Abstract
Context
The use of radioactive iodine (RAI) for low-risk thyroid cancer is common, and variation in its use exists, despite the lack of benefit for low-risk disease and potential harms and costs.
Objective
To simultaneously assess patient- and physician-level factors associated with patient-reported receipt of RAI for low-risk thyroid cancer.
Methods
This population-based survey study of patients with newly diagnosed differentiated thyroid cancer identified via the Surveillance Epidemiology and End Results (SEER) registries of Georgia and Los Angeles County included 989 patients with low-risk thyroid cancer, linked to 345 of their treating general surgeons, otolaryngologists, and endocrinologists. We assessed the association of physician- and patient-level factors with patient-reported receipt of RAI for low-risk thyroid cancer.
Results
Among this sample, 48% of patients reported receiving RAI, and 23% of their physicians reported they would use RAI for low-risk thyroid cancer. Patients were more likely to report receiving RAI if they were treated by a physician who reported they would use RAI for low-risk thyroid cancer compared with those whose physician reported they would not use RAI (adjusted OR: 1.84; 95% CI, 1.29-2.61). The odds of patients reporting they received RAI was 55% lower among patients whose physicians reported they saw a higher volume of patients with thyroid cancer (40+ vs 0-20) (adjusted OR: 0.45; 0.30-0.67).
Conclusions
Physician perspectives and attitudes about using RAI, as well as patient volume, influence RAI use for low-risk thyroid cancer. Efforts to reduce overuse of RAI in low-risk thyroid cancer should include interventions targeted toward physicians, in addition to patients.
Keywords: decision making, thyroid cancer, overtreatment, radioactive iodine
Thyroid cancer is one of the most rapidly rising cancers in the United States, mostly due to increased detection of small, low-risk tumors. In recent years clinical guidelines have evolved regarding the use of radioactive iodine (RAI) as part of the initial treatment of low-risk disease. This change has been in response to the low risk of recurrence (3%-10%) (1, 2), its limited benefit in this context, the potential harms, and growing evidence of its overuse (1, 3). However, RAI use in low-risk disease still remains common, despite being either not recommended or only selectively recommended by clinical guidelines, and notable variation in its use persists (4-6).
Strategies to reduce the overtreatment of low-risk thyroid cancer with RAI are needed, given its limited benefit and associated costs and side effects, including damage to salivary glands and lacrimal ducts (7). Prior research suggests that patient factors influence the receipt of RAI, including age, race/ethnicity, and worry about death, as well as clinical characteristics such as tumor size, stage, and histology (8, 9). Previously, we found that many patients with thyroid cancer feel they do not have a choice about whether or not to receive RAI, and this perception is greatest among those whose physician strongly recommended RAI (10). Physician perceptions about thyroid cancer treatment decision-making likely also play a significant role in the use of more intensive treatment for low-risk disease. Patient volume, physician specialty, and training all have been found to correlate with treatment intensity, and surgeons who favor greater extent of surgery also favor more RAI use for low-risk disease (11, 12). Thyroid cancer management also requires the involvement of multiple physician specialties, and prior research suggests who the primary physician decision maker is also influences the use of RAI in low-risk disease (13). Designing effective interventions to reduce the use of more intensive treatment in low-risk thyroid cancer may therefore require targeting interventions toward both the physician and patient levels. However, to date, studies in thyroid cancer have focused solely on individually assessing patient or physician factors that drive overuse or more intensive treatment (13-15).
The interplay between physicians and patients in determining RAI use remains understudied, yet is critical to tailoring appropriate care, and to designing and tailoring interventions to reduce the overuse of RAI in the context of low-risk thyroid cancer. Therefore, we assessed the association of physician-level factors, including physician-reported propensity to use RAI for low-risk thyroid cancer, and patient-level factors, with patient-reported receipt of RAI in a large and diverse population-based sample of patients with low-risk differentiated thyroid cancer and their treating endocrinologists and surgeons.
