Abstract
We present a multimodality pictorial review of axillary lymphadenopathy in patients recently vaccinated against COVID-19. As the mass vaccination programme continues to be rolled out worldwide in an effort to combat the pandemic, it is important that radiologists consider recent COVID-19 vaccination in the differential diagnosis of unilateral axillary lymphadenopathy and are aware of typical appearances across all imaging methods. We review current guidelines on the management of unilateral axillary lymphadenopathy in the context of recent COVID-19 vaccination.
Introduction
The Medicines and Healthcare products Regulation Agency (MHRA) approved the Pfizer/BioNTech vaccine for use in the UK on 2 December 20201 followed by the Oxford–AstraZeneca vaccine on 30 December 2020.2 There followed a mass vaccination programme, which aims to offer all adults in the UK the first dose of a COVID-19 vaccination by the end of July 2021. Although the MHRA reported lymphadenopathy as an uncommon side effect of both vaccines (occurring in ≥0.1% to <1% of patients),1 , 2 their widespread use has resulted in benign unilateral axillary lymphadenopathy becoming an increasingly encountered side effect.3 , 4 Although the link between vaccination and unilateral axillary lymphadenopathy and vaccination is not new,5, 6, 7 early experiences show that the incidence is higher with the COVID-19 vaccine compared to other vaccines as it invokes a strong immune response.8
This review aims to demonstrate the imaging appearances of benign reactive hyperplasia across various imaging techniques in patients with a recent history of COVID-19 vaccination. We will then discuss current guidelines and management strategies.
The patients in this series were selected from two institutions in January and February 2021, shortly after the vaccination programme commenced. The cases include patients with a known malignancy who underwent staging or surveillance imaging, as well as patients without a history of malignancy presenting to symptomatic breast clinic.
Ultrasound
Ultrasound offers the most thorough assessment of the size and morphology of axillary lymph nodes due to its high soft-tissue resolution. Axillary lymphadenopathy post-COVID-19 vaccination may be discovered as part of assessment of a palpable axillary lump or as an incidental finding, for example, as part of breast ultrasound. In the latter, this may cause a diagnostic conundrum as other differentials include metastases from breast cancer.
Sonographic appearances of COVID-19 vaccine-related lymphadenopathy are non-specific and include lymph node enlargement as measured by increased short-axis diameter, a thickened cortex (Figure 1, Figure 2, Figure 3 ) and loss of the normal fatty hilum (Fig 4 ). The affected lymph node may be hypervascular on colour Doppler (Fig 1b). The contralateral axillary lymph nodes should be of normal size in the absence of concurrent pathology.
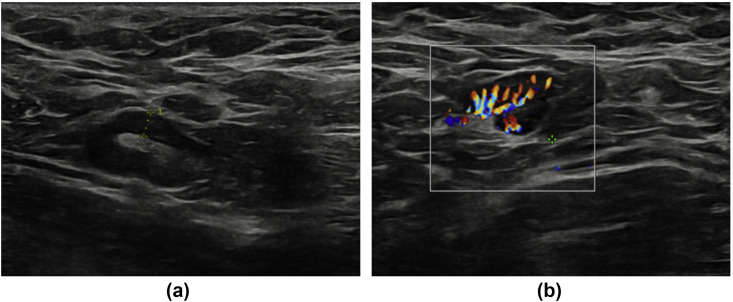
Figure 1.
A 29-year-old woman with no significant past medical history presented to breast clinic with nodularity in both breasts. An ultrasound of both breasts and axilla was performed. No abnormality was detected in either breast; however, grey-scale (a) and colour Doppler (b) ultrasound images of the left axilla revealed a lymph node with uniform cortical thickening measuring up to 3.9 mm. On questioning, the patient revealed that she has received the COVID-19 vaccine in her left arm 7 days earlier.
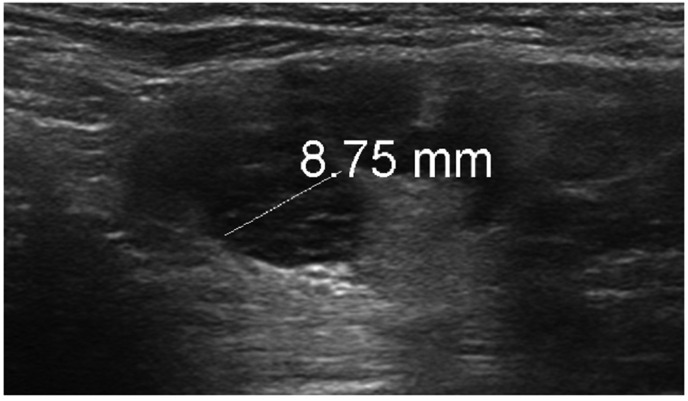
Figure 2.
A 37-year-old woman with bilateral subpectoral implants but no other significant past medical history presented to breast clinic with a 2-week history of palpable left axillary lump. Grey-scale ultrasound of the left axilla revealed a lymph node with cortical thickening measuring up to 8.75 mm. An ultrasound-guided core biopsy of the lymph node was performed, which confirmed reactive changes only. The patient had received the COVID-19 vaccine in the left arm approximately 1-week prior to the lymph node becoming clinically palpable.
