Abstract
Osteochondral repair remains a significant clinical challenge due to the multiple tissue phenotypes and complex biochemical milieu in the osteochondral unit. To repair osteochondral defects, it is necessary to mimic the gradation between bone and cartilage, which requires spatial patterning of multiple tissue-specific cues. To address this need, we have developed a facile system for the conjugation and patterning of tissue-specific peptides by melt extrusion of peptide-functionalized poly(ε-caprolactone) (PCL). In this study, alkyne-terminated PCL was conjugated to tissue-specific peptides via a mild, aqueous, and Ru(II)-catalyzed click reaction. The PCL-peptide composites were then 3D printed by multimaterial segmented printing to generate user-defined patterning of tissue-specific peptides. To confirm the bioactivity of 3D printed PCL-peptide composites, bone- and cartilage-specific scaffolds were seeded with mesenchymal stem cells and assessed for deposition of tissue-specific extracellular matrix in vitro. PCL-peptide scaffolds successfully promoted osteogenic and chondrogenic matrix deposition, with effects dependent on the identity of conjugated peptide.
Keywords: bioconjugation, scaffold, osteochondral, 3D printing, extrusion, patterning
1. Introduction
Osteochondral defects produce a significant clinical burden around the world, with hundreds of thousands of surgical procedures performed annually and a persistently increasing number of total patients [1]. The osteochondral unit is uniquely heterogeneous, consisting of a mineralized and highly vascularized subchondral bone layer, an acellular and avascular articular cartilage layer, and a gradient of tissue layers with biochemical and physical properties between those of bone and cartilage [2]. Successful repair of osteochondral defects thus requires the replacement or regeneration of multiple tissue phenotypes, presenting significant clinical and scientific challenges in replicating the heterogenous properties of the osteochondral unit [3]. Tissue engineering has shown significant promise for the regeneration of bone and cartilage tissue, but traditional constructs such as monolayer scaffolds and injectable hydrogels often fall short of replicating the complex spatial patterning of biochemical cues seen in native osteochondral tissue [3]. The interface between articular cartilage and subchondral bone, for instance, requires spatial patterning of multiple biomolecules in order to recapitulate the gradual transition from cartilage to calcified cartilage to bone [2]. Furthermore, currently established methods of biomolecule delivery such as controlled release suffer from off-site diffusion, which can result in loss of the original spatial localization of biochemical cues [4]. Thus, it is of longstanding interest to develop tissue engineering constructs and fabrication methods that can present heterogeneous, user-defined distributions of multiple regenerative cues for bone and cartilage.
Three-dimensional (3D) printing has recently emerged as a versatile set of technologies for the fabrication of constructs with user-defined distributions of multiple materials [4]. Melt extrusion printing, in particular, has enabled the deposition of polyesters commonly utilized in tissue engineering such as poly(ε-caprolactone) (PCL) and poly(lactide-co-glycolide) (PLGA) [3]. However, these polyesters and other commonly extruded synthetic polymers are largely bioinert, requiring the secondary deposition of bioactive components or the modification of these polymers with tissue-specific moieties [4]. Bioconjugation strategies such as activated ester chemistry and click chemistry have thus gained recent interest for their potential to enable the biological functionalization of 3D printed scaffolds [5,6]. Nevertheless, applications of bioconjugation to 3D printing have so far been primarily limited to the bulk modification of scaffolds after printing [5–7], restricting the ability to spatially control biochemical cues or to pattern multiple biomolecules. In some cases, synthetic polyesters such as PCL and biological polymers such as gellan gum have been modified with the cell-adhesive arginyl-glycyl-aspartic acid (RGD) sequence prior to printing, which allows for the deposition and patterning of cell-adhesive fibers [8,9]. However, such strategies have utilized non-bioorthogonal chemistry such as activated ester and maleimide-based reactions that are reactive towards biologically prevalent amines and thiols, and thus have a limited selection of compatible biomolecules [7]. Moreover, the functionalization and patterning of tissue-specific moieties and peptide sequences remains an outstanding goal.
To address a longstanding need for the spatial patterning of tissue-specific biochemical cues, we developed a facile strategy for the click conjugation and melt extrusion of PCL-peptide composites (Figure 1).
Figure 1:
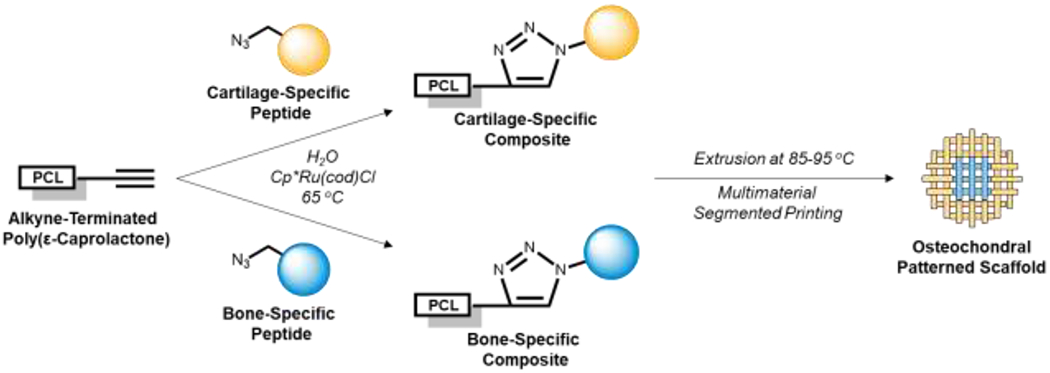
Click conjugation and three-dimensional printing of cartilage- and bone-specific composites. The tissue-specific polymers can be spatially patterned by multimaterial segmented printing.
Our PCL-peptide conjugation scheme utilizes a mild, aqueous, and chloro(pentamethylcyclopentadienyl)(cycloocta-diene)ruthenium(II) (Cp*Ru(cod)Cl) catalyzed alkyne-azide cycloaddition (RuAAC) that has been previously investigated by our laboratory and demonstrated to be bioorthogonal, non-cytotoxic, and compatible with biomolecules of diverse size and chemical character [10–12]. To generate bone-specific composites, we utilized the osteogenic peptides bone morphogenetic protein mimetic peptide (BMPm) and glycine-histidine-lysine peptide (GHK), which have been implicated in the osteogenesis of osteoblasts and mesenchymal stem cells (MSCs), respectively [13,14]. To generate cartilage-specific composites, we used the N-cadherin peptide (NC), which mimics the N-cadherin protein that forms cell-cell contacts between MSCs during early chondrogenesis [15]. In this study, we investigated the utility of click conjugated PCL-peptides for osteochondral tissue engineering by characterizing their printability, fabricating heterogeneous scaffolds with a user-defined distribution of peptides, and investigating construct bioactivity in promoting bone-and cartilage-specific extracellular matrix (ECM) deposition in vitro.
