Abstract
Background
Recent studies have indicated that a high‐fat diet (HFD) and/or HFD‐induced obesity may influence prostate cancer (PCa) progression, but the role of HFD in PCa microenvironment is unclear. This study aimed to delineate the molecular mechanisms of PCa progression under HFD milieus and define the stromal microenvironment focusing on macrophage inhibitory cytokine‐1 (MIC‐1) activation.
Methods
We investigated the effects of HFD on PCa stromal microenvironment and MIC‐1 signaling activation using PC‐3M‐luc‐C6 PCa model mice fed with HFD or control diet. Further, we explored the effect of periprostatic adipocytes derived from primary PCa patients on activation and cytokine secretion of prostate stromal fibroblasts. Expression patterns and roles of MIC‐1 signaling on human PCa stroma activation and progression were also investigated.
Results
HFD stimulated PCa cell growth and invasion as a result of upregulated MIC‐1 signaling and subsequently increased the secretion of interleukin (IL)‐8 and IL‐6 from prostate stromal fibroblasts in PC‐3M‐luc‐C6 PCa mouse model. In addition, periprostatic adipocytes directly stimulated MIC‐1 production from PC‐3 cells and IL‐8 secretion in prostate stromal fibroblasts through the upregulation of adipose lipolysis and free fatty acid release. The increased serum MIC‐1 was significantly correlated with human PCa stroma activation, high serum IL‐8, IL‐6, and lipase activity, advanced PCa progression, and high body mass index of the patients. Glial‐derived neurotrophic factor receptor α‐like (GFRAL), a specific receptor of MIC‐1, was highly expressed in both cytoplasm and membrane of PCa cells and surrounding stromal fibroblasts, and the expression level was decreased by androgen deprivation therapy and chemotherapy.
Conclusion
HFD‐mediated activation of the PCa stromal microenvironment through metabolically upregulated MIC‐1 signaling by increased available free fatty acids may be a critical mechanism of HFD and/or obesity‐induced PCa progression.
Keywords: macrophage inhibitory cytokine‐1, tumor microenvironment, high‐fat diet, prostate cancer, metabolism
MIC‐1 production was increased in PCa cells, which was affected by adipocyte infiltration and adipolysis. MIC‐1 directly stimulated the surrounding PrSC cells to secrete protumorigenic cytokines such as IL‐8 and IL‐6 in the PCa stromal microenvironment, especially under a HFD condition. These upregulated functional cytokines directly and/or indirectly stimulated PCa cell proliferation, invasion, and metastasis.
Abbreviations
- MIC‐1
Macrophage inhibitory cytokine‐1;
- rMIC‐1
Recombinant MIC‐1
- GFRAL
Glial‐derived neurotrophic factor receptor α‐like;
- PrSC
prostate stromal fibroblasts;
- FFA
Free fatty acid;
- IL‐8/IL‐6
Interleukin (IL)‐8/ Interleukin (IL)‐6;
- αSMA
α smooth muscle actin;
- siRNA
Small interfering RNA;
- siMIC‐1
Macrophage inhibitory cytokine‐1 siRNA;
- siGFRAL
Glial‐derived neurotrophic factor receptor α‐like siRNA;
- PCa
Prostate cancer;
- HFD
High‐fat diet;
- CD
Control diet;
1. BACKGROUND
Prostate cancer (PCa) is a commonly diagnosed cancer and a leading cause of cancer‐related death in the United States and Japan [1, 2]. Recent epidemiological studies have indicated that a high‐fat diet (HFD) and/or HFD‐induced obesity is associated with PCa tumorigenesis and progression [3, 4]. Several studies, including our own, have shown that a HFD and dietary components may alter gene expression and cellular activity and induce other important changes in circulating biological factors related to PCa aggressiveness [5, 6, 7].
The prostate stromal microenvironment is an interconnected network that consists of various types of stromal cells and has emerged as a key factor in the growth and development of PCa [8]. Prostate stromal fibroblasts are a common component of the prostate stroma, and their crosstalk with adjacent cancer cells may influence tumor progression by the secretion of proinflammatory cytokines and growth factors [9, 10]. Alpha smooth muscle actin (αSMA) is a key marker of cancer‐associated fibroblasts and reactive stroma, and αSMA‐overexpressing prostate stroma has been shown to be directly correlated with prostate stromal dysfunction, human prostate tumorigenesis, and PCa progression [11, 12]. Macrophage inhibitory cytokine‐1 (MIC‐1) is a divergent member of the transforming growth factor‐β family of cytokines and is a multifunctional and secretory molecule that can exist as either dimeric or monomeric forms [13]. In addition, MIC‐1 is a protumorigenic and progressive marker of PCa [14], and recent reports have shown that MIC‐1 is expressed in PCa tissues, where it can significantly influence cancer cell growth and invasion [15, 16, 17]. Interestingly, the functional MIC‐1 receptor glial‐derived neurotrophic factor receptor α‐like (GFRAL) family has been shown to interact with MIC‐1 and induce important metabolic signaling in mice with HFD‐induced obesity [18].
Recent studies have shown that HFD‐induced obesity significantly promoted PCa progression through the induction of periprostatic inflammation [19] and secretion of cytokines and other factors [20, 21]. In addition, adipocyte infiltration in the ovarian cancer microenvironment has been shown to markedly stimulate cancer cell homing, migration, and invasion through the induction of pro‐tumorigenic cytokines such as interleukin (IL)‐8 and IL‐6 [22]. A HFD and/or HFD‐induced obesity may bring physical and chemical changes that modify the PCa microenvironment to promote cancer progression. However, the molecular mechanisms that exist in the altered crosstalk between cancer cells and surrounding stromal cells in the HFD‐induced milieu remain poorly understood.
In this study, we aimed to delineate the molecular mechanisms and role of the stromal microenvironment in HFD‐induced PCa progression, focusing on MIC‐1 activation.
2. MATERIALS AND METHODS
2.1. Cell culture and reagents
Human PCa PC‐3, DU145, and LNCaP cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). The luciferase‐expressing PC‐3M‐luc‐C6 cell line derived from metastatic PC‐3M cells was purchased from PerkinElmer (Waltham, MA, USA). The cells were maintained in RPMI‐1640 or Dulbecco's Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) and 1% penicillin‐streptomycin. The normal prostate stromal cells (PrSC) and optimized culture media (Clonetics™ SCGM™ Stromal Cell Growth Medium) were purchased from Lonza (Walkersville, MD, USA). Three types of fatty acids (FAs), palmitic acid (PA), oleic acid (OA), and linoleic acid (LA), were purchased from Sigma (St. Louis, MO, USA).
The FAs were dissolved in water with 2% of Bovine Serum Albumin Fraction V (BSAFV, Sigma‐Aldrich, St. Louis, MO, USA) and filtered through a 0.22‐μm filter (SLGV004SL; Millipore, Billerica, MA, USA) before use. To assess gene expression in the cultured PCa cells after FA induction, a total of 4 × 105 cells were seeded in a 35‐mm dish and treated a day later with various FAs (0.125 mmol/L of PA, 0.25 mmol/L of OA, or 0.15 mmol/L of LA) in serum‐free DMEM for 24 h. FA‐free (2%) BSA was used as a vehicle for exogenous FFA experiments. Mitogen‐activated protein kinase inhibitor (U0126, used for cell signaling analysis at 1 μmol/L) and recombinant human MIC‐1 (rMIC‐1, used at 50 ng/mL) were purchased from Cell Signaling Technology (Boston, MA, USA) and R&D Systems (Minneapolis, MN, USA), respectively. The nicotinic acid (NA, Fujifilm, Tokyo, Japan) was used at 100 μmol/L as a lipolysis inhibitor.
