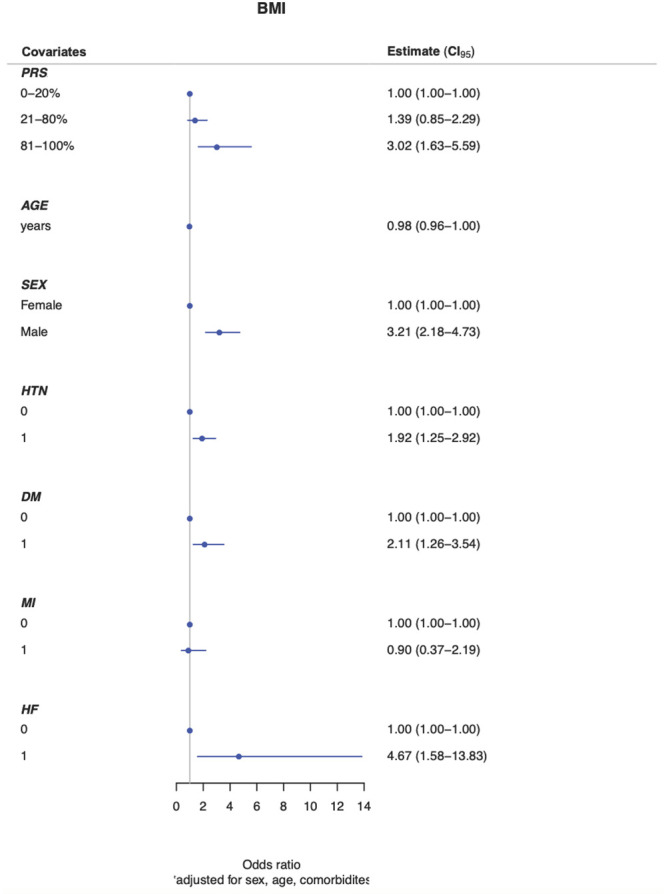
When adding the effects of the common variants, we performed a logistic regression model for known sex, age and clinical risk factors (HTN, DM, MI, CHF) for obesity. In the AA cohort, the intermediate and high genetic risk groups were associated with a 1.39-fold (OR 1.39; 95% [CI]: 0.85–2.29) and 3.02-fold (OR 3.02; 95% [CI]: 1.63–5.59) increase risk in obesity compared to the low genetic risk group. There were no significant differences observed between the different risk categories (intermediate or high compared with low genetic risk) in the H/L cohort (OR 1.09; 95% [CI]: 0.70–1.72) and (OR 1.07; 95% [CI]: 0.62–1.85) respectively (Figs 1 and 2).
Fig 1. Adjusted polygenic risk score for BMI groups in African Americans.
Abbreviations: BMI, body mass index; CI, confidence interval; HTN, hypertension; DM, diabetes mellitus; MI, myocardial infarction; HF, heart failure.
Fig 2. Adjusted polygenic risk score for BMI groups in Hispanics/Latinos.
Abbreviations: BMI, body mass index; CI, confidence interval; HTN, hypertension; DM, diabetes mellitus; MI, myocardial infarction; HF, heart failure.
Discussion
The association between obesity risk variants and BMI in ethnic minority populations is poorly understood. Here, we genotyped 26 obesity-associated SNPs previously identified in whites of European descent in AA and H/L individuals. We showed that rs8050136 (in FTO) in AAs and rs2272383 (in TUB) in H/Ls were associated with obesity. However, none of the SNPs in multivariable analysis of either AA or H/L cohorts were significant when corrected for multiple testing. We also showed that AA individuals with an intermediate or high PRS, were at a three-fold increased risk of developing obesity but this association was not found in the H/L group. Importantly, the FTO SNP has previously been reported as a significant contributor to early-onset childhood obesity in whites.