Methods
Study Population
Patients
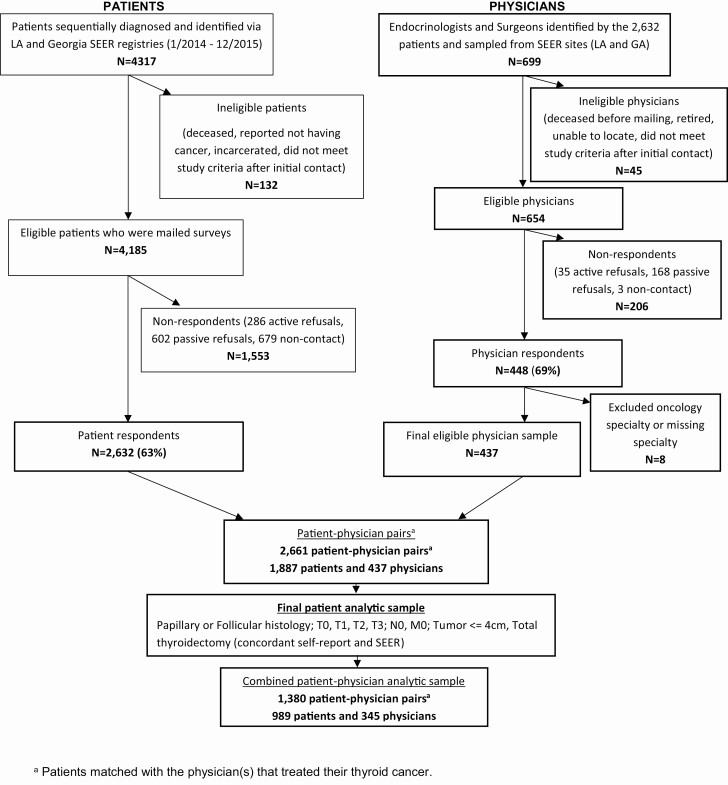
As part of a large and diverse population-based survey study of patients with differentiated thyroid cancer (papillary, follicular, Hürthle cell), we identified patients with incident thyroid cancer aged 18 to 79 years, as reported to the Surveillance Epidemiology and End Results (SEER) registries of Georgia and Los Angeles County from January 1, 2014 to December 31, 2015. Patients completed surveys in 2017-2018 about their treatment experiences. A modified Dillman method was used to encourage response, which included follow-up phone calls, tracing, and remailing of materials, and an unconditional $20 cash incentive (16). All materials were sent in both English and Spanish to those with Spanish surnames. Responses to the survey were merged with clinical cancer information from the SEER registries to create a de-identified patient analytic dataset. Of the 4317 patients identified, 4185 were eligible and mailed a survey and 2632 responded (63% response rate) (10) (Fig. 1).
Figure 1.
Flow diagram illustrating cohort selection.
Physicians
Patient participants were asked to identify the endocrinologists and surgeons involved in their thyroid cancer management. Of the 699 physicians identified, 45 were ineligible due to retirement, unable to be located, deceased prior to the initial mailing, or did not meet study screening criteria. All physicians identified by more than one patient (N = 482) and a random sample of those identified by only one patient (N = 172) were sent surveys in 2018-2019. To enhance response rates, a $50 incentive was included in the initial mailing and nonresponders were followed up with phone, fax, or email. Of the 448 respondents (69% response rate), 8 were later excluded due to missing or ineligible specialty information (ie, oncology), resulting in an eligible physician sample of 437 physicians (Fig. 1). A waiver of written informed consent was obtained for all subjects. Both survey studies were approved by the institutional review board of the University of Michigan, University of Southern California, Emory University, the California Committee for the Protection of Human Subjects, the California Cancer Registry, and the Georgia Department of Public Health.
Combined patient-physician dataset
The physician data was merged with the patient data, resulting in 2661 patient-physician pairs. Physicians were not asked about treatment of specific patients, nor were the participating patients identified to the physicians. The analytic sample was selected as follows: Patients with follicular or papillary histology, who had tumor sizes ≤ 4 cm, T0, T1, T2, or T3 disease, and no evidence of gross extrathyroidal extension, lymph node or distant metastases (N0, M0) were eligible for inclusion. We further limited the analytic sample to those who received a total thyroidectomy, where self-reported surgical treatment was concordant with the SEER registry treatment data (as was the case for 89%). The inclusion criteria were chosen to include only patients in whom either no RAI or selective RAI use was recommended by the 2009 American Thyroid Association recommendations for RAI use (the clinical standards at the time of diagnosis) (3). The final analytic sample includes 989 patients linked to 345 physicians (Fig. 1).
Measures
The questionnaire content was informed by a conceptual framework, our hypotheses, and our prior work in other favorable prognosis cancers, including thyroid cancer (10, 17-24).
Patient-Reported Receipt of RAI
Patient-reported receipt of RAI was defined by asking patients, “If you had radioactive iodine, you may remember eating a low-iodine diet, becoming hypothyroid or getting a shot of Thyrogen in your buttocks, and then seeing a doctor who gave you a radioactive pill to swallow. Did you receive radioactive iodine after your thyroid surgery (yes/no)?”