2.2. Animal study
The Institutional Review Board of the Institutional Animal Care and Use Committee of Akita University Graduate School of Medicine approved all animal experiments. Male 8‐week‐old BALB/c‐nu/nu mice were obtained from Japan SLC (Shizuoka, Japan). A total of 2 × 106 PC‐3M‐luc‐C6 cells were resuspended in 0.1 mL of ice‐cold phosphate‐buffered saline (PBS, Invitrogen) and inoculated by intraperitoneal injection into the mice. Two weeks after inoculation, the mice were randomly assigned to the control diet (CD) and HFD groups (5 per group). The mice in the HFD group were fed with an HFD consisting of 59.9% calories from fats, 21.4% from carbohydrates, and 18.6% from proteins (Purina Mills Test Diets, Richmond, IN, USA), whereas the mice in the CD group were fed with a CE‐2 diet (Japan SLC, Shizuoka, Japan) consisting of 12.1% calories from fats, 58.8% from carbohydrates, and 29.1% from proteins. In vivo bioluminescence was used to follow the PC‐3M‐luc‐C6 cell inoculation and invasiveness by the intraperitoneal injection of luciferin (200 μL at 15 mg/mL in PBS), and the reflective total flux (60‐s exposure) was measured by the Xenogen IVIS™ imaging system (Los Angeles, CA, USA) 4 weeks after injection of the cells. The mice were then euthanized, and the tumor burdens in the peritoneal organs were macroscopically evaluated. The tumors were excised and subjected for histopathology, quantitative real‐time PCR (qRT‐PCR), and intratumoral lipid activity analyses. Blood samples were then collected from the orbital sinus at 4 weeks after cell injection, and sera were separated, filtered, and stored at −80 °C until use.
2.3. Small‐interfering RNA (siRNA)‐mediated gene knockdown
MIC‐1 siRNA (siMIC‐1, SI03098480) and GFRAL siRNA (siGFRAL, GS389400) were purchased from Qiagen (Valencia, CA, USA), and luciferase siRNA (siCtrl, SI03650353, Qiagen) was used as a control. The siRNAs were transfected into tumor cells and PrSC cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cells were treated with 50 nmol/L siRNAs in a reducing serum RPMI‐1640 for 12 h and 24 h. MIC‐1 and GFRAL knockdown was verified by quantitative real‐time PCR (qRT‐PCR).
2.4. Human periprostatic adipocyte preparation and co‐culture assay
Human periprostatic adipose tissues were collected from 13 PCa patients after radical prostatectomy. Approximately 1 g of adipose tissues surrounding the prostate was collected, and fibrosis, clots, and small vessels were macroscopically removed. The adipose tissue was washed twice with cold PBS before being minced and incubated with 0.075% collagenase (Type 1, Funakoshi Co., Ltd, Japan) at 37 °C for 30 min. Then, the mixtures were centrifuged at 500 × g for 1 min after dilution with DMEM containing 2% FBS, and the supernatant was removed and cultured with adipocyte culture medium (Zen‐Bio Inc., Durham, NC, USA) as described previously [23]. To investigate the role of periprostatic adipocytes on lipid activities and cytokine production by co‐culture periprostatic adipocytes with various prostate cells, the isolated adipocytes were directly incubated in 6‐well plates that were previously seeded with 2 × 105 PC‐3 cells and/or 4 × 104 PrSC cells in 2 mL of DMEM containing 2% FBS without antibiotics for 48 h. To evaluate the effect of MIC‐1 knockdown on the co‐cultured tumor cells and periprostatic adipocytes, PC‐3 cells were pretreated with 50 nmol/L siMIC‐1 for 12 h. The conditioned medium (CM) from the monoculture or co‐culture plates was then collected and stored at ‐80 °C until use.
2.5. Adipose lipolysis assay
The periprostatic adipocyte lipolysis of the co‐cultured cells was quantified by the amount of glycerol released into the CM, which is proportional to the level of triglyceride storage and the degree of adipose lipolysis using the Adipolyis assay kit (Abcam, Cambridge, UK) according to the manufacturer's instructions. Briefly, 25 μL of the CM from the co‐culture plates was incubated with 100 μL of free glycerol assay reagent for 15 min at room temperature. Then, the absorbance was measured at 540 nm using an ELISA reader (Bio‐Rad, Tokyo, Japan), and the glycerol concentration was calculated using the standard curve method.
2.6. Lipase activity assay
Lipase activity in the sera of PCa patients and sera and xenograft extracts from mice were quantified using the Lipase assay kit (Abcam, Cambridge, UK) to quantify the hydrolyzation of a triglyceride substrate enzymatically. Briefly, the glycerol standard and diluted serum samples were incubated with 100 μL reaction mix, and the absorbance was measured at 570 nm every 3 min for 30 min. The concentration of glycerol was determined by the standard curve, and the lipase activity was calculated by the linked change of glycerol. The lipase activity in the sera and xenograft tumor extracts of the mice was measured using the kit.
2.7. Free fatty acid (FFA) quantification
The levels of FFAs in the extracts of the xenograft and the sera from mice were quantified in triplicate using the FFA quantification kit (Biovision, Mountain View, CA, USA) according to the manufacturer's instructions. Briefly, 10 mg of the xenografts were homogenized with 200 μL chloroform‐triton X‐100 (1% Triton X‐100 in pure chloroform), and the extracts were centrifuged at 500 rpm for 1 min. The purified lipids in the lower phase were collected, air dried and dissolved in 200 μL FA assay buffer. FAs were converted to their coenzyme A (CoA) derivatives, and the subsequent oxidized reaction and concomitant generated color were measured at 570 nm. The concentration was calculated using a PA standard curve, and the secreted FFA in the CM of the co‐culture plates of the cells was measured.
2.8. qRT‐PCR
Total RNAs were extracted from the cultured cells using the TRIzol® reagent (Invitrogen). The following RT‐PCR primers were used: IL‐8, forward 5′‐ATGACTTCCAAGCTGGCCGTGG‐3′, reverse 5′‐CATAATTTCTGTTTGGCGCAGTGTGG‐3′; IL‐6, forward 5′‐GCTTTAAGGAGTTCCTGC‐3′, reverse 5′‐GGTAAGCCTACACTTTCCA‐3′; MIC‐1, forward 5′‐CGCGCAACGGGGACCACT‐3′, reverse 5′‐TGAGCACCATGGGATTGTAGC‐3′; GFRAL, forward 5′‐GGAGAGTAATGGAAGATGCCTGC‐3′, reverse 5′‐GAAGTCATCAGTGCAAAGACACTC‐3′; transforming growth factor‐β (TGF‐β), forward 5′‐GACATCAACGGGTTCAC‐3′, reverse 5′‐GAAGTTGGCATGGTAGC‐3′; β‐actin, forward 5′‐ATCTGGCACCACACCTTCTA‐3′, reverse 5′‐CGTCATACTCCTGCTTGCTGATCC‐3′. The polymerase chain reaction condition was 90°C for 30 s, 60°C for 30 s, and 72°C for 45 s. The experiments were performed in triplicate.
2.9. Cytokine analysis
A total of 4 × 104 PrSC cells were seeded in a 35‐mm dish containing the optimal growth medium and cultured with or without 50 ng/mL rMIC‐1 for 24 h. The PrSC cells were co‐cultured with 2 × 105 PC‐3 cells that were previously treated with 50 nmol/L siCtrl or siMIC‐1 for 24 h. Cytokine levels in the CM described above and in the serum of mice and humans were measured using a cytometric bead array (CBA™, BD Biosciences, San Jose, USA), which can simultaneously measure six pro‐inflammatory cytokines IL‐1β, IL‐6, IL‐8, IL‐10, IL‐12p70, and tumor necrosis factor (TNF)‐α. The procedure was carried out according to the manufacturer's instructions. Briefly, 50 μL of chemokine capture bead mixture was incubated with 50 μL of each recombinant standard or sample and 50 μL phycoerythrin (PE)‐conjugated detection antibody for 2 h at room temperature. The mixture was then washed to remove unbound PE detection reagent, and the data were acquired using a FACSCalibur flow cytometer (BD Biosciences, Bedford, MA). The levels of cytokines were quantified using CellQuest software (BD Biosciences).