Common genetic variants in the FTO locus have consistently been associated with obesity mostly among whites of European descent [12–14]. In 2007, three independent groups identified multiple SNPs in FTO located on chromosome 16q12.2 to be strongly associated with obesity in both adults and children of European descent. The strongest signal resided in the first and second intronic regions of FTO, which most likely modulates nearby genes [14, 19, 20]. The closest neighboring gene to the FTO locus is the RPGRIP1L and this is responsible for regulating the trafficking of leptin receptors in cilia to maintain the ciliary function in the body [21, 22]. Defects in this process may lead to obesity, but the underlying mechanisms are poorly understood. A more recent study showed that variants in FTO can alter adipocyte function by targeting the ARID5B, IRX3 and IRX5 thermogenesis pathway [4]. The result of this alteration leads to conversion of the energy-dissipating beige adipocytes to energy-storing white adipocytes, fostering lipid storage and ultimately leading to weight gain.
Some studies have investigated selected FTO SNPs in obese individuals from non-European ethnic populations [15, 23]. While several studies in H/L populations replicated the European findings, identifying the causal FTO variants in AAs has been more challenging [12, 24–28]. It is thought that this may in part be related to the lower levels of linkage disequilibrium within the variants at the FTO locus [29]. A recent meta-analysis identified two novel variants in the FTO locus in AAs and this may provide insights into the underlying mechanisms [30, 31].
The TUB gene was discovered in the 1990’s as a relevant marker for obesity in mice. It has been hypothesized that this gene, which is highly expressed in the hypothalamus, encodes for a transcription factor that regulates energy homeostasis [32–36]. This process is initiated by the secretion of leptin from adipocyte tissue and binds to receptors in the hypothalamus to inhibit the orexigenic and stimulate the anorexigenic pathways in response to eating food and energy expenditure [37]. Defects in these pathways are postulated to be critical for the obesity phenotype. Despite these advancements, the underlying molecular function of this gene remains unclear. Given the limited number of human studies that have examined the association between TUB SNPs and obesity, unraveling the differences in common genetic variation across racial and ethnic groups is important. With improved understanding of the underlying pathophysiologic mechanisms by which TUB SNPs increase the risk of obesity will not only provide important insights into the obesity epidemic in the H/L population but also identify potentially therapeutic targets.
Over the last decade, genetic studies have uncovered signaling pathways important in the pathogenesis of obesity. However, translating this knowledge to tackle the obesity epidemic has been limited. Therapeutic targeting of the thermogenesis and orexigenic or anorexigenic pathways that are regulated by the FTO and TUB genes respectively has important clinical implications for the obesity epidemic especially in ethnic minorities.
This study has a number of limitations that should be addressed. First, this was an institutional cohort reflective of a metropolitan population in a large city and our findings may not necessarily reflect rural or other populations where other SNPs may show stronger associations with obesity in minority populations. Second, we did not estimate admixture in the obese and non-obese groups. Third, there were significantly more women in the obese than non-obese groups most noticeably in AAs and less so in H/Ls. Nonetheless, there remained a significant association between the rs8050136 and rs2272383 SNPs after multiple risk factor adjustments. Fourth, several previously reported common SNPs associated with obesity in whites of European decent were not replicated in AAs and H/Ls. This may be in part be explained by the heterogeneity in ethnic minorities and because the linkage disequilibrium structure varies across race and ethnic groups. Fifth, we selected SNPs focused on genetic targets thought to be directly associated with obesity and common genetic variation associated with obesity risk factors was not evaluated. Replication of the associations of obesity with FTO and TUB in independent cohorts is warranted given the limited sample sizes.
Conclusion
In conclusion, our study confirms the association between common genetic variants in the FTO and TUB genes and increased risk for developing obesity in individuals of African and Hispanic descent respectively. However, none of the SNPs in multivariable analysis of either AA or H/L cohorts survived correction for multiple testing. Furthermore, implementing a PRS into clinical practice may be considered a useful tool for physicians assessing at-risk individuals. These findings highlight the importance of genetic variation in the pathogenesis of obesity in ethnic minority populations in order to understand individualized genetic risk factors for obesity, both in terms of more accurate risk stratification and mechanistic studies. Further validation and functional studies are needed to confirm our findings and to determine the underlying mechanisms by which FTO and TUB SNPs modulate susceptibility to obesity across racial and ethnic groups.
Data Availability
All data are available on Figshare (DOI: 10.6084/m9.figshare.14448003.v1).