Physician Propensity to Use RAI for Very-Low-Risk and Low-Risk Thyroid Cancer
Physician propensity (or intention) to use RAI for low-risk thyroid cancer was ascertained via the following clinical vignette: “A 65-year-old female patient underwent total thyroidectomy for a 1.5-cm papillary thyroid cancer with no extrathyroidal extension, no known lymph node metastases, and no vascular invasion.” Physicians were then asked the following, “How likely are you to recommend radioactive iodine treatment for this patient,” with response categories of extremely unlikely, unlikely, likely, and extremely likely. Physician propensity to use RAI was then categorized into would use (likely/extremely likely) versus would not use (extremely unlikely, unlikely) for analyses. Physicians were also presented with a clinical vignette for a very-low-risk thyroid cancer (a 0.9-cm tumor), and the proportion who reported they would use RAI was low (8%), limiting our ability to draw meaningful conclusions about this clinical scenario.
Covariates
Patient and physician-level demographics collected via survey included sex (male/female), race (White, Non-White/Multiracial), ethnicity (Hispanic/Non-Hispanic). Physician-level covariates included years in practice (1-9, 10-19, 20-29, 30+), practice setting (private/other), number of patients with thyroid cancer in the past 12 months (0-20, 21-40, 40+), and specialty (endocrinology, general surgery/otolaryngology). Patient-level covariates included educational attainment (less than high school, high school graduate, some college or more), insurance status (private, other insurance), number of comorbidities (0, 1, and 2 or more) and worry about thyroid cancer (somewhat/quite a bit/a lot vs never/a little) (25). Patient characteristics collected from SEER included age at diagnosis (years), and thyroid cancer clinical characteristics such as tumor size, histology, and stage at diagnosis (26).
Statistical analyses
We first evaluated the bivariate distributions of patient-reported receipt of RAI across levels of patient-level characteristics, and physician propensity to use RAI for low-risk thyroid cancer across physician-level characteristics, using Rao-Scott chi-square tests. We then estimated the bivariate association of patient-reported receipt of RAI by physician-reported propensity to use RAI for low-risk thyroid cancer, stratified by tumor size using Rao-Scott chi-square tests. The associations of patient-level and physician-level characteristics with patient-reported receipt of RAI were then estimated using multivariable, weighted logistic regression, accounting for the clustering of patients within physicians, and linearized standard errors. Sensitivity analyses excluding those remaining patients diagnosed with T3 disease (N = 94) in whom RAI would have been selectively recommended were conducted to confirm the robustness of our results.
All statistical analyses incorporated weights to allow our statistical inference to be more representative of the target population and to reduce potential bias as a result of nonresponse. This included the use of design weights to account for differential probability of patient sample selection and nonresponse weights to account for disproportionate nonresponse rates across different patient subgroups. We also included physician nonresponse weights and performed an adjustment so that the weights summed to the effective sample size (27). All analyses were performed using STATA, and two-sided tests, and a P value of < 0.05 was considered statistically significant.
Results
Table 1 displays the distributions of patient-level characteristics by patient-reported receipt of RAI. In this sample, 48% of patients reported receiving RAI, with 34% receiving 1 dose, 12% receiving 2 doses, and 3.5% receiving 3 or more doses. No significant differences in the receipt of RAI were seen across the patient demographic characteristics. Tumor size was positively associated with receipt of RAI, with a greater proportion of patients with tumors of 2 to 4 cm reporting they received RAI (80%), compared with patients with tumor sizes ≤ 1 cm (26%) (P < 0.001). A greater proportion of patients with follicular histology, while comprising only 3% of the overall sample, reported receiving RAI (87%), compared with those with papillary histology (47%) (P < 0.001) (Table 1).