2.10. Cell proliferation assay
A total of 1 × 104 cells were seeded in a 96‐well plate and cultured in DMEM containing 2% FBS without antibiotics. The cells were pretreated with 50 nmol/L of siGFRAL for 12 h. Then, the cells were treated with 50 ng/mL of rMIC‐1 for 24 h. Cell proliferation was assessed using a nonradioactive 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT)‐based cell proliferation assay kit (Roche, Basel, Switzerland).
2.11. Matrigel invasion assay
The in vitro invasion assay was performed in triplicate using growth factor‐reduced BD BioCoat Matrigel Invasion Chambers (BD Biosciences) according to the manufacturer's instructions. Briefly, PCa cells were pretreated with 50 nmol/L siGFRAL for 12 h, then 5 × 104 cells were seeded in the upper chamber and treated with or without 50 ng/mL rMIC‐1. DMEM containing 20% FBS was then placed in the lower chamber and incubated for 24 h. Following incubation, the non‐invading cells in the upper chamber were removed, and the membranes were stained with a Diff–Quik cell‐staining kit (Sysmex, Kobe, Japan) to count the total number of invading cells under a light microscope.
2.12. Western blotting
Proteins were extracted from the cultured cells using Complete Lysis‐M buffer (Roche, Switzerland). Equal amounts (10 μg) of protein were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride filter (ATTO), and hybridized with corresponding antibodies. The following antibodies were used: anti‐MIC‐1 (Cell Signaling), anti‐α smooth muscle actin (αSMA, Abcam), anti‐extracellular signal‐regulated kinase (ERK) 1/2, anti‐phospho‐ERK1/2 (pERK1/2, Thr202/Tyr204, Cell Signaling), and anti‐β‐actin antibodies (Cell Signaling).
2.13. Determination of serum level of MIC‐1
Serum samples were obtained from xenograft mice and another cohort of 67 patients with PCa who underwent radical prostatectomy between 2006 and 2010 at Akita University Hospital. The serum MIC‐1 levels were measured using an MIC‐1 ELISA Kit (Oxford Biomedical Research, Inc., Oxford, MI, USA) according to the manufacturer's instructions.
2.14. Histopathological analysis
Prostate tissue samples were obtained from the 67 patients with PCa. The mean age and serum MIC‐1 level of the patients with PCa were 66.4±4.5 years and 947.5 ± 271.5 pg/mL, respectively. A brief summary of clinicopathological data of the patients is shown in Supplementary Table S1. The expression of GFRAL, MIC‐1, and αSMA in human PCa tissues were evaluated using a rabbit anti‐human GFRAL polyclonal antibody (1:200, Abcam), rabbit anti‐human MIC‐1 polyclonal antibody (1:100, Cell Signaling), and rabbit anti‐human αSMA (1:200, Abcam). The immunohistochemical staining was performed as previously described [24]. The expression levels were scored by two investigators (MH and HN) who were blinded to the patients’ background and clinicopathological information. The GFRAL and MIC‐1 staining intensities in cancer cells and stromal cells were scored on a semi‐quantitative scale as follows: 1, negative or low; 2, moderate; and 3, strong staining. The percentage of αSMA‐positive cells in the stroma was evaluated and classified as low (≤5%), moderate (6%–20%), and high (>20%).
Hematoxylin and eosin staining was performed in the in vivo xenograft tumors, and the degree of invasiveness of the inoculated tumor cells into the intraperitoneal organs was microscopically evaluated. The percentage of Ki67‐positive cells in 400‐500 tumor cells in the area containing the highest density of the xenograft tumor was evaluated using an anti‐Ki67 antibody (1:800; Cell Signaling Technology).
2.15. Ethical approval
This study was approved by the Institutional Review Board of the Akita University School of Medicine. Human samples were collected after written informed consent was obtained from all patients.
2.16. Statistical analyses
All statistical analyses were performed using SPSS version 12 software (IBM Japan, Tokyo, Japan). All the values are presented as mean ± standard deviation. Statistical significance was evaluated by an unpaired Student's t‐test or analysis of variance repeated measurement for comparison between two or three means in each experiment. Differences were considered statistically significant at P < 0.05.
3. RESULTS
3.1. HFD enhances PCa progression, adipocyte infiltration, and adipose lipolysis in the tumor microenvironment in vivo
The mouse PCa model was generated by intraperitoneally inoculating PC‐3M‐luc‐C6 cells with stable luciferase expression. At four weeks after cancer cell injection, the mean weight of mice in the HFD group was higher than that in the CD group (20.4 ± 2.1 g vs. 18.0 ± 1.9 g, P = 0.075); the tumor burden, as evaluated by the total flux, was significantly higher in the HFD group than in the CD group (Figure 1A). Immunohistochemistry‐assessed Ki67 positivity was significantly higher in the HFD group than in the CD group (P = 0.027; Figure 1B). In addition, the hematoxylin and eosin staining of the mouse peritoneal tumor demonstrated a higher transmigration of PC‐3M‐luc‐C6 PCa cells into the peritoneal stroma in the HFD group than in the CD group (arrow in the middle panel, Figure 1C). Interestingly, HFD markedly stimulated adipocyte infiltration to the xenograft tumor microenvironment (Figure 1C, right panel). The adipocytes may provide many types of nutrients, such as FAs, for growing tumor cells [25]. Then, we investigated the role of adipocytes in the tumor microenvironment by measuring lipase activity in both the xenograft tumor and the serum. The lipase activities in both sample types were markedly higher in the HFD group than in the CD group (P = 0.033, Figure 1D; P = 0.018; Figure 1E). In addition, the FFA levels in both sample types were significantly higher in the HFD group than in the CD group (P = 0.025, Figure 1F; P = 0.008, Figure 1G). These findings suggest that a HFD influences PCa progression, and enhances adipocyte infiltration and lipolysis in the PCa microenvironment.
FIGURE 1.
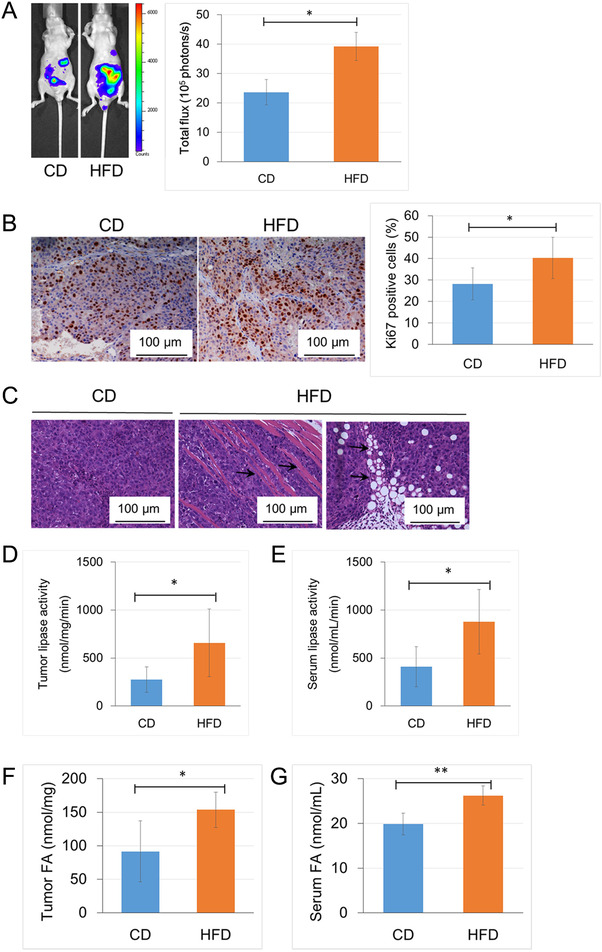
HFD influenced PCa progression and enhanced adipocyte infiltration and adipose lipolysis in the PCa microenvironment. PC‐3M‐luc‐C6 cells were intraperitoneally injected into mice which were randomly assigned to the CD group or the HFD group (5 mice per group). A. Tumor burden and the total flux were measured by the Xenogen IVIS™ imaging system with an intraperitoneal injection of luciferin 4 weeks after injection of the cells. B. Xenograft tumor sections from mice in the HFD and CD groups were subjected to immunohistological staining with an anti‐Ki67 antibody (bar, 100 μm). C. The slides of mouse xenograft tumor tissue were stained with hematoxylin and eosin. The mouse peritoneal tumors in the HFD group showed higher transmigration of PCa cells into the peritoneal stroma (middle panel, arrow) and stimulated adipocyte infiltration in the tumor microenvironment (right panel, arrow). D and E. Lipase activity in the xenograft tumors and the sera of mice were measured using a Lipase activity assay kit. G and F. FFA concentration in the tumor extract and sera of the xenograft mice was measured with a FFA quantification kit. *P < 0.05, **P < 0.01. Abbreviations: PCa, prostate cancer; HFD, high‐fat diet; CD, control diet; FFA, free fatty acid.