Funding Statement
This work was supported in part by grants from the AHA 17MCPRP33420153 (Brandon Chalazan) and NIH/NHLBI R01 HL138737 and T32 HL139439 (Dawood Darbar).
References
- 1.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–84. 10.1001/jama.2012.14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.cdc.gov/nchs/data/factsheets/factsheet_nhanes.htm. National Health and Nutrition Examination Survey. 2017.
- 3.Corella D, Lai CQ, Demissie S, Cupples LA, Manning AK, Tucker KL, et al. APOA5 gene variation modulates the effects of dietary fat intake on body mass index and obesity risk in the Framingham Heart Study. J Mol Med (Berl). 2007;85(2):119–28. 10.1007/s00109-006-0147-0 [DOI] [PubMed] [Google Scholar]
- 4.Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med. 2015;373(10):895–907. 10.1056/NEJMoa1502214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Klaauw AA, Keogh JM, Henning E, Stephenson C, Kelway S, Trowse VM, et al. Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nat Commun. 2016;7:13055. 10.1038/ncomms13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiri-Sverdlov R, Custers A, van Vliet-Ostaptchouk JV, van Gorp PJ, Lindsey PJ, van Tilburg JH, et al. Identification of TUB as a novel candidate gene influencing body weight in humans. Diabetes. 2006;55(2):385–9. 10.2337/diabetes.55.02.06.db05-0997 [DOI] [PubMed] [Google Scholar]
- 7.Wassel CL, Pankow JS, Rasmussen-Torvik LJ, Li N, Taylor KD, Guo X, et al. Associations of SNPs in ADIPOQ and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis (MESA). Obesity (Silver Spring). 2011;19(4):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katira A, Tan PH. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol Med. 2016;13(1):101–19. 10.28092/j.issn.2095-3941.2015.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins MC, Trujillo J, Farias DR, Kac G. Polymorphisms in the leptin (rs7799039) gene are associated with an increased risk of excessive gestational weight gain but not with leptin concentration during pregnancy. Nutr Res. 2017;47:53–62. 10.1016/j.nutres.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 10.Yang MM, Wang J, Fan JJ, Ng TK, Sun DJ, Guo X, et al. Variations in the Obesity Gene "LEPR" Contribute to Risk of Type 2 Diabetes Mellitus: Evidence from a Meta-Analysis. J Diabetes Res. 2016;2016:5412084. 10.1155/2016/5412084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanabe A, Yanagiya T, Iida A, Saito S, Sekine A, Takahashi A, et al. Functional single-nucleotide polymorphisms in the secretogranin III (SCG3) gene that form secretory granules with appetite-related neuropeptides are associated with obesity. J Clin Endocrinol Metab. 2007;92(3):1145–54. 10.1210/jc.2006-1808 [DOI] [PubMed] [Google Scholar]
- 12.Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10(1):51–61. 10.1038/nrendo.2013.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi Q, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet. 2014;23(25):6961–72. 10.1093/hmg/ddu411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. 10.1126/science.1141634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratigopoulos G, Martin Carli JF, O’Day DR, Wang L, Leduc CA, Lanzano P, et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 2014;19(5):767–79. 10.1016/j.cmet.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O’Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12(5):561–74. 10.1093/hmg/ddg057 [DOI] [PubMed] [Google Scholar]
- 17.Richards JB, Waterworth D, O’Rahilly S, Hivert MF, Loos RJ, Perry JR, et al. A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 2009;5(12):e1000768. 10.1371/journal.pgen.1000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Li Y, Lange EM, Croteau-Chonka DC, Kuzawa CW, McDade TW, et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet. 2010;19(24):4955–64. 10.1093/hmg/ddq423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders CL, Chiodini BD, Sham P, Lewis CM, Abkevich V, Adeyemo AA, et al. Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity (Silver Spring). 2007;15(9):2263–75. 10.1038/oby.2007.269 [DOI] [PubMed] [Google Scholar]