Table 1.
Patient Demographic and Clinical Characteristics by Patient Receipt of Radioactive Iodine (RAI) (N = 989)
| N (%) | Received RAI N (%) |
Did Not Receive RAI N (%) |
P value | |
|---|---|---|---|---|
| Sex | 0.422 | |||
| Male | 170 (16.7) | 88 (51.0) | 82 (49.0) | |
| Female | 819 (83.3) | 391 (47.6) | 428 (52.4) | |
| Age at diagnosis | 0.832 | |||
| 18–44 | 326 (36.1) | 159 (48.5) | 167 (51.5) | |
| 45–54 | 247 (24.0) | 124 (49.6) | 123 (50.4) | |
| 55–64 | 229 (21.9) | 110 (48.2) | 119 (51.8) | |
| 65–79 | 187 (18.0) | 86 (45.1) | 101 (54.9) | |
| Race | 0.355 | |||
| White | 703 (71.9) | 332 (46.9) | 371 (53.1) | |
| Non-White/Multiracial | 226 (28.1) | 115 (50.5) | 111 (49.5) | |
| Ethnicity | 0.634 | |||
| Hispanic | 135 (15.7) | 69 (50.1) | 66 (49.9) | |
| Non-Hispanic | 809 (84.3) | 388 (47.8) | 421 (52.2) | |
| Education | 0.594 | |||
| High school and below | 208 (21.0) | 106 (50.6) | 102 (49.4) | |
| Some college | 297 (30.2) | 149 (49.4) | 148 (50.6) | |
| College degree and above | 476 (48.8) | 222 (46.7) | 254 (53.3) | |
| Health insurance | 0.364 | |||
| Private | 722 (74.7) | 344 (47.3) | 378 (52.7) | |
| Other | 246 (25.3) | 125 (50.7) | 121 (49.3) | |
| Tumor size | <0.001 | |||
| ≤ 1cm | 460 (47.4) | 118 (25.7) | 342 (74.3) | |
| 1–2 cm | 316 (31.6) | 192 (60.6) | 124 (39.4) | |
| 2–4 cm | 213 (21.0) | 169 (79.9) | 44 (20.1) | |
| Histology | <0.001 | |||
| Papillary | 959 (97.0) | 453 (46.9) | 506 (53.1) | |
| Follicular | 30 (3.0) | 26 (87.3) | 4 (12.7) | |
| AJCC-7 T | <0.001 | |||
| T1 | 709 (71.8) | 261 (36.3) | 448 (63.7) | |
| T2 | 186 (18.2) | 144 (78.1) | 42 (21.9) | |
| T3 | 94 (10.0) | 74 (77.9) | 20 (22.1) | |
| Number of comorbidities | 0.463 | |||
| 0 | 538 (55.7) | 270 (49.9) | 268 (50.1) | |
| 1 | 289 (28.5) | 134 (46.3) | 155 (53.7) | |
| 2 or more | 162 (15.8) | 75 (45.3) | 87 (54.7) | |
| Worry about thyroid cancer | 0.006 | |||
| Less worry | 316 (32.6) | 135 (28.3) | 181 (36.7) | |
| More worry | 645 (67.4) | 332 (71.7) | 313 (63.3) | |
| Site | 0.075 | |||
| Georgia | 587 (58.9) | 299 (50.5) | 288 (49.5) | |
| Los Angeles County | 402 (41.1) | 180 (44.7) | 222 (55.3) |
Table 2 displays the distribution of physician-level characteristics by physician-reported propensity to use RAI for low-risk thyroid cancer. Overall, 23% of physicians reported they would use RAI for low-risk disease. No significant differences in the distribution of the physician demographics were seen when comparing those who reported they would use RAI versus those who reported they would not use RAI for low-risk thyroid cancers. However, a greater proportion of physicians who were in private practice (30%) reported they would use RAI compared with those in other practice settings (14%) (P < 0.001). The volume of patients with thyroid cancer was strongly and inversely correlated with physician propensity to use RAI, with only 8% of physicians who saw more than 40 patients in the past year reporting they would use RAI, compared with 32% of those who saw fewer (0 to 20) patients (P < 0.001). A greater proportion of general surgeons/otolaryngologists (29%) reported they would use RAI compared with endocrinologists (15%) (P = 0.004) (Table 2).
Table 2.
Physician Characteristics by Physician Propensity to Use Radioactive Iodine (RAI) for Low-Risk Thyroid Cancer (N = 345)
| N (%) | Would not use RAI N (%) |
Would use RAI N (%) |
P value | |
|---|---|---|---|---|
| Sex | 0.109 | |||
| Male | 242 (70.4) | 178 (74.6) | 61 (25.4) | |
| Female | 100 (29.6) | 82 (82.7) | 17 (17.3) | |
| Race | 0.537 | |||
| White | 216 (65.6) | 165 (78.0) | 47 (22.0) | |
| Non-White/Multiracial | 116 (34.4) | 87 (74.9) | 29 (25.1) | |