3.2. HFD and exogenous FFAs enhance the expression and secretion of MIC‐1 in vivo and in vitro
MIC‐1 is a divergent member of the transforming growth factor‐β family [26] and has been shown to be expressed in both PCa cells and prostate stromal fibroblasts [15, 16]. Therefore, we investigated the expression of MIC‐1 and GFRAL, the cognate receptor of MIC‐1, semi‐quantitatively by immunohistochemistry using the mouse xenograft tumor samples. Although the expression level of MIC‐1 was significantly higher in the HFD group than in the CD group (P = 0.031), there was no significant difference in the expression of GFRAL between the two groups (Figure 2A). In addition, the mean serum level of MIC‐1 in mice was significantly higher in the HFD group than in the CD group (P = 0.043; Figure 2B).
FIGURE 2.
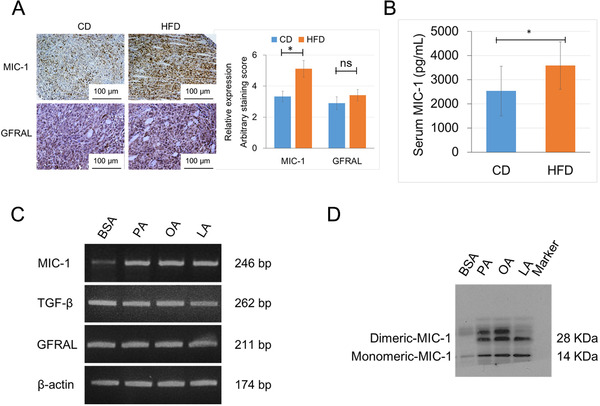
HFD and exogenous FFAs stimulated the expression and secretion of MIC‐1. A. The xenograft tumors were stained with antibodies to MIC‐1 and GFRAL, and the staining intensity was semi‐quantitatively evaluated. The staining level of MIC‐1 was significantly higher in the HFD group than in the CD group, but there was no significant difference in the staining level of GFRAL between the two groups. B. The mean serum level of MIC‐1 was significantly higher in the HFD group than in the CD group as measured by an MIC‐1 ELISA kit. C and D. PC‐3 cells were cultured with 0.125 mmol/L of PA, 0.25 mmol/l of OA, 0.15 mmol/l of LA, or 2% BSA (as the control) for 24 h. Cells were harvested, total RNA was extracted, and quantitative RT‐PCR was performed to detect MIC‐1, TGF‐β, GFRAL, and β‐actin. Proteins from equal volumes of the CM in these cells were subjected to Western blotting analysis using an anti‐human MIC‐1 antibody, and the expression of monomeric and dimeric MIC‐1 was markedly increased in the CM of PC‐3 cells treated with FFAs than that of the PC‐3 cells cultured with 2% BSA. *P < 0.05. Abbreviations: ns, not significant; MIC‐1, macrophage inhibitory cytokine‐1; GFRAL, glial‐derived neurotrophic factor receptor α‐like; PA, palmitic acid; OA, oleic acid; LA, linoleic acid; BSA, bovine serum albumin; CM, conditioned medium, ns: not significant.
Considering the accumulating evidence suggesting that PA stimulates MIC‐1 expression in many types of cancer cells [7, 27], we examined the role of increased FFAs on MIC‐1 expression under a HFD condition. The mRNA expression level of MIC‐1, but not GFRAL and TGF‐β, was significantly higher in PCa LNCaP, PC‐3, and DU145 cells after treatment with 0.125 mmol/L of PA, 0.25 mmol/L of OA, or 0.15 mmol/L of LA (Supplementary Fig. S1 and Figure 2C). In addition, the expression of monomeric and dimeric MIC‐1 was markedly increased in the CM of PC‐3 cells treated with FFAs compared with that of the PC‐3 cells cultured with 2% BSA as control (Figure 2D). The proliferation rate was increased in the PC‐3 and PC‐3M‐luc‐C6 cells following treatment with 50 ng/mL rMIC‐1, and the effect was attenuated by pretreatment with 50 nmol/L siGFRAL for 12 h (Supplementary Fig. S2a). Similarly, the invasive capacity of PC‐3 and PC‐3M‐luc‐C6 cells was significantly increased by treatment with rMIC‐1, and the effect was abrogated by pretreatment with siGFRAL (Supplementary Fig. S2b). These findings suggest that enhanced FFA release in the tumor microenvironment through upregulated adipose lipolysis would stimulate the expression and secretion of MIC‐1 in tumor cells under the HFD condition, and that the MIC‐1‐GFRAL signaling stimulated PCa progression.
3.3. Activation and cytokine secretion in PCa stromal fibroblasts by upregulated MIC‐1 in the HFD condition
The expression level of αSMA, an activation marker of stromal fibroblasts, was significantly higher in the xenograft tumor of HFD group than in the CD group (P = 0.022; Figure 3A). We measured the mouse serum cytokine levels using a human cytokine cytometric bead array for IL‐8, IL‐1β, IL‐6, IL‐10, TNFα, and IL‐12p70. The mean serum IL‐8 level was significantly higher in the HFD group than in the CD group (P = 0.019; Figure 3B). Next, we evaluated the relationship between the increased cytokine secretion in PrSC cells and the upregulated MIC‐1 in PCa cells. The IL‐8 and IL‐6 levels were significantly increased by 3.4 and 3.2 folds, respectively, in the CM of PrSC cells treated with 50 ng/mL rMIC‐1 and by 4.7 and 5.4 folds in the CM of PrSC cells co‐cultured with PC‐3 cells compared to untreated PrSC cells. However, the effect was markedly abrogated by pretreatment of the PC‐3 cells with 50 nmol/L siMIC‐1 for 12 h (Figure 3C). Similarly, the IL‐8 and IL‐6 levels were significantly higher in the CM of the PrSC cells co‐cultured with DU145 cells or co‐cultured with siMIC‐1‐pretreated DU145 cells for 12 h compared to untreated PrSC cells (Supplementary Fig. S3). In addition, the expression levels of αSMA and pERK1/2 were significantly upregulated in PrSC cells by treatment with 50 ng/mL rMIC‐1 (Figure 3D). The mRNA expression levels of IL‐8 and IL‐6 were markedly increased in PrSC cells by treatment with 50 ng/mL rMIC‐1, and the effect was attenuated by pretreatment with 1 μmol/L U0126, an ERK kinase inhibitor (Figure 3E). These findings suggest that upregulated MIC‐1 in PCa cells stimulates the surrounding stromal fibroblasts, resulting in increased secretion of pro‐tumorigenic cytokines such as IL‐8 and IL‐6 through activation of the ERK signaling pathway in the PCa microenvironment under a HFD condition.