- 20.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724–6. 10.1038/ng2048 [DOI] [PubMed] [Google Scholar]
- 21.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. 10.1371/journal.pgen.0030115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17(18):1586–94. 10.1016/j.cub.2007.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SL, Cheng I, Pendergrass SA, Kucharska-Newton AM, Lim U, Ambite JL, et al. Association of the FTO obesity risk variant rs8050136 with percentage of energy intake from fat in multiple racial/ethnic populations: the PAGE study. Am J Epidemiol. 2013;178(5):780–90. 10.1093/aje/kwt028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villalobos-Comparan M, Teresa Flores-Dorantes M, Teresa Villarreal-Molina M, Rodriguez-Cruz M, Garcia-Ulloa AC, Robles L, et al. The FTO gene is associated with adulthood obesity in the Mexican population. Obesity (Silver Spring). 2008;16(10):2296–301. 10.1038/oby.2008.367 [DOI] [PubMed] [Google Scholar]
- 25.Song Y, You NC, Hsu YH, Howard BV, Langer RD, Manson JE, et al. FTO polymorphisms are associated with obesity but not diabetes risk in postmenopausal women. Obesity (Silver Spring). 2008;16(11):2472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wing MR, Ziegler JM, Langefeld CD, Roh BH, Palmer ND, Mayer-Davis EJ, et al. Analysis of FTO gene variants with obesity and glucose homeostasis measures in the multiethnic Insulin Resistance Atherosclerosis Study cohort. Int J Obes (Lond). 2011;35(9):1173–82. 10.1038/ijo.2010.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeyemo A, Chen G, Zhou J, Shriner D, Doumatey A, Huang H, et al. FTO genetic variation and association with obesity in West Africans and African Americans. Diabetes. 2010;59(6):1549–54. 10.2337/db09-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassanein MT, Lyon HN, Nguyen TT, Akylbekova EL, Waters K, Lettre G, et al. Fine mapping of the association with obesity at the FTO locus in African-derived populations. Hum Mol Genet. 2010;19(14):2907–16. 10.1093/hmg/ddq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hester JM, Wing MR, Li J, Palmer ND, Xu J, Hicks PJ, et al. Implication of European-derived adiposity loci in African Americans. Int J Obes (Lond). 2012;36(3):465–73. 10.1038/ijo.2011.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters U, North KE, Sethupathy P, Buyske S, Haessler J, Jiao S, et al. A systematic mapping approach of 16q12.2/FTO and BMI in more than 20,000 African Americans narrows in on the underlying functional variation: results from the Population Architecture using Genomics and Epidemiology (PAGE) study. PLoS Genet. 2013;9(1):e1003171. 10.1371/journal.pgen.1003171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, Lange LA, et al. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Genet. 2013;45(6):690–6. 10.1038/ng.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan LJ, Zhu H, He H, Wu KH, Li J, Chen XD, et al. Replication of 6 obesity genes in a meta-analysis of genome-wide association studies from diverse ancestries. PLoS One. 2014;9(5):e96149. 10.1371/journal.pone.0096149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 1990;81(6):424–7. 10.1093/oxfordjournals.jhered.a111019 [DOI] [PubMed] [Google Scholar]
- 34.Kleyn PW, Fan W, Kovats SG, Lee JJ, Pulido JC, Wu Y, et al. Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell. 1996;85(2):281–90. 10.1016/s0092-8674(00)81104-6 [DOI] [PubMed] [Google Scholar]
- 35.Nies VJM, Struik D, Wolfs MGM, Rensen SS, Szalowska E, Unmehopa UA, et al. TUB gene expression in hypothalamus and adipose tissue and its association with obesity in humans. Int J Obes (Lond). 2018;42(3):376–83. 10.1038/ijo.2017.214 [DOI] [PubMed] [Google Scholar]
- 36.Boggon TJ, Shan WS, Santagata S, Myers SC, Shapiro L. Implication of tubby proteins as transcription factors by structure-based functional analysis. Science. 1999;286(5447):2119–25. 10.1126/science.286.5447.2119 [DOI] [PubMed] [Google Scholar]
- 37.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010;6(10):578–88. 10.1038/nrendo.2010.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available on Figshare (DOI: 10.6084/m9.figshare.14448003.v1).