Abstract
Context
Erythrocytosis is a known side effect of testosterone therapy that can increase the risk of thromboembolic events.
Objectives
To study the prevalence and determinants in the development of erythrocytosis in trans men using testosterone.
Methods
A 20-year follow-up study in adult trans men who started testosterone therapy and had monitoring of hematocrit at our center (n = 1073).
Results
Erythrocytosis occurred in 11% (hematocrit > 0.50 L/L), 3.7% (hematocrit > 0.52 L/L), and 0.5% (hematocrit > 0.54 L/L) of trans men. Tobacco use (odds ratio [OR] 2.2; 95% CI, 1.6-3.3), long-acting undecanoate injections (OR 2.9; 95% CI, 1.7-5.0), age at initiation of hormone therapy (OR 5.9; 95% CI, 2.8-12.3), body mass index (BMI) (OR 3.7; 95% CI, 2.2-6.2), and pulmonary conditions associated with erythrocytosis and polycythemia vera (OR 2.5; 95% CI, 1.4-4.4) were associated with hematocrit > 0.50 L/L. In the first year of testosterone therapy hematocrit increased most: 0.39 L/L at baseline to 0.45 L/L after 1 year. Although there was only a slight continuation of this increase in the following 20 years, the probability of developing erythrocytosis still increased (10% after 1 year, 38% after 10 years).
Conclusion
Erythrocytosis occurs in trans men using testosterone. The largest increase in hematocrit was seen in the first year, but also after the first years a substantial number of people present with hematocrit > 0.50 L/L. A reasonable first step in the care for trans men with erythrocytosis while on testosterone is to advise them to quit smoking, to switch to a transdermal administration route, and if BMI is high, to lose weight.
Keywords: erythrocytosis, hematocrit, trans men, hormone treatment, gender dysphoria, time relation
People diagnosed with gender dysphoria (trans people) experience distress due to an incongruence between their gender identity and the sex assigned at birth (1). To reduce this distress, gender affirmative hormone therapy can be given to induce physical and mental changes toward the experienced sex (2). In trans men, who are assigned as female at birth, testosterone therapy induces virilization (3, 4). This includes deepening of the voice, an increase in facial and body hair, a more masculine body composition, and psychological and sexual changes (5-7). Also, hematological and biochemical changes occur due to the treatment, and therefore regular laboratory monitoring is recommended (3).
It is known from studies in hypogonadal cis men (birth-assigned male, male gender identity) that testosterone has a dose-dependent stimulating effect on erythropoiesis (8). Hence, one of the changes due to testosterone therapy is the rise in hematocrit levels. This results in secondary erythrocytosis, a potentially serious adverse effect of testosterone therapy as it is associated with an increased risk of thromboembolic events by an increase in blood viscosity (9, 10). Hematocrit is one of the major determinants of blood viscosity with an increased risk of coronary heart disease (relative risk of 1.16) (11) and unprovoked venous thromboembolisms (2.4-fold increased risk in the 20th highest percentile compared with lowest 40th percentile in men) (12, 13).
Erythrocytosis is previously described in both cis men and trans men on testosterone therapy (14). Prevalence of erythrocytosis (hematocrit > 0.50) in testosterone-treated hypogonadal cis men is described between 5% and 66%. The largest increase was seen in the first year after initiation of testosterone therapy (14-17). Prevalence of erythrocytosis (hematocrit > 0.50) in testosterone-treated trans men is 11.5% in previous literature (18). Studies in testosterone-treated hypogonadal cis men described highest risk of erythrocytosis with injectable testosterone therapy (both short-acting esters and long-acting undecanoate) when compared with transdermal administration. Studies in testosterone-treated trans men also described the highest risk of erythrocytosis (hematocrit > 0.50) with injectable testosterone (both short-acting esters and long-acting undecanoate) compared with gel and the biggest increase in the first 3 months to 1 year (follow-up of 1 to 5 years in these studies) (19-21).
Current guidelines on the management of secondary erythrocytosis in trans men on testosterone therapy refer to the guideline for testosterone-treated hypogonadal cis men and consider hematocrit levels > 0.50 L/L as potentially dangerous, as it has a very high risk of adverse outcome (3, 22). This guideline advices a cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy when hematocrit levels exceed 0.54 L/L to reduce the risk of adverse events. For levels between 0.50 and 0.54 L/L no clear advice is given in these guidelines. The question remains whether these guidelines are applicable to trans men, as the duration of testosterone therapy is much longer in trans men and hormone treatment can often not be discontinued.