FIGURE 3.
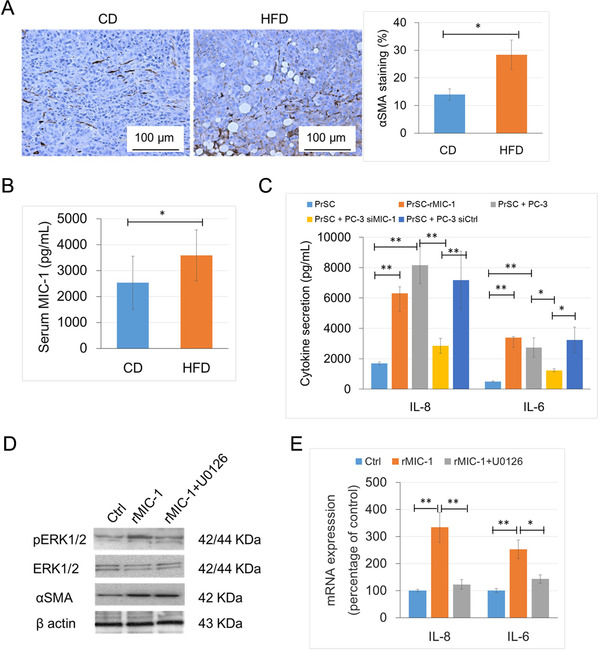
Activation and cytokine secretion in PCa stromal fibroblasts by the upregulation of MIC‐1 under a HFD conditions. A. The xenograft tumors were stained with an αSMA antibody, and the staining level was semi‐quantitatively evaluated. B. The serum IL‐8 level of mice was measured by a cytometric bead array kit. C. Cytokine secretion in PrSC cells stimulated by MIC‐1. PrSC cells were treated with 50 ng/mL rMIC‐1 or co‐cultured with PC‐3 cells for 24 h. Some of the PC‐3 cells were pretreated with 50 nmol/L siMIC‐1 or siCtrl for 12 h. D and E. PrSC cells were cultured in the presence or absence of 1 μmol/L U0126 for 1 h before treatment with 50 ng/mL rMIC‐1 for 3 h. D. Equal amounts of proteins (10 μg) from the cells were subjected to anti‐pERK1/2, anti‐ERK1/2, anti‐αSMA, and anti‐β‐actin antibodies. E.IL‐8 and IL‐6 mRNA levels were measured by quantitative RT‐PCR, normalized to the mRNA levels of β‐actin. *P < 0.05, and ** P < 0.01. Abbreviations: PrSC, prostate stromal fibroblasts; αSMA, α smooth muscle actin; siMIC‐1, MIC‐1 siRNA; siGFRAL, GFRAL siRNA; siCtrl, control siRNA; PrSC, prostate stromal fibroblast; rMIC‐1, recombinant MIC‐1.
3.4. MIC‐1 secretion in PCa cells and subsequent IL‐8 secretion in PrSC in a direct co‐culture system
To address the functional role of periprostatic adipocytes on MIC‐1 and cytokine expression in the PCa stromal microenvironment, a co‐culture assay was performed by direct incubation of periprostatic adipocytes from 13 PCa patients with PC‐3 cells and/or PrSC cells. The clinical characteristics of these 13 patients are summarized in Supplementary Table S1. The levels of adipose lipolysis and FFA release were significantly higher in the CM harvested from the co‐culture of periprostatic adipocytes with PC‐3 cells than in the CM from the periprostatic adipocytes cultured alone, regardless of the presence or absence of PrSC cells (adipose lipolysis; P = 0.028 and P = 0.022; FFA release: P = 0.012 and P = 0.018; Figure 4A and 4B). Interestingly, the levels of adipose lipolysis and FFA release were also increased in the co‐culture of periprostatic adipocytes and siMIC‐1‐pretreated PC‐3 cells (Figure 4A and 4B). In addition, the MIC‐1 level was significantly higher in the CM of the PC‐3 cells co‐cultured with periprostatic adipocytes and/or PrSC cells compared to that of the PC‐3 cells cultured alone (P = 0.007 and P = 0.006). On the contrary, the MIC‐1 level was significantly decreased in the siMIC‐1‐pretreated PC‐3 cells co‐cultured with periprostatic adipocytes in the presence or absence of PrSC than that of untreated PC‐3 cells (P = 0.003 and P = 0.005; Figure 4C). Interestingly, the increased FFA release of PrAC cells was significantly abrogated in the presence of 100 μmol/L NA, an inhibitor of adipocyte lipolysis [28] (Supplementary Fig. S4a). Furthermore, the increased MIC‐1 expression in co‐culture of periprostatic adipocytes with PC‐3 cells was also abrogated in the presence of 100 μmol/L NA (Supplementary Fig. S4b). The IL‐8 level was significantly higher in the CM of the co‐culture of PrSC cells with periprostatic adipocytes and PC‐3 than in the CM of PrSC cells cultured alone or co‐cultured with periprostatic adipocytes (P = 0.002 and P = 0.007; Figure 4D). Furthermore, IL‐8 was markedly decreased in the CM of PrSC cells co‐cultured with periprostatic adipocytes and siMIC‐1‐pretreated PC‐3 cells compared to that of PrSC cells co‐cultured with untreated PC‐3 cells (P < 0.05; Figure 4D). These findings strongly suggest that the periprostatic adipocytes in the tumor microenvironment have bidirectional crosstalk with PCa cells and the surrounding stromal cells and could enhance PCa progression through the upregulation of MIC‐1 in PCa cells. This, in turn, is thought to enhance IL‐8 production from PrSC cells, especially under conditions of a HFD and/or HFD‐mediated obesity.
FIGURE 4.
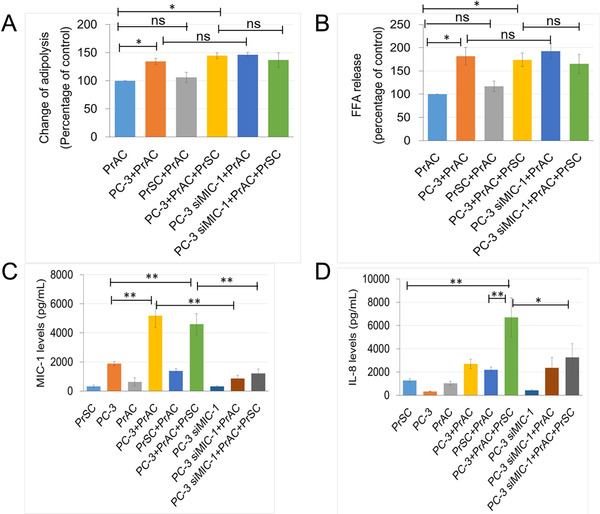
PrAC stimulates MIC‐1 secretion in PC‐3 cells and IL‐8 secretion in PrSC cells by upregulating adipose lipolysis and FFA release. The isolated PrAC from 13 patients who underwent radical prostatectomy were directly incubated with PC‐3 cells pretreated with 50 nmol/L siMIC‐1, and cultured in the presence or absence of PrSC for 48 h. A and B. The levels of adipose lipolysis and FFA release were significantly higher in the co‐culture of PrAC and PC‐3 cells than in PrAC cultured alone. C. The MIC‐1 level, as measured by an MIC‐1 ELISA kit, was significantly increased in the co‐culture of PC‐3 cells and PrAC, but decreased in the CM of the PC‐3 cells treated with siMIC‐1. D. The IL‐8 levels, as measured by an IL‐8 ELISA kit, were significantly higher in the CM of PrSC cells co‐cultured with PrAC and further in the presence of PC‐3 cells compared to those cultured alone or co‐cultured with PrAC only, but was significantly decreased in the CM of PrSC cells by co‐culture with PrAC and PC‐3 cells pretreated with siMIC‐1. *P < 0.05, ** P < 0.01. Abbreviations: ns, not significant; PrAC, periprostatic adipocytes; PrSC, prostate stromal fibroblasts; CM; conditioned medium.