2. Materials and Methods
2.1. Materials
Fetal bovine serum (FBS) was purchased from Gemini Bio-Products (Sacramento, CA). Low glucose Dulbecco’s modified eagle medium (DMEM), antibiotic-antimycotic, minimum essential medium alpha (α-MEM), Quant-iT PicoGreen dsDNA assay kit, and proteinase K were purchased from ThermoFisher Scientific (Waltham, MA). ITS+ Premix was purchased from Corning (Corning, NY). Phosphate buffered saline (PBS), ε-caprolactone, propargyl alcohol, Cp*Ru(cod)Cl, CDCL3 with 1% (v/v) trimethylsilane, tetrahydrofuran (THF), N,N-diisopropylethylamine (DIEA), ascorbic acid, dexamethasone, β-glycerol 2-phosphate, acetic acid, pepstatin A, iodacetamide, tris(hydroxymethyl aminomethane), ethylenediaminetetraacetic acid (EDTA), 1,9-dimethyl-methylene blue (DMMB) zinc chloride double salt, and Costar Ultra-Low Attachment 24-well plates were purchased from MilliporeSigma (St. Louis, MO). 5-(and-6)-carboxytetramethylrhodamine, succinimidyl ester (TAMRA) dye was purchased from AAT Bioquest (Sunnyvale, CA). Pacific Blue dye was purchased from AdipoGen Life Sciences (San Diego, CA). Arsenazo III assay kit was purchased from Pointe Scientific (Canton, MI). Ultrapure water was obtained from a Millipore Super-Q water system (Billerica, MA).
2.2. Synthesis of Tissue-Specific Composites
The BMPm, (“GGGRHVRISRSL”), GHK (“GGGGHKSP”), and NC (“GGGHAVDI”) peptide sequences with N-terminal azides were synthesized by solid phase peptide synthesis as described previously [11,16]. Alkyne-terminated PCL (PCL-alkyne) is available commercially, but in this case, was synthesized at a molar feed ratio of 200:1 ε-caprolactone:propargyl alcohol, as described in detail elsewhere [17]. PCL-peptide composites were synthesized by mixing PCL-alkyne, the peptide of interest, and Cp*Ru(cod)Cl at 1:1:1 molar ratio in ultrapure H2O at 65 °C for 24 h, with stirring of the suspension by a mechanical stirrer at 400 rpm. After reaction, the aqueous portion was decanted to remove unreacted peptide and the majority of Cp*Ru(cod)Cl, after which the non-soluble product was dissolved in THF and precipitated in methanol to remove residual Cp*Ru(cod)Cl. The precipitated product was then collected by vacuum filtration.
2.3. Polymer Characterization
1H nuclear magnetic resonance spectroscopy (NMR) was used to measure the conversion of PCL-alkyne to PCL-peptide products using a 600 MHz Bruker spectrometer (Billerica, MA). Samples were dissolved in CDCL3 with 1% (v/v) trimethylsilane, and spectra were processed using Bruker TopSpin software. Gel permeation chromatography (GPC) was used to characterize the molecular weight of PCL-alkyne and PCL-peptide composites before and after printing. Samples were dissolved in THF and analyzed using a Waters Acquity Advanced Polymer Chromatography system (Milford, MA), with comparison to polystyrene standards of known molecular weight.
2.4. Fabrication and Physical Characterization of 3D Printed Constructs
The 3D printed constructs were fabricated by melt extrusion with an EnvisionTEC 3D Bioplotter Manufacturer (Gladbeck, Germany), using techniques developed previously by our laboratory [18–20]. The polymer of interest was placed in a metal cartridge, heated to 85-95 °C, and extruded through a 27G needle at 4.9-5.1 bar pressure, 4.0 mm/s deposition speed, and 0.4 s pre-flow and post-flow (Table S1), with imaging after each layer. Constructs were printed as a cylindrical, alternating crosshatch pattern with 4 layers of 5 mm diameter and 220 μm thickness, producing scaffolds with total dimensions of 5 mm diameter and 0.88 mm height.
The porosity, average fiber diameter, and average pore diameter of each construct was assessed by microcomputed tomography (μCT) using a Bruker SkyScan 1272 (Billerica, MA) [19,20]. Scans were acquired at a voltage of 40 kV and a current of 250 μA, with a rotation step size of 0.4°, 8 μm/pixel, frame averaging of 6, and random movement of 10. Reconstruction, slicing, and analysis were performed in Bruker NRecon and CTAn software (Billerica, MA) and LabView (Austin, TX). For quantification of physical architectural properties, a proportional volume of interest was set at 75% scale of the x, y, and z axes, equally spaced from the scaffold edges.
2.5. PCL-Peptide Patterning and Imaging
For fluorescent visualization, BMPm and NC peptides were synthesized with an extra lysine group for dye attachment (“GGGRHVRISRSLK” and “GGGHAVDIK”), followed by conjugation to PCL-alkyne as described above. The PCL-BMPm and PCL-NC composites were then covalently linked to activated esters of Pacific Blue (405/455 nm excitation/emission) and TAMRA (543/572 nm excitation/emission), respectively. Briefly, the PCL-peptide composite, dye, and DIEA were dissolved in 1:1:1 molar ratio in THF, added to a N2 purged flask, and stirred for 24 h at room temperature in the dark. After reaction, the product was precipitated in methanol and collected by vacuum filtration.
To demonstrate patterning, Pacific Blue-tagged PCL-BMPm and TAMRA-tagged PCL-NC were printed within cylindrical scaffolds of 10 mm diameter and 0.88 mm height, with each layer containing a centered 5 mm × 5 mm square of PCL-BMPm surrounded by PCL-NC on all sides. The fluorescent scaffolds were imaged at 10× using a Nikon A1-Rsi confocal microscope (Tokyo, Japan), with automated stitching to generate a single image of the whole scaffold.