In light of these considerations, the aim of this study is twofold:
-
1.
To study the prevalence and determinants in the development of erythrocytosis in trans men on testosterone therapy.
-
2.
To study the time relation between duration of testosterone therapy and hematocrit levels over the course of 20 years.
Based on the results of this study we discuss how these findings affects current practices regarding erythrocytosis in trans men.
Methods
Study Design and Population
This study is part of the Amsterdam Cohort of Gender Dysphoria study. This is a large cohort study containing medical data of 6793 people who visited the Center of Expertise on Gender Dysphoria of the Amsterdam UMC, Vrije Universiteit, Amsterdam, from 1972 to 2015 (23). Laboratory measurements of these people between 2004 and 2018 were available for analysis.
For this current study, trans men who started testosterone and had at least one follow-up visit after starting testosterone therapy were included. Subjects were excluded if testosterone therapy was discontinued during follow-up, or if laboratory results or starting date of testosterone therapy were missing.
Information on medication use, medical history, duration of hormonal treatment, testosterone administration route, body mass index (BMI, calculated as the weight in kilograms divided by the square of the height in meters; kg/m2), tobacco use, alcohol use, and laboratory results of testosterone levels and hematocrit was obtained retrospectively from medical files. Chronic pulmonary diseases (eg, asthma, chronic obstructive pulmonary disease, chronic bronchitis), sleep apnea, and polycythemia vera were considered as a medical history predisposing to the development of erythrocytosis.
Hormonal Treatment
In our Center of Expertise on Gender Dysphoria, testosterone was most commonly started as transdermal gel, intramuscular injections (both short-acting esters and long-acting undecanoate), or, in the past, oral administration. The starting dose of transdermal testosterone gel was 50 to 60 mg. Intramuscular administration as a 250-mg short-acting testosterone ester mix was injected every 2 to 4 weeks. Long-acting intramuscular testosterone undecanoate injections were dosed at 1000 mg every 10 to 14 weeks. If used, oral administration was most frequently prescribed as testosterone undecanoate capsules in a dose ranging from 40 to 360 mg daily. Dosages and administration intervals were adjusted on an individual basis by monitoring testosterone levels, aiming for levels between 10 and 30 nmol/L for transdermal gel and levels of 10 to 15 nmol/L prior to injection for injectable administration routes.
Laboratory Measures
Blood samples of the included trans men were available from January 2004 until December 2018. Total testosterone levels and hematocrit levels were obtained by venous blood samples drawn when participants visited the endocrine outpatient clinic. The first year after initiation of testosterone therapy, trans men visited the center every 3 to 6 months. Thereafter the monitoring frequency was reduced to yearly, every 2 years, or more frequently if indicated. Therefore, the number of measurements per person varied.
Until January 2013, total testosterone levels were measured using a Coat-A-Count radioimmunoassay (RIA) (Siemens, USA) with a lower limit of quantitation of 1 nmol/L and an interassay coefficient of variation (CV) of 7% to 20%. Thereafter, the Architect competitive immunoassay (Abbott, USA) was used to measure testosterone levels with a lower limit of quantitation of 0.1 nmol/L and an interassay CV of 6% to 10%. Coat-A-Count RIA values measured before 2013 were converted to Architect values using 2 formulas. For testosterone levels < 8 nmol/L, the formula Architect = 1.1*RIA + 0.2 was used. For testosterone levels > 8 nmol/L, the formula Architect = 1.34*RIA−1.65 was used.
Hematocrit was measured using a CELL-DYN Sapphire (Abbott, USA), with an interassay CV of 1.1%. In our center, the upper limit of normal of hematocrit levels for cis men is 0.50 L/L.
Determinants
Clinical and laboratory parameters were studied as possible determinants of development of erythrocytosis. Studied determinants were: route of testosterone administration; age at initiation of testosterone therapy; duration of testosterone therapy; BMI; tobacco use; testosterone levels; and a medical history predisposing to develop erythrocytosis, including chronic obstructive pulmonary disease, asthma, chronic bronchitis, or polycythemia vera.