3.5. Overexpression and secretion of MIC‐1 was correlated with cancer stroma activation and advanced human PCa progression
To further determine the role of MIC‐1 in human PCa progression, we performed MIC‐1 and αSMA immunohistochemistry in specimens of 67 PCa patients treated by radical prostatectomy. The clinical characteristics of these 67 patients are summarized in Supplementary Table S2. MIC‐1 was highly expressed in PCa cells and was also expressed in the surrounding tumor stromal cells (Supplementary Fig. S5). αSMA was predominantly expressed in the PCa stroma (Figure 5A). The 67 PCa patients were divided into the MIC‐1‐low group (735.0 ± 67.1 pg/mL, n = 33) and the MIC‐1‐high group (1206.9 ± 274.3 pg/mL, n = 34) according to the median serum level of MIC‐1 (881.2 pg/mL). In the MIC‐1‐low group, 51.5%, 36.4%, and 12.1% patients had low, moderate, and high αSMA staining, respectively, while 29.4%, 38.2%, and 32.4% of patients in the MIC‐1‐high group had low, moderate, and high αSMA staining (P = 0.011; Figure 5B). In addition, the mean serum levels of IL‐8 and IL‐6 were significantly higher in the MIC‐1‐high group than in the MIC‐1‐low group (P = 0.035 and P = 0.044, Figure 5C and 5D). In addition, the associations of serum MIC‐1 levels with clinicopathological characteristics of PCa patients were analyzed. High serum MIC‐1 levels were associated with high PSA levels (P = 0.009; Figure 5E), high Gleason score (P = 0.074; Figure 5F), high body mass index (P = 0.037; Figure 5G), and high serum lipase activity (P = 0.001; Figure 5H and Supplementary Table S2). These findings strongly suggest that the overexpression and secretion of MIC‐1 may play an important role in PCa progression by enhanced secretion of proinflammatory cytokines through the activation of stromal fibroblasts.
FIGURE 5.
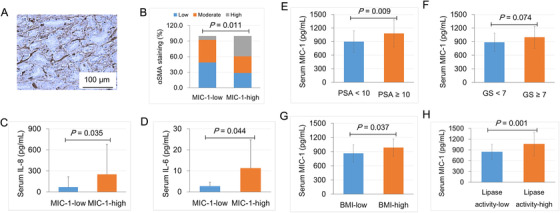
Overexpression and secretion of MIC‐1 was significantly correlated with cancer stroma activation and advanced PCa progression. The serum MIC‐1 levels in 67 patients with localized PCa were measured using an MIC‐1‐specific ELISA kit. A. The αSMA is expressed in the stroma of the PCa tumor section. B. The 51.5%, 36.4%, and 12.1% patients in the MIC‐1‐low group and 29.4%, 38.2%, and 32.4% in the MIC‐1‐high group were classified as having low, moderate, and high αSMA staining, respectively (P = 0.011). C and D. The serum cytokine concentration was measured by a cytometric bead array kit. E‐H The associations of serum MIC‐1 levels with clinicopathological characteristics of PCa patients were analyzed. High serum MIC‐1 levels were associated with high PSA levels (P = 0.009; E), high GS (P = 0.074; F), high BMI (P = 0.037; G), and high serum lipase activity (P = 0.001; H). Abbreviations: αSMA, α smooth muscle actin; PSA, prostate‐specific antigen; GS, Gleason score; BMI, body mass index.
3.6. GFRAL is expressed in PCa stroma and associated with androgen deprivation therapy and chemotherapy
To determine the role of GFRAL, a MIC‐1 functional receptor, in PCa progression, we performed GFRAL immunohistochemistry in specimens from the 67 PCa patients. GFRAL was predominantly expressed in the cytoplasm and on the membrane of PCa cells, and also in the surrounding stromal fibroblasts (Figure 6A and 6B). The staining level of GFRAL in cancer cells was not associated with neoadjuvant therapy (P = 0.208; Figure 6C), whereas the GFRAL staining level in the stromal fibroblasts was significantly lower in patients who received neoadjuvant chemotherapy and hormonal therapy than in those who did not (P = 0.017; Figure 6C). In addition, as shown in Supplementary Fig. S6, the upregulated mRNA levels of IL‐8 and IL‐6 in PrSC cells by rMIC‐1 were significantly abrogated by pretreatment with 50 nmol/L siGFRAL. Thus, GFRAL expression may be essential for the stimulation of cytokine expression in stromal fibroblasts and the secretion of MIC‐1 from PCa cells. Although the MIC‐1 was mainly expressed and secreted in the PCa cells (Supplementary Fig. S7), our data also suggest that MIC‐1‐GFRAL signaling in the tumor microenvironment plays a critical role in PCa progression by affecting the interaction between PCa cells and stromal fibroblasts.
FIGURE 6.
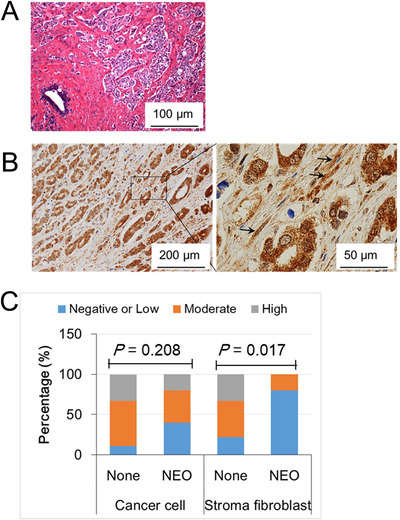
GFRAL is expressed in prostate cancer cells and the surrounding stromal fibroblasts. A. A representative image of hematoxylin and eosin staining of a high‐grade tumor. B. GFRAL immunostaining shows that GFRAL was predominantly expressed in the cytoplasm and membrane of cancer cells and stromal fibroblasts (arrow). C. The association of GFRAL expression with neoadjuvant treatment. Of the 19 patients, 10 did not receive neoadjuvant treatment (None), and 9 received NEO therapy. The GFRAL staining level in stromal fibroblasts was lower in the NEO group than in the None group (P = 0.017). Abbreviations: None, not receive neoadjuvant treatment; NEO, neoadjuvant androgen deprivation therapy and/or chemotherapy.
4. DISCUSSION
In the present study, we investigated the role of MIC‐1 signaling in PCa progression under HFD milieus and found that PCa stromal fibroblasts were activated and secreted IL‐8 and IL‐6 by the upregulation of MIC‐1 in PCa cells, in vivo and in vitro.