2.6. MSC Harvest and Culture
Rat bone marrow derived MSCs were harvested by aspiration of bone marrow from the tibia of five 6-8 week old male Fischer 344 rats from Charles River Laboratories (Wilmington, MA), in accordance with protocols approved by the Rice Institutional Animal Care and Use Committee and in agreement with the animal care and use guidelines set forth by the National Institutes of Health [21]. Cells from all rats were pooled to minimize inter-animal variability. Adherent cells were cultured in growth medium (DMEM with 10% v/v FBS and 1% v/v antibiotic-antimycotic) inside a humidified incubator at 37 °C and 5% CO2, and cryopreserved in liquid N2 until point of usage. All MSCs were used at Passage 3.
2.7. Cell Seeding and In Vitro Study Design
Printed constructs were sterilized by exposure of both sides to UV light for 3 h, immersion in a sterile gradient (100%, 75%, 50%, 25%, 0%) of ethanol and PBS, and two additional washes in PBS. MSCs were seeded on the scaffolds by pipetting 30 μL of a 3.3 × 106 cells/mL suspension on top of each scaffold in a low-attachment 24-well plate. After 2 h, 970 μL of growth medium was gently added to each well. After 24 h of cell attachment, the growth medium was replaced with either chondrogenic, osteogenic, or osteochondral medium.
Parallel chondrogenic (PCL-alkyne, PCL-NC) and osteogenic (PCL-alkyne, PCL-GHK) studies were conducted, in which scaffolds were cultured in both tissue-specific medium as well as mixed osteochondral medium. Chondrogenic medium contained DMEM supplemented with 1% v/v ITS+ Premix (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenous acid, 5.35 μg/mL linoleic acid and 1.25 μg/mL bovine serum albumin), 50 mg/L ascorbic acid, 10−7 M dexamethasone, and 1% v/v antibiotic-antimycotic, while osteogenic medium contained α-MEM supplemented with 10% v/v FBS, 50 mg/L ascorbic acid, 10−8 M dexamethasone, 10 mM β-glycerol 2-phosphate, and 1% v/v antibiotic-antimycotic [12]. The mixed osteochondral medium consisted of a 1:1 mixture of the osteogenic and chondrogenic formulations [22]. Scaffolds were cultured for up to 28 days, with replacement of medium every 2-3 days.
2.8. Biochemical Assays
After 0, 7, 14, or 28 days of culture, scaffolds were digested and assayed for cellularity and tissue-specific ECM deposition using the PicoGreen assay (DNA content), DMMB assay (cartilage-specific sulfated glycosaminoglycans), and Arsenazo III assay (bone-specific mineralization) [12]. Scaffolds were washed in PBS for 10 min at 37 °C and then transferred to sterile pre-weighed polystyrene tubes containing 5 mm stainless steel beads. After weighing, each scaffold was frozen at −20 °C until ready for characterization. Thawed samples were homogenized in 300 μL of proteinase K solution (PicoGreen, DMMB) or 300 μL of 0.5M acetic acid (Arsenazo III) using a Qiagen TissueLyser II (Hilden, Germany) at 30 s−1 for 5 min. The proteinase K solution consisted of 1 mg/mL proteinase K, 10 μg/mL pepstatin A, and 185 μg/mL iodoacetamide in tris–EDTA solution (6.055 mg/mL tris(hydroxymethyl aminomethane), 0.372 mg/mL EDTA, pH 7.6). After homogenization, the proteinase K and acetic acid suspensions were allowed to digest for 16 h at 65 °C and room temperature, respectively, followed by quantification of biochemical content using assay kits. All biochemical data were normalized to acellular controls that were cultured under the same conditions.
2.9. Statistical Analysis
GPC data (n=3) were analyzed using the Student’s t-test for assessment of differences in molecular weight parameters before and after peptide conjugation, as well as before and after printing. μCT data (n=3) were analyzed using one-way analysis of variance, with post-hoc testing by Tukey’s honestly significant difference (HSD) to assess differences in architectural parameters between each printed scaffold material. Data from biochemical assays (n=3) were analyzed using two-way analysis of variance with post-hoc testing by Tukey’s HSD. All tests were performed at α=0.05.
3. Results
3.1. Synthesis of Tissue-Specific PCL-Peptide Composites
Bone- and cartilage-specific PCL-peptide composites were synthesized by the click conjugation of the azide-terminated peptides, BMPm, GHK, and NC, to PCL-alkyne. PCL-BMPm, PCL-GHK, and PCL-NC were synthesized with yields of 86.5%, 84.8%, and 85.7%, respectively. Given that peptide conjugation (Figure 2a) represents the terminal modification of a large PCL-alkyne macromer (~22 kDa) with relatively smaller peptides (778-1380 Da), the bulk chemical structure does not undergo any significant changes (Figure 2b). An area of interest from 4.6-5.2 ppm (Figure 2c), however, can be used to assess reaction conversion by both the disappearance of an alkyne-adjacent peak (“f”) and the appearance of new peaks corresponding to the histidine side chain (“g”, “h”). For all PCL-peptide composites, the alkyne-adjacent peak disappeared after reaction, indicating quantitative conversion of the alkyne group. Furthermore, peaks corresponding to the histidine side chain were observable on all PCL-peptide spectra. Since free BMPm, GHK, and NC peptides are insoluble in the CDCL3 solvent used for NMR, the appearance of histidine peaks indicates conjugation of the peptides to PCL.