Statistical Analysis
The results are presented as numbers, percentages, means with standard deviations, or medians with interquartile ranges. The prevalence of erythrocytosis is presented as the percentage of trans men with erythrocytosis. Erythrocytosis was defined as a hematocrit level exceeding the upper limit of the physiological range for men (>0.50 L/L twice) conforming to the guidelines for the hormonal treatment of trans people (3). Also, the prevalence of hematocrit levels of >0.52 L/L twice and >0.54 L/L twice were studied to give more insight into the prevalence of these high levels. For analysis of the determinants of erythrocytosis a hematocrit level of >0.50 L/L on a single measurement was used. Over the course of 20 years, trans men could switch route of testosterone administration. Hence, hematocrit levels were linked to the route of testosterone administration prior to the time of laboratory measurement.
To study the effect of clinical and laboratory parameters on the risk of erythrocytosis development (hematocrit > 0.50), logistic mixed-model analyses were performed, with erythrocytosis as outcome variable, and with tobacco use, age at initiation of testosterone therapy, BMI, testosterone levels, testosterone administration route, and predisposing medical history as determinants. Tobacco use was categorized to yes (for current and former smoking) or no. Age at initiation of testosterone therapy was categorized as <18, 18 to 30, 30 to 40, 40 to 50, and ≥50 years. BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5-25 kg/m2), overweight (25-30 kg/m2) and obese (≥30 kg/m2). Testosterone levels were categorized as under target range (0-10 nmol/L), within target range (10-20 nmol/L and 20-30 nmol/L), and above target range (>30 nmol/L). The results are presented as odds ratios (OR) with 95% CI. The analyses were adjusted for testosterone levels, testosterone administration route, predisposing medical history, tobacco use, age at initiation of testosterone therapy, and BMI by analyzing all these determinants in one model. Linear mixed-model analyses with measurements clustered within participants were performed to analyze the change in hematocrit levels over time.
Failure analysis for the development of hematocrit levels >0.50 L/L were performed on all trans men of whom baseline hematocrit measurements were available (testosterone therapy starting date after 2004)
Data analyses were performed using STATA Statistical Software, version 15.1 (StataCorp, College Station, Texas, USA).
Results
In this analysis, 1073 trans men were included. The Amsterdam Cohort of Gender Dysphoria study database consists of 2398 trans men; 431 trans men were excluded due to missing data for the starting date of hormone therapy and 894 were excluded due to lack of available laboratory results. Baseline characteristics of the study population are shown in Table 1. Of all hematocrit measurements, 1087 were during the use of testosterone gel, 1826 under the use of short-acting injections, 345 under long-acting undecanoate injections, 150 under oral testosterone administrations, and for 2120 measurements the testosterone administration route was unknown.
Table 1.
Baseline Characteristics
| Characteristic | Total (n = 1073) |
|---|---|
| Age at start of HT, median (IQR) | 22.5 (18.4-31.8) |
| BMI at start of HT, mean (SD) | 24.5 (±5.5) |
| Tobacco use, % yes | 38% |
| Conditions associated with erythrocytosis, % yes | 8.6% |
| Pulmonary condition associated with erythrocytosisa | 7.6% |
| Obstructive sleep apnea | 0.7% |
| Polycytemia vera | 0.4% |
Abbreviations: BMI, body mass index; HT, hormone therapy; IQR, interquartile range.
a Asthma, chronic obstructive pulmonary disease, chronic bronchitis
Prevalence
A single measurement of hematocrit > 0.50 L/L occurred in 24.0% of trans men, 11.1% had this measurement twice. Hematocrit levels of >0.52 L/L were seen in 7.6% of trans men in this cohort, 3.7% had this twice. Hematocrit levels of >0.54 L/L were seen in 2.2% once and 0.5% twice.
Determinants
Table 2 shows the results of different determinants on the occurrence of a hematocrit of >0.50 L/L and >0.52 L/L. Compared with testosterone gel, the use of long-acting undecanoate injection showed highest odds for hematocrit levels >0.50 (OR 2.9; 95% CI, 1.7-5.0). Short-acting ester injections (OR 1.1; 95% CI, 0.7-1.6) and oral administration (OR 0.4; 95% CI, 0.1-1.8) had similar odds for hematocrit levels >0.50 L/L compared with testosterone gel.