| Ethnicity | 0.774 | |||
| Hispanic | 11 (3.6) | 9 (80.0) | 2 (20.0) | |
| Non-Hispanic | 304 (96.4) | 229 (76.2) | 72(23.8) | |
| Years in practice | 0.458 | |||
| 1–9 years | 65 (19.3) | 54 (82.8) | 11 (17.2) | |
| 10–19 years | 116 (34.3) | 89 (78.2) | 25 (21.8) | |
| 20–29 years | 95 (28.0) | 70 (74.3) | 24 (25.7) | |
| 30 or more years | 64 (18.4) | 45 (71.9) | 18 (28.1) | |
| Practice setting | <0.001 | |||
| Private practice | 186 (54.3) | 128 (69.6) | 56 (30.4) | |
| Other | 159 (45.7) | 135 (86.4) | 22 (13.6) | |
| Number of patients with thyroid cancer | <0.001 | |||
| 0–20 | 173 (50.3) | 115 (67.6) | 55 (32.4) | |
| 21–40 | 76 (22.2) | 61 (79.9) | 15 (20.1) | |
| More than 40 | 92 (27.5) | 84 (91.6) | 8 (8.4) | |
| Specialty | 0.004 | |||
| General Surgery/Otolaryngology | 199 (56.1) | 139 (71.1) | 56 (28.9) | |
| Endocrinology | 146 (43.9) | 124 (84.9) | 22 (15.1) | |
| Site | 0.204 | |||
| Georgia | 162 (50.1) | 119 (74.3) | 42 (25.7) | |
| Los Angeles County | 183 (49.9) | 144 (80.2) | 36 (19.8) |
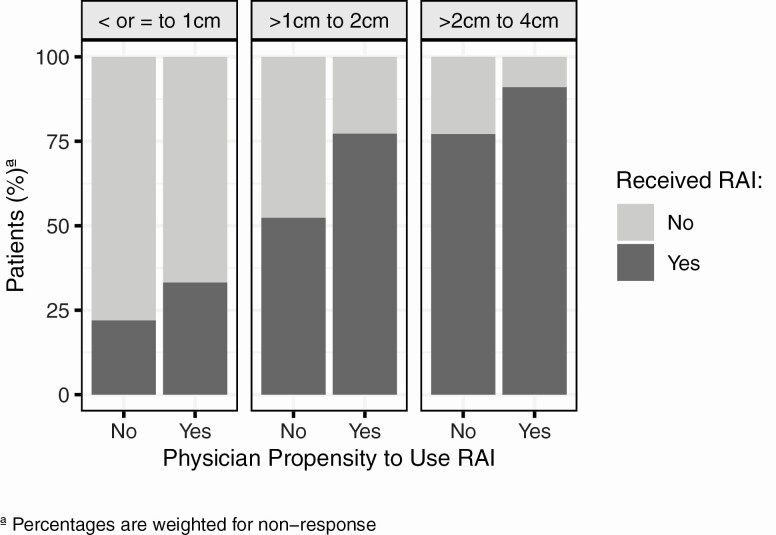
Figure 2 displays the association between physician propensity to use RAI for low-risk thyroid cancer and patient receipt of RAI, stratified by tumor size. A greater proportion of patients whose physicians reported they would use RAI for low-risk thyroid cancer reported receiving RAI, compared with those whose physicians reported they would not use it, and this association was consistent across tumor size. Among patients with 1- to 2-cm tumors, 77% of patients whose physicians reported they would use RAI reported they received RAI versus 52% of patients whose physicians reported they would not use RAI. For 2- to 4-cm tumors, the association was similar, albeit more patients received RAI, and the difference by physician propensity to use RAI was slightly attenuated (91% vs 77%). Similar trends were seen for the very-low-risk thyroid cancer scenario (tumor size 0.9 cm) (data not shown). While we would expect a difference in the 1- to 2-cm tumor size, which is consistent with the vignette presented in the survey, the persistent trend across tumor size, and similar findings in the very-low-risk vignette suggests that physician-reported propensity to use RAI in the 1.5-cm scenario is reflective of their underlying propensity to use RAI for very-low-risk and low-risk thyroid cancer more broadly as well.
Figure 2.
Relationship between physician propensity to use radioactive iodine (RAI) for low-risk thyroid cancer and patient-reported receipt of RAI stratified by patient tumor size ≤1 cm, >1 to 2 cm, and >2 to 4 cm.
Table 3 displays the adjusted associations between physician and patient-level characteristics with patient-reported receipt of RAI. Patients whose physicians reported they would use RAI for low-risk thyroid cancer were more likely to report receiving RAI compared with those whose physician reported they would not use RAI (adjusted OR: 1.84; 95% CI, 1.29-2.61). The odds of patients reporting they received RAI was 55% lower among patients whose physicians reported they saw a higher volume of thyroid cancer patients (40+ vs 0-20) (adjusted OR: 0.45; 0.30-0.67). Among the patient-level characteristics, only tumor size was significantly associated with patient receipt of RAI. Patients with a tumor of 2 to 4 cm had a 17-fold greater odds of reporting they received RAI compared to those with a tumor of ≤ 1 cm (adjusted OR: 17.63; 11.45-27.16). Similar associations were seen between physician-reported propensity to use RAI for very-low-risk thyroid cancer (tumor size 0.9 cm) with patient-reported receipt of RAI (data not shown), albeit given the small proportion of physicians who would use RAI for a 0.9-cm cancer, the association was not statistically significant (adjusted OR: 1.25; 95% CI, 0.66-2.39). When excluding the remaining patients with T3 disease (ie, patients with minimal extrathyroidal extension), the associations were very similar (results not shown).
Table 3.