Figure 2:
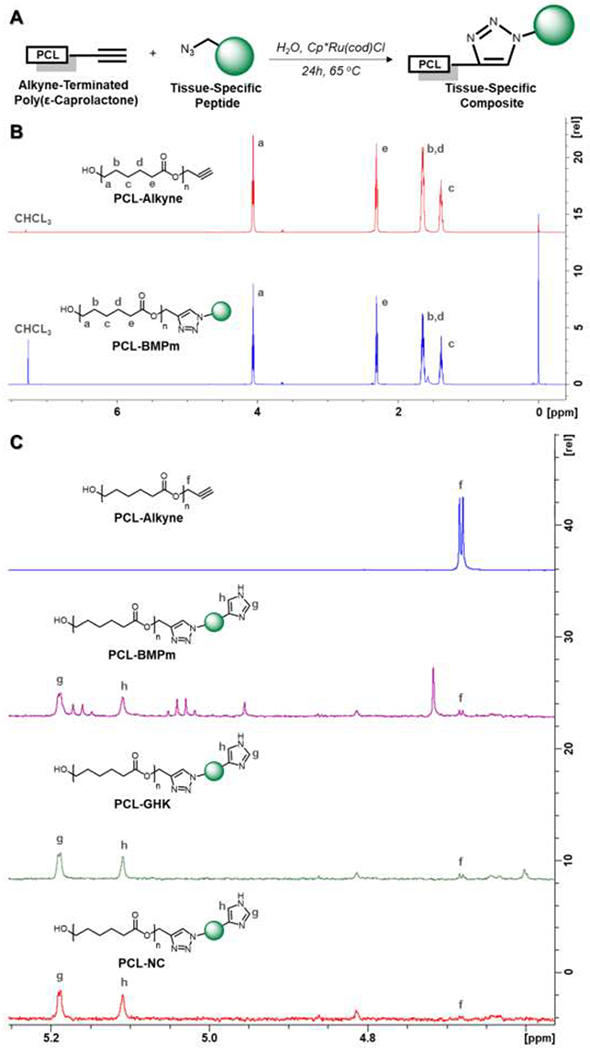
NMR characterization of PCL-alkyne and PCL-peptide composites, with (a) PCL-peptide conjugation scheme, (b) overall comparison of PCL-alkyne and PC-BMPm spectra, and (c) area of interest containing alkyne-adjacent peak and histidine side chain peaks for PCL-alkyne and PCL-peptide composites.
GPC was also used to assess changes in the molecular weight distribution before and after peptide conjugation to PCL-alkyne (Table 1), revealing statistically significant changes in number-average molecular weight (Mn) and polydispersity index (PDI) after the conjugation of BMPm, GHK, and NC to PCL-alkyne.
Table 1:
Molecular weight distributions of PCL-alkyne compared to PCL-peptide composites, as measured by GPC. Scaffold materials were also characterized after exposure to temperatures of 85-95 °C for 2 hours during melt extrusion printing. All data are reported as means ± standard deviation for a sample size of n=3.
| Scaffold Material |
Mn (kDa) Before Printing |
Mn (kDa) After Printing |
PDI Before Printing |
PDI After Printing |
|---|---|---|---|---|
| PCL-Alkyne | 22.3 ± 0.4 | 22.1 ± 0.3 | 1.33 ± 0.01 | 1.33 ± 0.01 |
| PCL-BMPm | 27.2 ± 0.5 | 27.9 ± 0.3 | 1.22 ± 0.01 | 1.22 ± 0.01 |
| PCL-GHK | 24.0 ± 0.5 | 23.7 ± 0.5 | 1.29 ± 0.01 | 1.28 ± 0.01 |
| PCL-NC | 24.6 ± 0.4 | 24.6 ± 0.5 | 1.26 ± 0.01 | 1.27 ± 0.01 |
3.2. Three-Dimensional Printing of PCL-Peptide Constructs
After confirming the successful synthesis of PCL-peptide composites, each material was printed by melt extrusion through 27G needles at 85-95 °C, 4.9-5.1 bar pressure, 4.0 mm/s deposition speed, and 0.4 s pre-flow and post-flow in a cylindrical crosshatch pattern, generating scaffolds with total dimensions of 5 mm diameter and 0.8 mm height (Figures 3a–d). As shown by μCT analysis, there were no significant differences in porosity, average fiber diameter, or average pore diameter between different scaffold materials, indicating that constructs could be reproducibly printed under similar conditions (Figure 3e).
Figure 3:
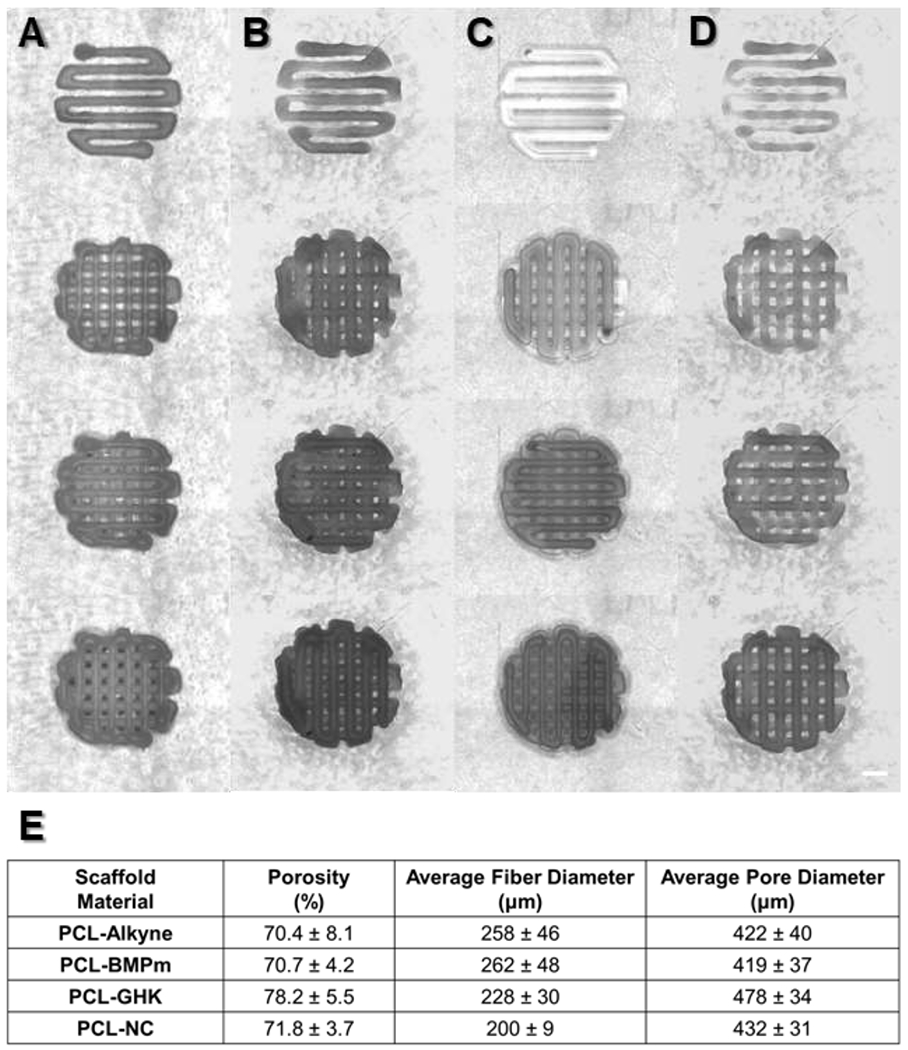
Layer-by-layer images of melt extruded (a) PCL-alkyne, (b) PCL-BMPm, (c) PCL-GHK, and (d) PCL-NC scaffolds. Scale bar represents 1 mm. (e) Physical parameters of printed scaffolds, as measured by μCT. All data are reported as means ± standard deviation for a sample size of n=3.