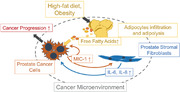
Although several reports have shown that HFD enhanced PCa progression through cytokine signaling, such as MIC‐1 [7], monocyte chemotactic protein‐1 [6], and IL‐8 and IL‐6 [29], the underlying mechanism of increased cytokine secretion has not been fully delineated. Prostate stromal fibroblasts represent a major component of prostate stroma and may play an important role in PCa tumorigenesis and cancer progression via the secretion of proinflammatory cytokines [9]. A recent report has shown that many types of cytokines, such as chemokine (C‐X‐C motif) ligand 1 (CXCL‐1), CXCL‐2, CXCL‐3, and IL‐8, are produced by prostate stromal cells via stimulating the secretion of IL‐1 by prostate epithelial cells [10]. In addition, periprostatic adipocytes may facilitate the metastatic extension of PCa cells by enhanced CCR3/CCL7 signaling in HFD‐induced obesity [19]. In the present study, IL‐8 and IL‐6 were secreted in activated prostate stromal fibroblasts as a consequence of elevated MIC‐1 signaling in PCa cells affected by HFD‐mediated adipocyte infiltration and FFA release in the tumor microenvironment of the PCa xenograft model mice. In addition, IL‐8 secretion was significantly increased in PrSC cells by MIC‐1 secreted by PC‐3. These findings suggest that a HFD enhances MIC‐1 expression and secretion in PCa cells and that the enhanced MIC‐1 stimulates the prostate stromal cells to increase the expression and secretion of pro‐tumorigenic cytokines such as IL‐8 and Il‐6, as shown in Figure 7. According to the accumulating evidence of the involvement of IL‐6 and IL‐8 in the progression of PCa [30, 31, 32, 33], our results indicate that the tumor‐promoting crosstalk between PCa cells and the stroma of the tumor microenvironment is caused by HFD‐induced PCa phenotype alteration (Figure 7).
FIGURE 7.
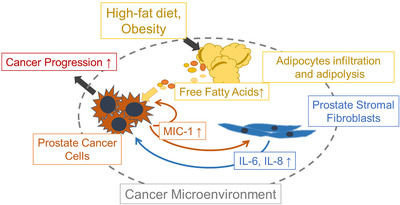
Schematic role of MIC‐1 signaling in the prostate cancer microenvironment under a HFD milieu. MIC‐1 production was increased in PCa cells, which was affected by adipocyte infiltration and adipose lipolysis. MIC‐1 directly stimulated surrounding PrSC cells to secrete protumorigenic cytokines such as IL‐8 and IL‐6 in the PCa stromal microenvironment, especially under a HFD condition. These upregulated functional cytokines directly and/or indirectly stimulated PCa cell proliferation, invasion, and metastasis. Abbreviations: MIC‐1, macrophage inhibitory cytokine‐1.
MIC‐1 is a TGF‐β family molecule, and the overexpression and enhanced secretion of MIC‐1 has been shown to stimulate PCa cell proliferation[14] and invasion [34], and be involved in anti‐cancer therapy resistance [35]. In addition, MIC‐1 is a known stress‐responsible molecule, and its expression and secretion have been shown to be affected by multiple factors or conditions such as dietary PA [7], hypoxia [34], drugs [35], and tumor suppressor molecule P53 [36]. In the present study, HFD was associated with prominent adipocyte infiltration to the xenograft stroma and significantly increased lipase activity and FFA release in both the tumor tissue and serum of the PCa xenograft mouse. In addition, the expression and secretion of MIC‐1 were directly stimulated by several exogenous FFAs such as PA, OA, and LA in PCa cells. Furthermore, the enhanced FFA release in periprostatic adipocytes significantly stimulated the expression and secretion of MIC‐1 in PCa cells following direct co‐culture with periprostatic adipocytes. Our data also showed that MIC‐1 is expressed and secreted with a much higher level from PCa cells than stromal cells (Supplementary Fig. S7). Although the underlying mechanism of upregulated adipocyte infiltration and adipose lipolysis in the xenograft tumor microenvironment is unclear, a recent report also showed that dietary enhanced expression of lipoprotein lipase and the subsequent increase of FFA secretion were required for the growth of PCa cells and that the alteration of adipocytes may be influenced by PCa cells [25]. These findings, including those of the current study, may suggest that the increase in FFAs in the tumor microenvironment by enhanced periprostatic adipocyte lipolysis is one of mechanisms that promote MIC‐1 expression in PCa cells. Moreover, metabolically activated MIC‐1 signaling may play an important role in HFD‐induced PCa progression.
The MIC‐1 functional receptor GFRAL has been shown to be highly expressed in the hindbrain and at a low‐level in adipose tissue, and may play a critical role in resistance to obesity in HFD‐fed mice [37, 38]. In addition, the binding of MIC‐1 with GFRAL can activate many intracellular signaling cascades, such as AKT (protein kinase B) and ERK1/2, through the tyrosine kinase co‐factor rearranged during transfection (RET) [38]. In the present study, we found that GFRAL was expressed in PCa cells and surrounding stromal fibroblasts, and the expression of GFRAL was linked with the effect of cancer neoadjuvant chemotherapy. Interestingly, while there was no significant difference in GFRAL expression in the xenograft PCa tumor tissue of the two diet groups, the MIC‐1‐mediated PCa cell proliferation, invasiveness, and cytokine secretion of PrSC were significantly abrogated by treatment with siGFRAL. These findings strongly suggest that MIC‐1/GFRAL signaling could indirectly stimulate PCa progression by promoting the expression and secretion of pro‐tumorigenic cytokines from cancer stromal cells in the tumor microenvironment and enhance the PCa progression through the direct and/or indirect signaling cascade.
A recent report has shown that the administration of rMIC‐1 significantly inhibited adiposity, metabolic dysfunction, and cytokine secretion through reduced food intake and body weight in mice fed with a HFD [37]. However, in the present study, there was no significant difference in the level of adipose lipolysis and FFA release in isolated periprostatic adipocytes following direct co‐culture with PCa PC‐3 cells compared to that of the PC‐3 cells pretreated with MIC‐1 siRNA (Figure 4A and 4B). A previous study suggested that the direct interaction of MIC‐1 with the cognate receptor GFRAL in the brain is required for metabolic effects [18]. Therefore, while MIC‐1 may have a role as an endocrine signaling molecule for systemic metabolic effects by the engagement of GFRAL in the brain, it may also promote cancer progression by directly stimulating cancer cells in an autocrine manner and by activating the cancer‐promoting interaction between cancer cells and stromal cells in the tumor microenvironment.
The major limitation of the present study was that the detailed mechanism of how FAs modulate MIC‐1 secretion could not be delineated. FAs are not only important metabolic substrates but also recognized to have many modulatory roles in a wide variety of intracellular processes [39]. Therefore, a further study needs to be conducted to elucidate the underlying mechanism of MIC‐1 secretion stimulated by each type of FA. Secondly, the present study did not discriminate intra‐ or extra‐cellular lipolysis and did not define the origin of FAs which modulate the MIC‐1 pathway and PCa progression by HFD. Thirdly, the in vivo adipocyte infiltration into xenograft tumors was pathologically determined with hematoxylin and eosin staining. Adipocyte‐specific immunohistochemistry may be a more promising way to determine adipose infiltration in tumors of animal models. Finally, although the high expression of αSMA was observed in the surrounding stroma in the HFD‐treated PCa xenograft mice, it remains unknown whether the effect was driven directly by MIC‐1 or indirectly via other cytokine signals such as IL‐6 and IL‐8.
5. CONCLUSIONS
In summary, we found that HFD facilitated adipocyte infiltration and FFA secretion in the tumor microenvironment and enhanced MIC‐1 expression in PCa cells. High levels of MIC‐1 subsequently activated PCa stromal fibroblasts to secrete protumorigenic cytokines, including IL6 and IL‐8, in the PCa microenvironment and accelerated PCa progression. Further, higher activity in MIC‐1/GFRAL signaling was clinically associated with PCa progression through activation and cytokine production in prostate stromal fibroblasts in human PCa specimens. Thus, MIC‐1 signaling in the PCa microenvironment could be an important target for chemoprevention and therapy for PCa.
DECLARATION
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Institutional Review Board of the Akita University School of Medicine approved all experiments, and all experiments were performed after obtaining individual written informed consent.