Adjusted Odds Ratio (95% CI) of the Associations of Patient and Physician-level Characteristics With Patient Receipt of RAI for Low-Risk Thyroid Cancer
| Adjusted OR | 95% CI | |
|---|---|---|
| PHYSICIAN CHARACTERISTICS | ||
| Physician propensity to use RAI for low-risk thyroid cancer | ||
| Would not use it | 1.0 | ref |
| Would use it | 1.84 | 1.29–2.61 |
| Practice setting | ||
| Private Practice | 1.0 | ref |
| Other | 0.73 | 0.51–1.06 |
| Number of patients with thyroid cancer | ||
| 0–20 | 1.0 | ref |
| 21–40 | 0.62 | 0.40–0.96 |
| More than 40 | 0.45 | 0.30–0.67 |
| Specialty | ||
| Endocrinology | 1.0 | ref |
| General Surgery/Otolaryngology | 0.71 | 0.51–0.99 |
| PATIENT CHARACTERISTICS | ||
| Sex | ||
| Female | 1.0 | ref |
| Male | 0.96 | 0.66–1.39 |
| Age at diagnosis | ||
| 18–44 | 1.0 | ref |
| 45–54 | 1.69 | 1.16–2.45 |
| 55–64 | 1.21 | 0.81–1.81 |
| 65–79 | 1.00 | 0.60–1.64 |
| Race | ||
| White | 1.0 | ref |
| Non-White/Multiracial | 0.96 | 0.66–1.38 |
| Ethnicity | ||
| Hispanic | 1.0 | ref |
| Non-Hispanic | 0.91 | 0.55–1.49 |
| Education | ||
| High school and below | 1.0 | ref |
| Some college | 1.20 | 0.74–1.95 |
| College degree and above | 0.88 | 0.56–1.37 |
| Health insurance | ||
| Private | 1.0 | ref |
| Other | 1.10 | 0.80–1.51 |
| Tumor size | ||
| ≤ 1cm | 1.0 | ref |
| 1–2 cm | 5.83 | 4.10–8.28 |
| 2–4 cm | 17.63 | 11.45–27.16 |
| Number of comorbidities | ||
| 0 | 1.0 | ref |
| 1 | 0.82 | 0.62–1.10 |
| 2 or more | 1.03 | 0.71–1.49 |
| Worry about thyroid cancer | ||
| Less worry | 1.0 | ref |
| More worry | 1.27 | 0.94–1.71 |
Abbreviations: OR, odds ratio; RAI, radioactive iodine.
Discussion
In this population-based sample of patients with low-risk thyroid cancer and their treating physicians, the use of RAI was common, with almost half of the patients reporting they received it. Patient receipt of RAI was associated with tumor size, physician-reported propensity to use RAI for low-risk thyroid cancer, and inversely related to physician-reported volume of patients with thyroid cancer. These findings suggest that in addition to tumor characteristics, physicians are an important driver of the use of more intensive treatments for low-risk thyroid cancer.
To our knowledge, this study is the first to characterize that physician perspectives and attitudes about using RAI for low-risk thyroid cancer independently influence patient receipt of RAI. This study, which uniquely assessed both patient and physician factors that influence RAI use, is both timely and necessary, given the ongoing costs and potential harms associated with the overuse of RAI for low-risk thyroid cancer. These findings extend upon our prior work which found that many patients with thyroid cancer feel that they did not have a choice about whether or not to receive RAI, and that those who reported their physicians strongly recommended RAI were most likely to receive it (10). It also builds upon our prior findings that the specialty of the primary physician decision maker influences RAI use in hospital settings (13). Our findings also align with existing evidence from other low-risk cancers such as breast and prostate cancer, that support the notion that physicians are a key influencer in the use of more intensive cancer treatments (21, 23, 28, 29).
Patients with treating physicians who saw a higher volume of patients with thyroid cancer were also less likely to report they received RAI in our sample. More experience with managing low-risk thyroid cancer may lead to increased physician understanding that patients with low-risk disease often do well with less intensive treatment. This is consistent with findings from other cancers, as volume is known to be strongly correlated with the delivery of guideline-concordant cancer treatment and care (30–32). Therefore, interventions targeted toward reducing more intensive use of RAI for low-risk thyroid cancer management should not only be targeted toward patients, but also their physicians.
While there was little to no variation seen across the patient-level demographic characteristics in this diverse sample, increasing tumor size was strongly and independently associated with a greater likelihood of receiving RAI. This aligns with the American Thyroid Association clinical guidelines, where RAI is not recommended for use in tumors <1 cm but is recommended for selective use in tumor sizes of 1 to 4 cm for patients with other risk factors for recurrence or death (1, 3). Additionally, when we descriptively assessed the concordance of physician propensity to use RAI for very-low- and low-risk thyroid cancer, our results suggested different preference thresholds for RAI use. Virtually all physicians who would treat a 0.9-cm cancer with RAI would also treat a 1.5-cm cancer with RAI, whereas just a third of those who would treat a 1.5-cm cancer with RAI would treat a 0.9-cm cancer with RAI. This highlights the propensity of some physicians to more intensively treat thyroid cancer, despite guidelines moving toward less intensive use of RAI for these tumor sizes. Therefore, efforts to increase the dissemination of the current treatment guidelines to physicians who treat patients with thyroid cancer, and physician-targeted interventions that support the de-implementation of inappropriate RAI use may help to reduce the overuse of RAI in low-risk thyroid cancer. In addition, because some content from current guidelines is based on insufficient or conflicting data, additional well-designed randomized trials are necessary, and multiple trials are currently in process for low-risk differentiated thyroid cancer.