To confirm the thermal stability of PCL-peptide conjugates, GPC was used to assess the molecular weight distribution of scaffold materials after their exposure to temperatures of 85-95 °C for 2 hours during the melt extrusion process (Table 1). No significant changes in Mn or PDI occurred as a result of melt extrusion, and NMR spectra also had no observable changes to chemical structure (data not shown).
3.4. Patterning of PCL-Peptide Composites
After confirming the printability of PCL-peptide scaffolds, we fabricated heterogeneous scaffolds with user-defined patterning of multiple materials. While vertical patterning has been readily achieved by simple layer-by-layer deposition [3,4,23], we sought instead to produce more difficult lateral patterning within individual layers. By adapting multimaterial segmented printing that our laboratory previously developed [18,24], we were able to fabricate heterogeneous scaffolds with lateral patterning across horizontal layers and even individual fibers, as shown by fluorescently tagged PCL-BMPm and PCL-NC (Figure 4).
Figure 4:
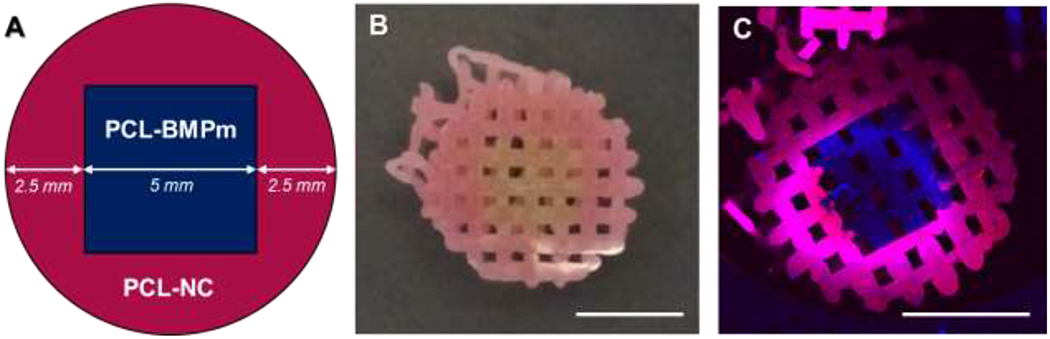
User-defined lateral patterning of fluorescently tagged PCL-peptide composites. (a) Heterogeneous design of construct layers, (b) image of printed construct, and (c) fluorescent image of printed construct. All scale bars represent 5 mm.
3.5. In Vitro Characterization of Scaffold Bioactivity
Lastly, we investigated the bioactivity of PCL-peptide composites by seeding MSCs on 3D printed constructs and investigating their ability to promote bone- and cartilage-specific ECM deposition in vitro (Figure 5). Calcium and sGAG deposition were normalized to cellular content to represent the amount of tissue-specific ECM deposition per cell, indicating tissue-specific differentiation (Figures 5b, 5d), and were also normalized to scaffold mass to represent the proportion of the overall scaffold (Figures 5e, 5e).
Figure 5:
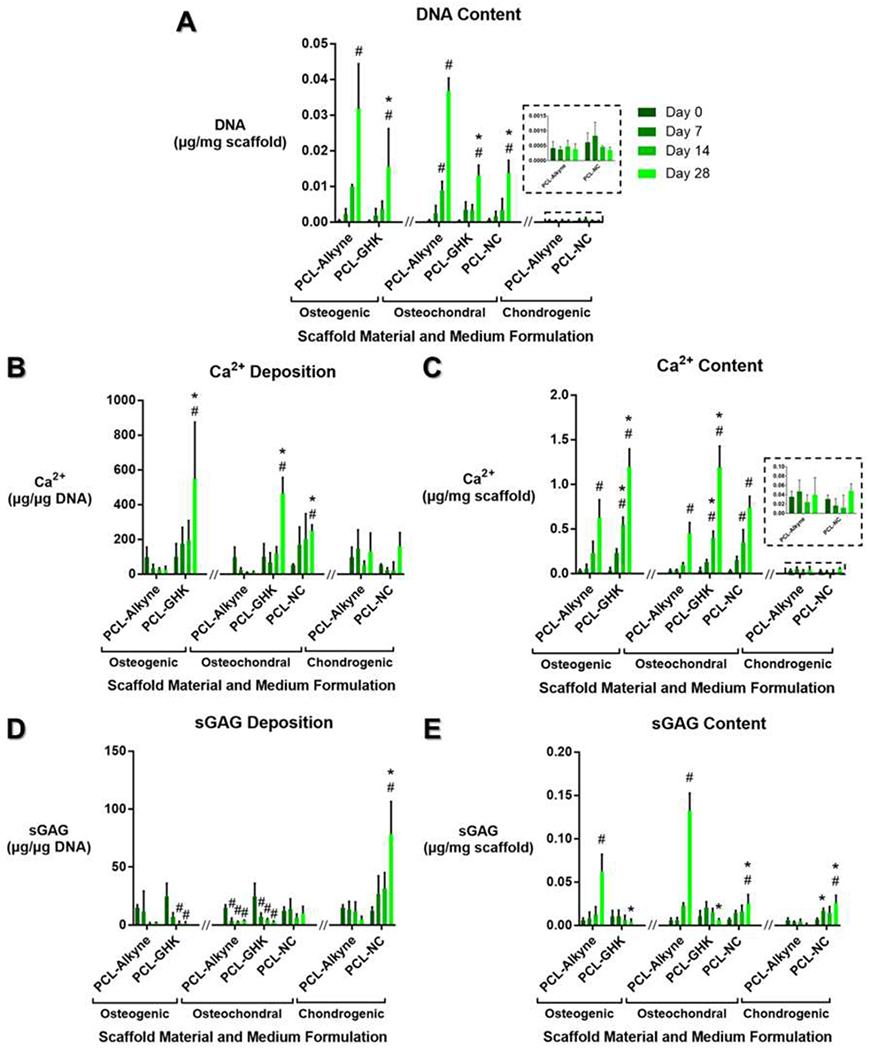
Assessment of cellularity and tissue-specific matrix deposition in PCL-peptide scaffolds cultured for 0-28 days. (a) DNA content, representing cellularity. (b-c) Bone-specific calcium content, normalized to cellular content and to scaffold mass. (d-e) Cartilage-specific sulfated glycosaminoglycan (sGAG) content, normalized to cellular content and to scaffold mass. All data are reported as means ± standard deviation for a sample size of n=3. * indicates statistical significance compared to a peptide-free PCL-alkyne control in the same medium formulation and same timepoint, # indicates significance compared to the same scaffold material and medium formulation at Day 0, representing progression over time.