Animal studies were approved by the Committee for Ethics in Animal Experimentation of Akita University School of Medicine and were performed according to the Guideline for Animal Experiments, and these studies met the ethical standards required by the law and the guidelines on the use of experimental animals in Japan.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS, Grant No.: 16H02679, 16K10992, 19K09663) and AMED‐CREST, Japan Agency for Medical Research and Development (AMED).
AUTHOR CONTRIBUTIONS
M.H. and T.H. conceived the project and designed experiments. M.H., A.K. T.N., and H.N. performed the experiments. M.H., S.N., A.K., T.N., S.S. H.N., and T.H. analyzed the data. M.H., S.N., and T.H. wrote the manuscript.
Supporting information
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
ACKNOWLEDGMENTS
Not applicable.
Huang M, Narita S, Koizumi A, et al. Macrophage inhibitory cytokine‐1 induced by a high‐fat diet promotes prostate cancer progression by stimulating tumor‐promoting cytokine production from tumor stromal cells. Cancer Commun. 2021;41:389–403. 10.1002/cac2.12137
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Divella R, Daniele A, Savino E, Paradiso A. Anticancer Effects of Nutraceuticals in the Mediterranean Diet: An Epigenetic Diet Model. Cancer Genomics Proteomics. 2020;17(4):335‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsushita M, Fujita K, Nonomura N. Influence of Diet and Nutrition on Prostate Cancer. Int J Mol Sci. 2020;21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625‐38. [DOI] [PubMed] [Google Scholar]
- 4. Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narita S, Tsuchiya N, Saito M, Inoue T, Kumazawa T, Yuasa T, et al. Candidate genes involved in enhanced growth of human prostate cancer under high fat feeding identified by microarray analysis. The Prostate. 2008;68(3):321‐35. [DOI] [PubMed] [Google Scholar]
- 6. Huang M, Narita S, Numakura K, Tsuruta H, Saito M, Inoue T, et al. A high‐fat diet enhances proliferation of prostate cancer cells and activates MCP‐1/CCR2 signaling. The Prostate. 2012;72(16):1779‐88. [DOI] [PubMed] [Google Scholar]
- 7. Huang M, Narita S, Inoue T, Tsuchiya N, Satoh S, Nanjo H, et al. Diet‐induced macrophage inhibitory cytokine 1 promotes prostate cancer progression. Endocr Relat Cancer. 2014;21(1):39‐50. [DOI] [PubMed] [Google Scholar]
- 8. Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocrine‐related cancer. 2012;19(6):R187‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross‐talk between paracrine‐acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244‐53. [DOI] [PubMed] [Google Scholar]
- 10. Kogan‐Sakin I, Cohen M, Paland N, Madar S, Solomon H, Molchadsky A, et al. Prostate stromal cells produce CXCL‐1, CXCL‐2, CXCL‐3 and IL‐8 in response to epithelia‐secreted IL‐1. Carcinogenesis. 2009;30(4):698‐705. [DOI] [PubMed] [Google Scholar]
- 11. Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61(22):8135‐42. [PubMed] [Google Scholar]
- 12. Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical cancer research: an official journal of the American Association for Cancer Research.. 2002;8(9):2912‐23. [PubMed] [Google Scholar]
- 13. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC‐1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF‐beta superfamily. Proc Natl Acad Sci U S A. 1997;94(21):11514‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, et al. Role of macrophage inhibitory cytokine‐1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66(10):4983‐6. [DOI] [PubMed] [Google Scholar]
- 15. Bruzzese F, Hagglof C, Leone A, Sjoberg E, Roca MS, Kiflemariam S, et al. Local and systemic protumorigenic effects of cancer‐associated fibroblast‐derived GDF15. Cancer Res. 2014;74(13):3408‐17. [DOI] [PubMed] [Google Scholar]
- 16. Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, et al. The propeptide mediates formation of stromal stores of PROMIC‐1: role in determining prostate cancer outcome. Cancer Res. 2005;65(6):2330‐36. [DOI] [PubMed] [Google Scholar]
- 17. Jones AC, Antillon KS, Jenkins SM, Janos SN, Overton HN, Shoshan DS, et al. Prostate field cancerization: deregulated expression of macrophage inhibitory cytokine 1 (MIC‐1) and platelet derived growth factor A (PDGF‐A) in tumor adjacent tissue. PLoS One. 2015;10(3):e0119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017;23(10):1215‐19. [DOI] [PubMed] [Google Scholar]
- 19. Laurent V, Guerard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duong MN, Geneste A, Fallone F, Li X, Dumontet C, Muller C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget. 2017;8(34):57622‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li‐Ning‐Tapia EM, et al. CXCL1 mediates obesity‐associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell‐Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carswell KA, Lee MJ, Fried SK. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol Biol. 2012;806:203‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang M, Koizumi A, Narita S, Inoue T, Tsuchiya N, Nakanishi H, et al. Diet‐induced alteration of fatty acid synthase in prostate cancer progression. Oncogenesis. 2016;5:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10(3):427‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine‐1 in cancer. J Cell Physiol. 2010;224(3):626‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim JH, Kim KY, Jeon JH, Lee SH, Hwang JE, Lee JH, et al. Adipocyte culture medium stimulates production of macrophage inhibitory cytokine 1 in MDA‐MB‐231 cells. Cancer Lett. 2008;261(2):253‐62. [DOI] [PubMed] [Google Scholar]
- 28. Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, et al. PUMA‐G and HM74 are receptors for nicotinic acid and mediate its anti‐lipolytic effect. Nat Med. 2003;9(3):352‐5. [DOI] [PubMed] [Google Scholar]
- 29. Huang M, Narita S, Inoue T, Koizumi A, Saito M, Tsuruta H, et al. Fatty acid binding protein 4 enhances prostate cancer progression by upregulating matrix metalloproteinases and stromal cell cytokine production. Oncotarget. 2017;8(67):111780‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Culig Z, Puhr M. Interleukin‐6 and prostate cancer: Current developments and unsolved questions. Mol Cell Endocrinol. 2018;462(Pt A):25‐30. [DOI] [PubMed] [Google Scholar]
- 31. Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8‐CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maxwell PJ, Coulter J, Walker SM, McKechnie M, Neisen J, McCabe N, et al. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN‐deficient prostate carcinoma. Eur Urol. 2013;64(2):177‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peinado H, Zhang H, Matei IR, Costa‐Silva B, Hoshino A, Rodrigues G, et al. Pre‐metastatic niches: organ‐specific homes for metastases. Nat Rev Cancer. 2017;17(5):302‐17. [DOI] [PubMed] [Google Scholar]
- 34. Mimeault M, Johansson SL, Batra SK. Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen‐independent prostate cancer cells by downregulating macrophage inhibitory cytokine‐1. Br J Cancer. 2013;108(5):1079‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, et al. Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling. Cancer Res. 2009;69(19):7696‐703. [DOI] [PubMed] [Google Scholar]
- 36. Shim M, Eling TE. Protein kinase C‐dependent regulation of NAG‐1/placental bone morphogenic protein/MIC‐1 expression in LNCaP prostate carcinoma cells. J Biol Chem. 2005;280(19):18636‐42. [DOI] [PubMed] [Google Scholar]
- 37. Tsai VW, Zhang HP, Manandhar R, Lee‐Ng KKM, Lebhar H, Marquis CP, et al. Treatment with the TGF‐b superfamily cytokine MIC‐1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet‐induced obesity. Int J Obes (Lond). 2018;42(3):561‐71. [DOI] [PubMed] [Google Scholar]
- 38. Mullican SE, Lin‐Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23(10):1150‐7. [DOI] [PubMed] [Google Scholar]
- 39. Glatz JF, Luiken JJ. Fatty acids in cell signaling: historical perspective and future outlook. Prostaglandins Leukot Essent Fatty Acids. 2015;92:57‐62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Supporting infromation
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