While this population-based study of patients with thyroid cancer and their treating physicians provides unique insight into both physician- and patient-level factors that influence more intensive use of RAI for low-risk disease, there are potential limitations to consider. First, as patients were surveyed 2 to 4 years after initial treatment, their recall of their initial treatment may be subject to recall bias. However, prior studies suggest that the emotionally-charged nature of cancer increases the validity of recall about treatment experiences (33–35), and RAI receipt includes multiple memorable steps, including a low-iodine diet and either becoming hypothyroid or receiving a shot of recombinant thyroid stimulating hormone. Our prior work in this cohort also found that when compared with SEER-reported RAI use, patient-report was highly concordant (10). Second, physicians in this study were not asked about specific patients they treated, but instead were given 2 clinical scenarios. Therefore, physician propensity to use RAI does not necessarily reflect what they actually recommend to individual patients, but rather captures their general intention or perspectives on using RAI in these clinical contexts. Third, it is possible that other factors, such as microscopic details of the tumor or other characteristics of progression or recurrence, which were not available in this study may have influenced physicians’ treatment recommendations. However, only 1.8% of patients in this sample experienced recurrence, thus the potential for this to bias our results is limited. Fourth, because patients were diagnosed in 2014-2015 and physicians were surveyed a few years later, there is the potential for treatment recommendations to change with time and thus influence physician perspectives on the use of RAI. It is also unknown whether the patients in this sample received different recommendations from their surgeon and endocrinologist (13). However, the guidelines have become more conservative regarding their recommendations about using RAI in the clinical scenarios presented, thus current use of RAI would be an even clearer case of more intensive treatment. Finally, this study only included participants from 2 geographical areas of the United States. However, the diverse and representative patient cohort, and the multispecialty physician cohort, which included a diverse representation across practice settings, strengthen the generalizability to other settings.
Conclusions
Our findings suggest that RAI use in low-risk thyroid cancer is relatively common, and that use was independently influenced by patient volume, as well as physician propensity to use RAI for low-risk thyroid cancer, after adjustment for patient characteristics. Therefore, increased dissemination of clinical guidelines to clarify appropriate use and promote adherence, and the development of interventions targeted toward physicians may help to reduce the overuse of RAI for low-risk thyroid cancer.
Acknowledgments
Financial Support: This study is supported by the National Cancer Institute (NCI) Grant No. R01 CA201198 to Principal Investigator, Dr. Megan Haymart. Dr. Haymart also receives funding from R01 HS024512 from the Agency for Healthcare Research and Quality (AHRQ). Dr. Wallner’s time was also supported by NCI K07 CA201052, and Dr. Lubitz’s time by NCI R37 CA231957. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under Cooperative Agreement No. 5NU58DP003862-04/DP003862; the NCI’s SEER Program under Contract No. HHSN261201000035C awarded to the University of Southern California. The collection of cancer incidence data in Georgia was supported by Contract No. HHSN261201800003I, Task Order No. HHSN26100001 from the NCI, and Cooperative Agreement No. 5NU58DP003875-04 from the CDC. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California and State of Georgia Departments of Public Health, the NCI, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred.