Table 2.
Multivariable Analysis: Chance of High Hematocrit for Different Determinants
| Hematocrit > 0.50 L/L, crude OR (95% CI) | Adjusted OR (95% CI)b | Hematocrit > 0.52 L/L, crude OR (95%CI) | Adjusted OR (95% CI)b | |
|---|---|---|---|---|
| Tobacco use | 2.2 (1.6–3.3) | 2.0 (1.3–3.0) | 3.2 (1.7–6.2) | 2.7 (1.3–5.5) |
| Positive medical historya | 2.5 (1.4–4.4) | 2.0(1.1–3.8) | 5.3 (2.3–12.3) | 4.6 (1.9–11.2) |
| Age at initiation | ||||
| <18 | ref | ref | ref | ref |
| 18–30 | 2.5 (1.4–4.4) | 1.5(0.8–2.9) | 3.2 (1.0–9.9) | 1.9 (0.5–7.0) |
| 30–40 | 3.9 (2.0–7.5) | 1.9 (0.9–4.0) | 5.7 (1.7–19.4) | 2.7 (0.6–11.3) |
| 40–50 | 5.9 (2.8–12.3) | 3.1 (1.4–7.2) | 10.3 (2.8–38.8) | 4.9 (1.1–22.5) |
| ≥50 | 2.6 (0.8–8.6) | 1.2 (0.3–4.5) | 2.9 (0.3–26.4) | 1.1 (0.1–13.5) |
| BMI | ||||
| ≤18.5 | 0.4 (0.2–1.2) | 0.6 (0.2–1.9) | n/a | n/a |
| 18.5–25 | ref | ref | ref | ref |
| 25–30 | 3.4 (2.1–5.5) | 3.0 (1.8–5.1) | 5.3 (2.3–12.3) | 4.3 (1.7–10.8) |
| ≥30 | 3.7 (2.2–6.2) | 3.1 (1.7–5.6) | 6.1 (2.5–14.9) | 4.6 (1.7–12.3) |
| Route of testosterone | ||||
| T gel | ref | ref | ref | ref |
| Short-acting im | 1.1 (0.7–1.6) | 1.1 (0.7–1.7) | 1.5 (0.7–3.4) | 1.5 (0.6–3.7) |
| Long-acting im | 2.9 (1.7–5.0) | 3.1 (1.7–5.6) | 1.0 (0.3–3.4) | 1.3 (0.4–5.0) |
| Oral T | 0.6 (0.2–1.6) | 1.3 (0.4–3.7) | 0.2 (0.1–2.4) | 0.5 (0.1–5.6) |
| Unknown | 1.2 (0.8–1.7) | 1.9 (1.3–2.8) | 1.8 (0.9–3.8) | 2.9 (1.3–6.6) |
| Testosterone levels | ||||
| 0–10 nmol/L | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.2 (0.1–0.4) | 0.1 (0.1–0.4) |
| 10–20 nmol/L | ref | ref | ref | ref |
| 20–30 nmol/L | 1.3 (0.9–1.8) | 1.3 (0.9–1.8) | 1.3 (0.7–2.5) | 1.4 (0.7–2.7) |
| >30 nmol/L | 1.4 (1.0–1.9) | 1.5 (1.1–1.8) | 1.4 (0.7–2.5) | 1.6 (0.8–3.0) |
Abbreviations: BMI, body mass index; im, intramuscular; n/a, no data available; OR, odds ratio; T, testosterone.
a Chronic obstructive pulmonary disease/asthma, sleep apnea, chronic bronchitis, polycythemia vera
b Adjusted for all other determinants
Tobacco use showed higher odds for hematocrit levels of >0.50 L/L and >0.52 L/L compared with no tobacco use. Higher age at initiation of testosterone therapy, a high BMI, and a predisposing medical history were also associated with high hematocrit levels. Table 2 also shows adjusted odds ratios for all determinants. After adjustment, the odds of high hematocrit levels in the age range from 18 to 30and 30 to 40 years were much lower (OR 1.5; 95% CI, 0.8-2.8 and OR 1.9; 95% CI, 0.9-3.9). Lower than aimed-for testosterone levels had a low odds of hematocrit levels >0.50 L/L and >0.52 L/L compared with aimed-for levels. Higher than targeted levels of testosterone had similar and slightly higher odds of high hematocrit compared with target levels.