PCL-alkyne scaffolds showed a large amount of cell proliferation over time in osteogenic and osteochondral media, while PCL-GHK and PCL-NC scaffolds demonstrated statistically lower proliferation in both media (Figure 5a). All scaffolds cultured in serum-free chondrogenic medium did not undergo cell proliferation and maintained static levels of DNA over 28 days of culture.
PCL-GHK constructs promoted the greatest amount of bone-specific mineralization per cell in both osteogenic and mixed osteochondral media (Figure 5b) and also achieved the highest level of scaffold mineralization in both media (Figure 5c). Interestingly, PCL-NC also promoted greater mineralization per cell in the mixed osteochondral medium but did not produce statistically significant effects on scaffold mineralization. No scaffolds cultured in chondrogenic medium underwent statistically significant mineralization when normalized to either cellular content or scaffold mass.
PCL-NC produced a large spike in cartilage-specific sGAG deposition per cell by day 28 in chondrogenic medium, whereas PCL-alkyne did not exhibit any changes in cell-normalized sGAG deposition over time (Figure 5d). PCL-NC also maintained a constant level of sGAG deposition per cell in mixed osteochondral medium, in which PCL-alkyne and PCL-GHK both exhibited decreasing amounts of cell-normalized sGAG deposition over time (Figure 5d). PCL-alkyne scaffolds had higher proportions of sGAG content by scaffold mass in both osteogenic and mixed osteochondral media as a result of cell proliferation in these media, but they did not experience significant changes in mass-normalized sGAG content when cultured in serum-free chondrogenic medium (Figure 5e). PCL-NC, on the other hand, demonstrated a statistically significant increase in mass-normalized sGAG content when cultured in chondrogenic medium (Figure 5e).
4. Discussion
Patterning of tissue-specific biochemical cues remains a longstanding goal in tissue engineering research, especially for heterogeneous tissues such as the osteochondral unit. While controlled release has traditionally been used to deliver proteins and other biomolecules, these methods are highly prone to off-site diffusion and loss of spatial localization, compromising their utility for the repair of heterogeneous tissues [4]. While prior methods in the literature have utilized post-processing reactions to covalently tether osteogenic peptides to 3D printed scaffolds, these approaches only allow for homogeneous functionalization of the bulk scaffold, prohibiting any spatial patterning of multiple biochemical cues [5,6]. As an alternative approach, 3D printable inks can instead be pre-functionalized with biochemical cues and then spatially deposited, but very few biomaterial constructs have utilized this approach [8,9]. For instance, Lozano et al. utilized activated ester chemistry to attach cell-adhesive RGD peptides to 3D printable gellan gum for enhanced cell proliferation [9]. However, activated esters are highly reactive towards amines and can thus result in unwanted side reactions and loss of bioactivity for any lysine-containing peptides [25]. Camacho et al. demonstrated a highly promising approach in which PCL-maleimide polymers were conjugated to RGD peptides [8]. However, the maleimide group is similarly reactive towards both amines and thiols, compromising compatibility for lysine- and cysteine-containing peptides [25]. Along these lines, both strategies have only applied the non-tissue-specific peptide sequence of RGD for ink biofunctionalization, and they may also incorporate harsh, cytotoxic solvents such as hexafluoroisopropanol (HFIP) during the printing process [8,9,26]. Ultimately, prior methods for 3D printable ink functionalization are non-bioorthogonal, compatible with a restricted variety of peptides, and require potentially cytotoxic solvents during printing.
To address these needs, we have developed a versatile, bioorthogonal platform for the covalent tethering of tissue-specific peptides to 3D printable PCL, generating PCL-peptide composites that can be spatially patterned by multimaterial segmented printing. Compared to other methods of ink functionalization, our bioconjugation strategy offers well-established bioorthogonality, compatibility with virtually any peptide, and printability by solvent-free melt extrusion [10,11]. We demonstrated these features using a set of peptides of diverse chemical character and biological origin. Specifically, by using the Cp*Ru(cod)Cl-catalyzed RuAAC reaction, we successfully functionalized our polymer system with two hydrophilic and bone-specific peptides, BMPm and GHK, as well as a hydrophobic and cartilage-specific peptide, NC. The successful synthesis of these PCL-peptide composites, as well as their thermal stability after melt extrusion, were verified using GPC and NMR characterization. As shown by NMR, peptides were conjugated to PCL-alkyne with quantitative conversion after 24h of reaction, which is consistent with the click nature of the alkyne-azide cycloaddition reaction [10]. Additional investigation can further optimize the reaction conditions for click conjugation – for instance, by lowering the temperature or duration of reaction. GPC indicated an approximate increase in Mn after click conjugation, as expected from the attachment of peptides to PCL. The decrease in PDI as a result of click conjugation can be attributed to the quantitative conversion of PCL-alkyne to each PCL-peptide, which would cause variability within the molecular weight distribution to become relatively lower in proportion to the increased average molecular weight.
As evidenced by μCT analysis, the PCL-peptide composites retain excellent printability, with reproducible physical architecture under similar printing conditions. The average fiber diameter of each scaffold material, for instance, corresponded roughly to the 210 μm inner diameter of the 27G needles used for extrusion. Furthermore, all scaffold materials produced statistically similar porosity, average fiber diameter, and average pore diameter when printed under conditions that were nearly identical except for a 10 °C lower temperature and 0.2 bar lower extrusion pressure for PCL-alkyne (Figure S1), given its lower molecular weight compared to the PCL-peptides. Following the successful printing of single material scaffolds, we then printed heterogeneous scaffolds with lateral patterning of bone-specific PCL-BMPm and cartilage-specific PCL-NC. The multimaterial segmented printing of multiple peptides within horizontal layers and individual fibers shows potential for generation of complex spatial patterns, and this approach could be readily paired with traditional layer-by-layer vertical patterning to create fully 3D patterns of biochemical cues. Furthermore, fluorescent imaging indicated that the BMPm and NC peptides retained their user-defined stratification, even when these two composites were fused within a single fiber. Thus, the combination of RuAAC bioconjugation with multimaterial segmented printing could be used to pattern a variety of bone-specific, cartilage-specific, and non-osteochondral peptide sequences.