Glossary
Abbreviations
- RAI
radioactive iodine
- SEER
Surveillance, Epidemiology, and End Results
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper DS, Doherty GM, Haugen BR, et al. ; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer . Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214. [DOI] [PubMed] [Google Scholar]
- 4. Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. Jama. 2011;306(7):721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haymart MR, Banerjee M, Yang D, et al. Variation in the management of thyroid cancer. J Clin Endocrinol Metab. 2013;98(5):2001-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park KW, Wu JX, Du L, Leung AM, Yeh MW, Livhits MJ. Decreasing use of radioactive iodine for low-risk Thyroid Cancer in California, 1999 to 2015. J Clin Endocrinol Metab. 2018;103(3):1095-1101. [DOI] [PubMed] [Google Scholar]
- 7. Singer MC, Marchal F, Angelos P, et al. Salivary and lacrimal dysfunction after radioactive iodine for differentiated thyroid cancer: American Head and Neck Society Endocrine Surgery Section and Salivary Gland Section joint multidisciplinary clinical consensus statement of otolaryngology, ophthalmology, nuclear medicine and endocrinology. Head Neck. 2020;42(11):3446-3459. [DOI] [PubMed] [Google Scholar]
- 8. Papaleontiou M, Banerjee M, Yang D, Sisson JC, Koenig RJ, Haymart MR. Factors that influence radioactive iodine use for thyroid cancer. Thyroid. 2013;23(2):219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah SA, Adam MA, Thomas SM, et al. Racial disparities in differentiated thyroid cancer: have we bridged the gap? Thyroid. 2017;27(6):762-772. [DOI] [PubMed] [Google Scholar]
- 10. Wallner LP, Reyes-Gastelum D, Hamilton AS, Ward KC, Hawley ST, Haymart MR. Patient-perceived lack of choice in receipt of radioactive iodine for treatment of differentiated thyroid cancer. J Clin Oncol. 2019;37(24):2152-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haymart MR, Banerjee M, Yang D, et al. The relationship between extent of thyroid cancer surgery and use of radioactive iodine. Ann Surg. 2013;258(2):354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuessler KM, Banerjee M, Yang D, Stewart AK, Doherty GM, Haymart MR. Surgeon training and use of radioactive iodine in stage I thyroid cancer patients. Ann Surg Oncol. 2013;20(3):733-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haymart MR, Banerjee M, Yang D, Stewart AK, Koenig RJ, Griggs JJ. The role of clinicians in determining radioactive iodine use for low-risk thyroid cancer. Cancer. 2013;119(2): 259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawka AM, Straus S, Gafni A, et al. Thyroid cancer patients’ involvement in adjuvant radioactive iodine treatment decision-making and decision regret: an exploratory study. Support Care Cancer. 2012;20(3):641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sawka AM, Straus S, Rodin G, et al. Thyroid cancer patient perceptions of radioactive iodine treatment choice: Follow-up from a decision-aid randomized trial. Cancer. 2015;121(20):3717-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dillman DA. Mail and internet surveys: the tailored design method. John Wiley & Sons Inc; 2007. [Google Scholar]
- 17. Martinez KA, Li Y, Resnicow K, Graff JJ, Hamilton AS, Hawley ST. Decision regret following treatment for localized breast cancer: is regret stable over time? Med Decis Making. 2015;35(4):446-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinez KA, Resnicow K, Williams GC, et al. Does physician communication style impact patient report of decision quality for breast cancer treatment? Patient Educ Couns. 2016;99(12):1947-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawley ST, Griffith KA, Hamilton AS, et al. The association between patient attitudes and values and the strength of consideration for contralateral prophylactic mastectomy in a population-based sample of breast cancer patients. Cancer. 2017;123(23):4547-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jagsi R, Hawley ST, Griffith KA, et al. Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg. 2017;152(3):274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katz SJ, Janz NK, Abrahamse P, et al. Patient reactions to surgeon recommendations about contralateral prophylactic mastectomy for treatment of breast cancer. JAMA Surg. 2017;152(7):658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. Jama. 2009;302(14):1551-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papaleontiou M, Reyes-Gastelum D, Gay BL, et al. Worry in thyroid cancer survivors with a favorable prognosis. Thyroid. 2019;29(8):1080-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. [DOI] [PubMed] [Google Scholar]
- 27. Rabe-Hesketh S, Skrondal A. Multilevel modelling of complex survey data. J R Stat Soc: Ser A. 2006;169(4): 805-827. [Google Scholar]
- 28. Katz SJ, Hawley ST, Abrahamse P, et al. Does it matter where you go for breast surgery?: attending surgeon’s influence on variation in receipt of mastectomy for breast cancer. Med Care. 2010;48(10):892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollenbeck BK, Kaufman SR, Yan P, et al. Urologist practice affiliation and intensity-modulated radiation therapy for prostate cancer in the elderly. Eur Urol. 2018;73(4):491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. Jama. 2000;284(23): 3028-3035. [DOI] [PubMed] [Google Scholar]
- 32. Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18(11):2327-2340. [DOI] [PubMed] [Google Scholar]
- 33. Phillips KA, Milne RL, Buys S, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol. 2005;23(21):4679-4686. [DOI] [PubMed] [Google Scholar]
- 34. Clegg LX, Potosky AL, Harlan LC, et al. Comparison of self-reported initial treatment with medical records: results from the prostate cancer outcomes study. Am J Epidemiol. 2001;154(6):582-587. [DOI] [PubMed] [Google Scholar]
- 35. Schootman M, Jeffe DB, West MM, Aft R. Self-report by elderly breast cancer patients was an acceptable alternative to surveillance, epidemiology, and end results (SEER) abstract data. J Clin Epidemiol. 2005;58(12):1316-1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.