Time Relation
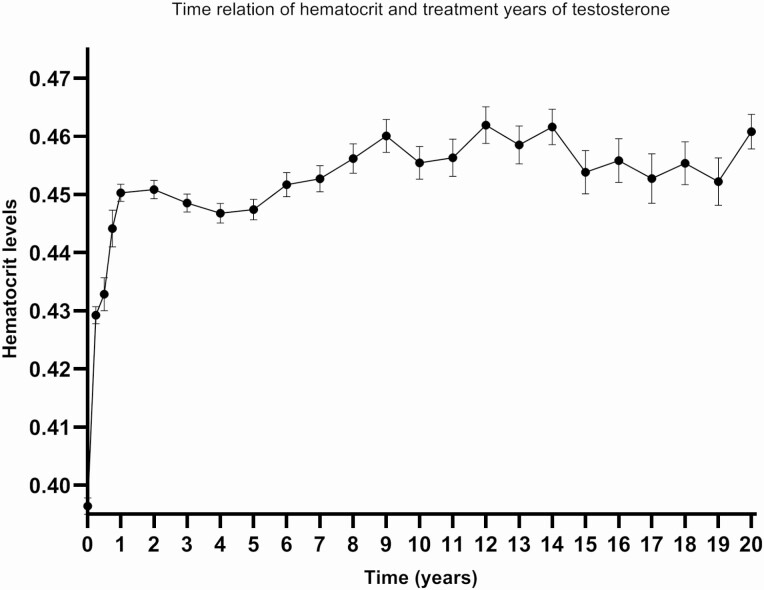
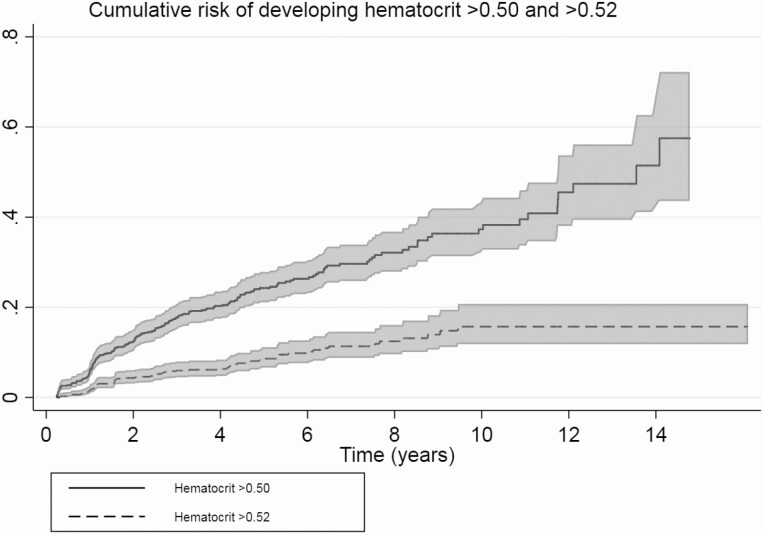
The time relation between the initiation of testosterone therapy and hematocrit is depicted in Fig. 1. The largest increase in hematocrit level was seen in the first year after initiation of testosterone therapy. A slight increase was seen over time until 20 years after initiation of testosterone therapy. Figure 2 shows the probability of developing hematocrit levels >0.50 and >0.52. In this subgroup analysis, 776 trans men were included. After 1 year the cumulative risk of developing hematocrit levels >0.50 was 8%, after 10 years 38% and at the end of the follow-up period, after 14 years, the cumulative incidence was 50%. For hematocrit levels of >0.52, the cumulative risk after 1 year was 4%, and it was 16% after 10 years.
Figure 1.
Time relation between hematocrit and duration of testosterone therapy (determined by mixed-model analysis; shown as mean with standard error of the mean).
Figure 2.
Nelson-Aalen curve for the cumulative risk of developing hematocrit levels >0.50 and >0.52. Restricted to participants starting testosterone therapy after 2004 (baseline hematocrit levels were present).