To support the utility of PCL-peptide scaffolds for tissue engineering applications, we investigated the bioactivity of constructs that were functionalized with GHK and NC peptide sequences, which have been demonstrated to promote osteogenesis and chondrogenesis, respectively, in MSCs [12]. PCL-GHK scaffolds consistently promoted bone-specific calcium deposition in both osteogenic medium and mixed osteochondral medium, indicating that the osteogenic effects of PCL-GHK were sufficiently potent to overcome chondrogenic supplements in the mixed osteochondral medium. Prior studies have established concentration-dependent promotion of osteogenesis by the GHK peptide [12], and it can be inferred that a relatively high concentration of GHK is present on these scaffolds given the quantitative conversion of PCL chains to PCL-GHK during click conjugation.
PCL-NC, on the other hand, strongly promoted cartilage-specific sGAG deposition when cultured in chondrogenic medium, but promoted both calcium and sGAG synthetic activity when cultured in mixed osteochondral medium. The N-cadherin protein has been implicated in the developmental processes of both cartilage and bone, and the “HAVDI” peptide sequence conjugated to PCL in this study has primarily been utilized for MSC chondrogenesis under highly chondrogenic culture conditions [15,27,28]. Under osteogenic culture conditions or in the presence of cell-adhesive moieties such as RGD that are found in serum, the “HAVDI” sequence can instead promote MSC osteogenesis by mimicking heterotypic cell-cell interactions [27], offering an explanation for the mixture of osteogenic and chondrogenic results promoted by PCL-NC in mixed osteochondral medium. Further investigation could explore how PCL-NC bioactivity is influenced by co-localization with PCL-GHK or other osteogenic and chondrogenic PCL-peptide composites.
Interestingly, PCL-GHK and PCL-NC scaffolds produced significantly less cell proliferation than PCL-alkyne scaffolds in both osteogenic and mixed osteochondral media, which we speculated could be a result of either mild cytotoxicity of the materials or loss of proliferative capacity from the differentiation of MSCs. Given that PCL-NC scaffolds supported statistically similar levels of cellular content to PCL-alkyne when cultured in the non-proliferative chondrogenic medium, it is much more plausible that these differences result from effects on proliferative capacity, given the established inverse correlation of stem cell differentiation and proliferation ability [29]. Ultimately, 3D printed PCL-peptide scaffolds demonstrated tissue-specific bioactivity in vitro, with differential promotion of osteogenesis or chondrogenesis depending on peptide identity.
While our work has established the suitability of PCL click conjugation for a variety of tissue-specific peptides, further investigation can elucidate how the click conjugation of carbohydrates, such as cartilage-specific sGAGs, and other macromolecules affect printability. While carbohydrates, for instance, demonstrate excellent thermal stability at the relatively moderate temperatures of 85-95 °C utilized during melt extrusion, their larger molecular weight will likely require a similarly larger molecular weight of PCL-alkyne in order to maintain the well-established physical properties and printability of PCL after click conjugation [30]. Furthermore, the covalent immobilization of certain peptides may affect their bioactivity, and further investigation is needed to determine how temporal effects such as scaffold degradation and the solubilization of previously immobilized peptides may affect scaffold bioactivity over time. Increasingly complex strategies may thus consider how the interplay of physical and biochemical properties such as material degradation time, mechanical properties, and biomolecule patterning influence tissue development within these click biofunctionalized scaffolds.
5. Conclusion
To address the need for spatial patterning of biochemical cues, we developed a facile system for the bioconjugation and 3D printing of PCL with tissue-specific peptides. PCL-peptide composites were successfully synthesized by the aqueous click conjugation of peptides with diverse chemical character and biological function. Following this, the composite materials could be reproducibly fabricated as single material scaffolds or as heterogeneous scaffolds with user-defined spatial patterning of peptides, by using multimaterial segmented printing. PCL-peptide scaffolds demonstrate strong in vitro bioactivity, with the ability to promote either osteogenic or chondrogenic ECM deposition by MSCs depending on the identity of conjugated peptide. Ultimately, this method of polymer click functionalization represents a versatile platform for the spatial patterning of tissue-specific biochemical cues and can offer substantial utility for the osteochondral unit and other heterogeneous tissues.
Supplementary Material
Acknowledgments:
We acknowledge support by the National Institutes of Health (R01 AR068073, P41 EB023833). L.D.-G. acknowledges support from the Consellería de Cultura, Educación e Ordenación Universitaria for a Postdoctoral Fellowship (Xunta de Galicia, ED481B 2017/063). S.M.B. acknowledges support from the National Science Foundation Graduate Research Fellowship Program.