Discussion
Eleven percent of trans men using testosterone showed erythrocytosis (>2 measurements of hematocrit > 0.50 L/L) during 20 years of follow-up. Determinants associated with hematocrit levels of >0.50 L/L besides testosterone therapy were smoking, higher age at initiation of testosterone therapy, higher BMI, and a predisposing medical history. Higher testosterone levels per se were not associated with an increased odds of hematocrit > 0.50 L/L.
The prevalence of erythrocytosis in this study is similar to that found in previous research in trans men (18). In testosterone-treated hypogonadal cis men the prevalence ranges from 5% to 66% depending on the cutoff values (most studies used a cutoff value of >0.50 L/L; one study used >0.52 L/L) and study design (14). Jones et al reported a 5% increase in hematocrit levels for long-acting undecanoate intramuscular injections and a 6.4% increase in hematocrit for short-acting ester intramuscular injections (17). In a study by Pastuzak et al, intramuscular injections had a very high prevalence (66%) of erythrocytosis (hematocrit levels > 0.50) (16). However, baseline hematocrit levels in this group were much higher. In addition, the dosage of testosterone was higher and the mean age was much higher than in our study.
In our cohort, long-acting undecanoate intramuscular injections showed the highest odds for hematocrit levels > 0.50 L/L. Other studies reported a similar increase with long-acting intramuscular injections (18, 21, 24, 25). However, some of these studies reported a higher or similar increase in hematocrit levels with short-acting intramuscular injections. All of these studies had smaller sample sizes and shorter follow-up periods. In line with previous studies, we observed that transdermal testosterone therapy showed the least increase in hematocrit levels. Moreover, as described earlier, tobacco use, high BMI, a medical history predisposing for erythrocytosis, and higher age at initiation of testosterone therapy showed increased risk of high hematocrit levels (26-30). After adjustment for BMI and smoking, the odds of high hematocrit levels for the age range from 18 to 40 years was much lower. Hence, higher weight and smoking partially caused increased odds in this age range. Previous research showed a dose-dependent stimulating effect of testosterone on erythropoiesis (31). In our study this dose-dependent effect was seen when comparing lower than aimed levels of testosterone (<10nmol/L) with aimed-for levels (10-20 and 20-30 nmol/L), our data also showed a slight increase in the odds of getting hematocrit levels > 0.50 with increased testosterone levels. It is possible that dose reduction has a lowering effect on hematocrit levels. Furthermore the target testosterone levels of 10 to 30 nmol/L for transdermal administration and 10 to 15 nmol/L prior to injection could give higher levels compared to levels in cis men (average 14-20 nmol/L in males in the age range 20-40 years) (32). This could give an increased risk of high hematocrit levels but no additional benefit. However, it has to be noted that it is difficult to aim for the exact levels seen in the cis population, due to the available testosterone administration types (especially with short-acting esters as these give a testosterone peak).
Currently, the guidelines in transgender men (3) for testosterone-induced erythrocytosis are based on the guidelines on hypogonadal cis men from 2008 (22). This guideline states that a hematocrit > 0.50 L/L has a moderate-high risk of adverse outcome. If hematocrit levels exceed the level of >0.54 L/L, testosterone therapy should be discontinued until hematocrit levels are normalized. However, the new guideline for testosterone-treated hypogonadal cis men from 2018 state that a hematocrit level of >0.48 L/L is associated with a moderate-high risk of adverse outcome (33). The lowering of this upper limit is based on studies that show that older men have a higher risk of developing erythrocytosis (31, 34). It is known from studies in general populations that high hematocrit levels give a higher risk of cardiovascular events (11). According to hematology guidelines (for people without polycythemia vera), the upper limit for hematocrit for cis males is defined as 0.52 L/L, for cis females this is 0.48 L/L (35). Whether this upper limit for cis males is also applicable for trans men is not known. It could be argued that the upper limit for cis females should be applied, as trans men generally are born with female genetics. This is a subject for further research, like the risk of thromboembolic events with different cutoff values. Furthermore, the difference among all these guidelines emphasizes the need for further research on this subject.
The results of this study show that the largest increase in hematocrit occurs in the first year after initiation of testosterone therapy, with a slight continuation of this increase up to 20 years. This is in line with previous literature and reflected in current guidelines (18, 21, 24, 36). Our study confirmed that this is also the case in trans men. The probability of developing first-time erythrocytosis increases over the course of 14 years. It indicates that a person who is stable on testosterone therapy for years still can develop first-time erythrocytosis. Therefore, regular control of hematocrit seems warranted as long as people use testosterone.
Strengths and Limitations
This is the first study in a large cohort of trans men on testosterone therapy with a follow-up period up to 20 years. Because of this large sample size, statistical analysis with many determinants could be performed. Also, because of the long follow-up period, a clear time relation between initiation of testosterone therapy and hematocrit can be shown in this study.
A limitation of this study is that data are collected retrospectively. Because data were obtained from medical files and not via a study design, some data were missing. Also, other possible confounders not present in our database could not be studied, and often the route of testosterone administration was unknown.
To reach acceptable group size for the analysis of the determinants of erythrocytosis, upper limits of >0.50 L/L and >0.52 L/L for hematocrit were chosen. Current guidelines describe a hematocrit > 0.50 L/L as a moderate-high risk of adverse outcome (3). If hematocrit levels exceed the level of >0.54 testosterone therapy should be discontinued until hematocrit levels are normalized. The group size of trans men with hematocrit levels > 0.54L/L was not sufficient to perform analysis on. Analysis on determinants were executed on hematocrit levels linked to testosterone administration route. Because there was a great variability in the amount of laboratory measurements per person, it was not possible to analyze subsequent hematocrit levels > 0.50 L/L. Therefore, these data give no insight into the duration of high hematocrit levels.
Recommendations for Clinical Practice
From this study, a few recommendations for clinical practice can be formulated. First, regular control of hematocrit seems warranted as long as people use testosterone. If hematocrit levels are between 0.50 and 0.54 L/L, reasonable first steps to prevent further increase would be:
• Consider switching injectable testosterone therapy to transdermal administration. We cannot exclude that dose reduction has the same effect.
• If BMI levels are > 25 kg/m2, the people should be advised to lose weight to reach a BMI in the healthy range (18.5-25 kg/m2).
• People should strongly be advised to stop smoking.
• Treatment optimization for chronic lung disease or sleep apnea should be pursued.
Acknowledgments
Financial Support: No funding was received for this study.
Glossary
Abbreviations
- BMI
body mass index
- CV
coefficient of variation
- RIA
radioimmunoassay
Additional Information
Disclosures: The authors report no conflict of interest.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2. Costa R, Colizzi M. The effect of cross-sex hormonal treatment on gender dysphoria individuals’ mental health: a systematic review. Neuropsychiatr Dis Treat. 2016;12:1953-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. . Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017; 102(11):3869-3903. [DOI] [PubMed] [Google Scholar]
- 4. Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017;5(4):301-311. [DOI] [PubMed] [Google Scholar]
- 5. Irwig MS, Childs K, Hancock AB. Effects of testosterone on the transgender male voice. Andrology. 2017;5(1):107-112. [DOI] [PubMed] [Google Scholar]
- 6. Fisher AD, Castellini G, Ristori J, et al. . Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. [DOI] [PubMed] [Google Scholar]
- 7. Klaver M, de Blok CJM, Wiepjes CM, et al. . Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178(2):163-171. [DOI] [PubMed] [Google Scholar]
- 8. Calof OM, Singh AB, Lee ML, et al. . Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451-1457. [DOI] [PubMed] [Google Scholar]
- 9. Walker RF, Zakai NA, MacLehose RF, et al. . Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern Med. 2020;180(2):190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood. 2017;130(16):1795-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danesh J, Collins R, Peto R, Lowe GDO. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J 2000;21(7):515-520. [DOI] [PubMed] [Google Scholar]