References:
- [1].D’Ambrosi R, Ragone V, Ursino N, What future in the treatment of osteochondral knee defects?, Annals of Translational Medicine. 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Di Luca A, Van Blitterswijk C, Moroni L, The osteochondral interface as a gradient tissue: from development to the fabrication of gradient scaffolds for regenerative medicine, Birth Defects Research Part C: Embryo Today: Reviews. 105 (2015) 34–52. [DOI] [PubMed] [Google Scholar]
- [3].Bittner SM, Guo JL, Melchiorri A, Mikos AG, Three-dimensional printing of multilayered tissue engineering scaffolds, Materials Today. 21 (2018) 861–874. 10.1016/j.mattod.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bittner SM, Guo JL, Mikos AG, Spatiotemporal control of growth factors in three-dimensional printed scaffolds, Bioprinting. 12 (2018) e00032. 10.1016/j.bprint.2018.e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saska S, Pires LC, Cominotte MA, Mendes LS, de Oliveira MF, Maia IA, da Silva JVL, Ribeiro SJL, Cirelli JA, Three-dimensional printing and in vitro evaluation of poly (3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering, Materials Science and Engineering: C. 89 (2018) 265–273. [DOI] [PubMed] [Google Scholar]
- [6].Li S, Xu Y, Yu J, Becker ML, Enhanced osteogenic activity of poly (ester urea) scaffolds using facile post-3D printing peptide functionalization strategies, Biomaterials. 141 (2017) 176–187. [DOI] [PubMed] [Google Scholar]
- [7].Guo JL, Kim YS, Mikos AG, Biomacromolecules for Tissue Engineering: Emerging Biomimetic Strategies, Biomacromolecules. 20 (2019) 2904–2912. 10.1021/acs.biomac.9b00792. [DOI] [PubMed] [Google Scholar]
- [8].Camacho P, Busari H, Seims KB, Schwarzenberg P, Dailey HL, Chow LW, 3D printing with peptide–polymer conjugates for single-step fabrication of spatially functionalized scaffolds, Biomaterials Science. 7 (2019) 4237–4247. [DOI] [PubMed] [Google Scholar]
- [9].Lozano R, Stevens L, Thompson BC, Gilmore KJ, Gorkin III R, Stewart EM, in het Panhuis M, Romero-Ortega M, Wallace GG, 3D printing of layered brain-like structures using peptide modified gellan gum substrates, Biomaterials. 67 (2015) 264–273. [DOI] [PubMed] [Google Scholar]
- [10].Destito P, Couceiro JR, Faustino H, López F, Mascareñas JL, Ruthenium-Catalyzed Azide–Thioalkyne Cycloadditions in Aqueous Media: A Mild, Orthogonal, and Biocompatible Chemical Ligation, Angewandte Chemie. 129 (2017) 10906–10910. 10.1002/ange.201705006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo JL, Kim YS, Xie VY, Smith BT, Watson E, Lam J, Pearce HA, Engel PS, Mikos AG, Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering, Science Advances. 5 (2019) eaaw7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guo JL, Li A, Kim YS, Xie VY, Smith BT, Watson E, Bao G, Mikos AG, Click functionalized, tissue-specific hydrogels for osteochondral tissue engineering, Journal of Biomedical Materials Research Part A. 108 (2020) 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kanie K, Kurimoto R, Tian J, Ebisawa K, Narita Y, Honda H, Kato R, Screening of osteogenic-enhancing short peptides from BMPs for biomimetic material applications, Materials. 9 (2016) 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Klontzas ME, Reakasame S, Silva R, Morais JC, Vernardis S, MacFarlane RJ, Heliotis M, Tsiridis E, Panoskaltsis N, Boccaccini AR, Oxidized alginate hydrogels with the GHK peptide enhance cord blood mesenchymal stem cell osteogenesis: A paradigm for metabolomics-based evaluation of biomaterial design, Acta Biomaterialia. 88 (2019) 224–240. [DOI] [PubMed] [Google Scholar]
- [15].Bian L, Guvendiren M, Mauck RL, Burdick JA, Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis, Proceedings of the National Academy of Sciences. 110 (2013) 10117–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aulisa L, Dong H, Hartgerink JD, Self-assembly of multidomain peptides: sequence variation allows control over cross-linking and viscoelasticity, Biomacromolecules. 10 (2009) 2694–2698. [DOI] [PubMed] [Google Scholar]
- [17].Tasdelen MA, Poly (epsilon-caprolactone)/clay nanocomposites via “click” chemistry, European Polymer Journal. 47 (2011) 937–941. [Google Scholar]
- [18].Diaz-Gomez L, Smith BT, Kontoyiannis PD, Bittner SM, Melchiorri AJ, Mikos AG, Multimaterial segmented fiber printing for gradient tissue engineering, Tissue Engineering Part C: Methods. 25 (2019) 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith BT, Bittner SM, Watson E, Smoak MM, Diaz-Gomez L, Molina ER, Kim YS, Hudgins CD, Melchiorri AJ, Scott DW, Multimaterial dual gradient three-dimensional printing for osteogenic differentiation and spatial segregation, Tissue Engineering Part A. 26 (2020) 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bittner SM, Smith BT, Diaz-Gomez L, Hudgins CD, Melchiorri AJ, Scott DW, Fisher JP, Mikos AG, Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering, Acta Biomaterialia. 90 (2019) 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vo TN, Ekenseair AK, Spicer PP, Watson BM, Tzouanas SN, Roh TT, Mikos AG, In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering, Journal of Controlled Release. 205 (2015) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee P, Tran K, Chang W, Fang Y-L, Zhou G, Junka R, Shelke NB, Yu X, Kumbar SG, Bioactive polymeric scaffolds for osteochondral tissue engineering: in vitro evaluation of the effect of culture media on bone marrow stromal cells, Polymers for Advanced Technologies. 26 (2015) 1476–1485. [Google Scholar]
- [23].Chia HN, Wu BM, Recent advances in 3D printing of biomaterials, Journal of Biological Engineering. 9 (2015) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Diaz-Gomez L, Kontoyiannis PD, Melchiorri AJ, Mikos AG, Three-dimensional printing of tissue engineering scaffolds with horizontal pore and composition gradients, Tissue Engineering Part C: Methods. 25 (2019) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McKay CS, Finn MG, Click chemistry in complex mixtures: bioorthogonal bioconjugation, Chemistry & Biology. 21 (2014) 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Capone R, Quiroz FG, Prangkio P, Saluja I, Sauer AM, Bautista MR, Turner RS, Yang J, Mayer M, Amyloid-β-induced ion flux in artificial lipid bilayers and neuronal cells: resolving a controversy, Neurotoxicity Research. 16 (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhu M, Lin S, Sun Y, Feng Q, Li G, Bian L, Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche, Biomaterials. 77 (2016) 44–52. [DOI] [PubMed] [Google Scholar]
- [28].Xu L, Meng F, Ni M, Lee Y, Li G, N-cadherin regulates osteogenesis and migration of bone marrow-derived mesenchymal stem cells, Molecular Biology Reports. 40 (2013) 2533–2539. [DOI] [PubMed] [Google Scholar]
- [29].Ruijtenberg S, van den Heuvel S, Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression, Cell Cycle. 15 (2016) 196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tomasik P, Pałasiński M, Wiejak S, The thermal decomposition of carbohydrates. Part I. The decomposition of mono-, di-, and oligo-saccharides, in: Advances in Carbohydrate Chemistry and Biochemistry, Elsevier, 1989: pp. 203–278. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