- 12. Braekkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95(2):270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells RE Jr, Merrill EW. Influence of flow properties of blood upon viscosity-hematocrit relationships. J Clin Invest. 1962;41:1591-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6(1):77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rotker KL, Alavian M, Nelson B, et al. . Association of subcutaneous testosterone pellet therapy with developing secondary polycythemia. Asian J Androl. 2018;20(2):195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pastuszak AW, Gomez LP, Scovell JM, Khera M, Lamb DJ, Lipshultz LI. Comparison of the effects of testosterone gels, injections, and pellets on serum hormones, erythrocytosis, lipids, and prostate-specific antigen. Sex Med. 2015;3(3):165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev. 2015;3(2):101-112. [DOI] [PubMed] [Google Scholar]
- 18. Defreyne J, Vantomme B, Van Caenegem E, et al. . Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. Andrology. 2018;6(3):446-454. [DOI] [PubMed] [Google Scholar]
- 19. Nolan BJ, Leemaqz SY, Ooi O, et al. . Prevalence of polycythaemia with different formulations of testosterone therapy in transmasculine individuals. Intern Med J Published online April 2020. doi: 10.1111/imj.14839 [DOI] [PubMed] [Google Scholar]
- 20. Gava G, Mancini I, Cerpolini S, Baldassarre M, Seracchioli R, Meriggiola MC. Testosterone undecanoate and testosterone enanthate injections are both effective and safe in transmen over 5 years of administration. Clin Endocrinol (Oxf). 2018;89(6):878-886. [DOI] [PubMed] [Google Scholar]
- 21. Pelusi C, Costantino A, Martelli V, et al. . Effects of three different testosterone formulations in female-to-male transsexual persons. J Sex Med. 2014;11(12):3002-3011. [DOI] [PubMed] [Google Scholar]
- 22. Bhasin S, Cunningham GR, Hayes FJ, et al. ; Task Force, Endocrine Society . Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. [DOI] [PubMed] [Google Scholar]
- 23. Wiepjes CM, Nota NM, de Blok CJM, et al. . The Amsterdam Cohort of Gender Dysphoria Study (1972–2015): trends in prevalence, treatment, and regrets. J Sex Med 2018;15(4):582-590. [DOI] [PubMed] [Google Scholar]
- 24. Jacobeit JW, Gooren LJ, Schulte HM. Safety aspects of 36 months of administration of long-acting intramuscular testosterone undecanoate for treatment of female-to-male transgender individuals. Eur J Endocrinol. 2009;161(5):795-798. [DOI] [PubMed] [Google Scholar]
- 25. Mueller A, Haeberle L, Zollver H, et al. . Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7(9):3190-3198. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen KM, Çolak Y, Ellervik C, Hasselbalch HC, Bojesen SE, Nordestgaard BG. Smoking and increased white and red blood cells. Arterioscler Thromb Vasc Biol. 2019;39(5):965-977. [DOI] [PubMed] [Google Scholar]
- 27. Teo KK, Ounpuu S, Hawken S, et al. ; INTERHEART Study Investigators . Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647-658. [DOI] [PubMed] [Google Scholar]
- 28. Lundy SD, Parekh NV, Shoskes DA. Obstructive sleep apnea is associated with polycythemia in hypogonadal men on testosterone replacement therapy. J Sex Med. 2020;17(7):1297-1303. [DOI] [PubMed] [Google Scholar]
- 29. McMullin MF, Harrison CN, Ali S, et al. ; BSH Committee . A guideline for the diagnosis and management of polycythaemia vera. A British Society for Haematology Guideline. Br J Haematol. 2019;184(2):176-191. [DOI] [PubMed] [Google Scholar]
- 30. Leslie WD, Dupont JO, Peterdy AE. Effect of obesity on red cell mass results. J Nucl Med. 1999;40(3):422-428. [PubMed] [Google Scholar]
- 31. Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93(3):914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chodick G, Epstein S, Shalev V. Secular trends in testosterone- findings from a large state-mandate care provider. Reprod Biol Endocrinol. 2020;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhasin S, Brito JP, Cunningham GR, et al. . Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018;103(5):1715-1744. [DOI] [PubMed] [Google Scholar]
- 34. Bhasin S, Woodhouse L, Casaburi R, et al. . Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90(2):678-688. [DOI] [PubMed] [Google Scholar]
- 35. McMullin MF, Bareford D, Campbell P, et al. ; General Haematology Task Force of the British Committee for Standards in Haematology . Guidelines for the diagnosis, investigation and management of polycythaemia/erythrocytosis. Br J Haematol. 2005;130(2):174-195. [DOI] [PubMed] [Google Scholar]
- 36. Chandra P, Basra SS, Chen TC, Tangpricha V. Alterations in lipids and adipocyte hormones in female-to-male transsexuals. Int J Endocrinol 2010;2010:945053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.