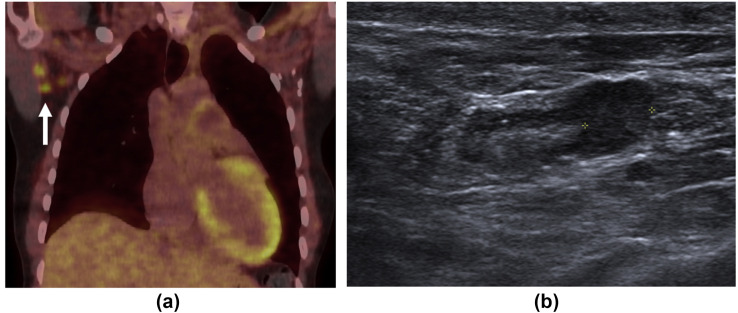
Figure 3.
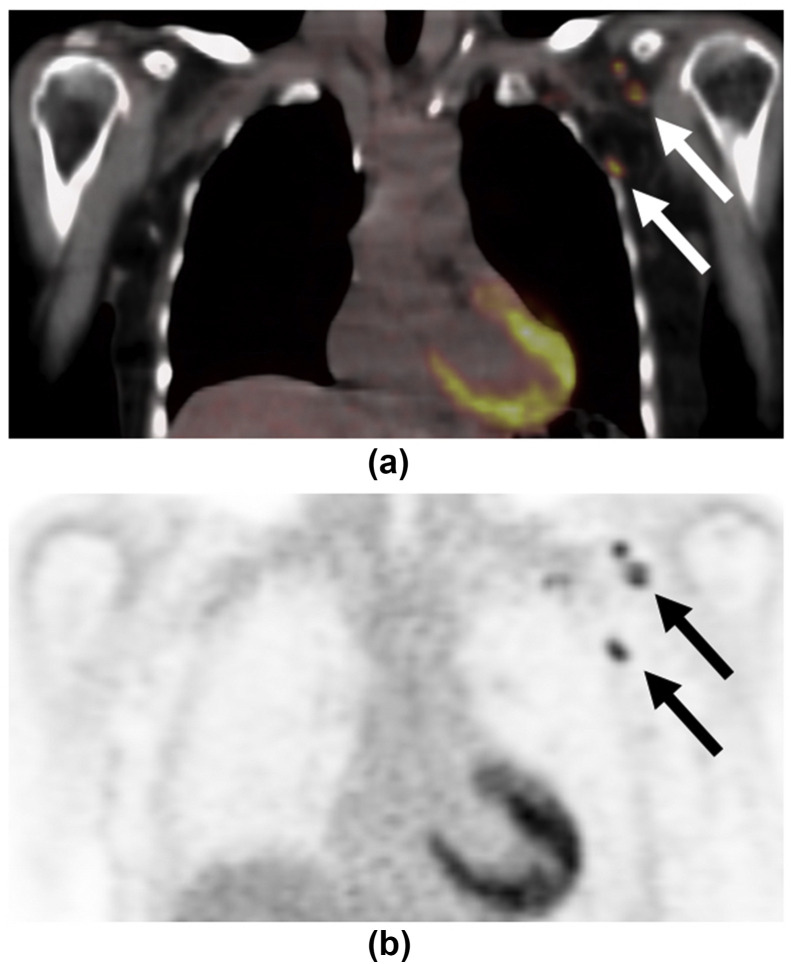
A 48-year-old woman with a history of left-sided breast cancer diagnosed 7 years previously and treated with left mastectomy and axillary node clearance presented for an 18F-FDG PET/CT restaging examination. (a) Coronal fused PET/CT demonstrated avid right axillary lymph nodes (arrow). (b) Grey-scale ultrasound demonstrated a right axillary lymph node with a thickened cortex measuring up to 6.5 mm.
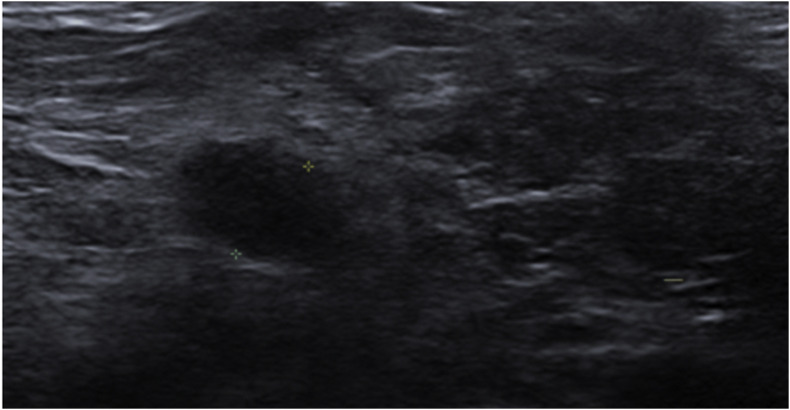
Figure 4.
A 74-year-old woman with a history of left-sided breast cancer treated with left mastectomy 8 years previously presented for surveillance imaging. Grey-scale ultrasound revealed no focal breast lesion but demonstrated a right axillary lymph node with a thickened cortex measuring 8 mm as shown. The patient had recently returned from Russia, where she had received the first dose of the Gam-COVID-Vac (Sputnik V vaccine) 4 weeks prior to imaging and her second dose 12 days prior to imaging.
Integrated positron-emission tomography with computed tomography
Following COVID-19 vaccination, integrated 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) positron-emission tomography with computed tomography (PET/CT) may demonstrate hypermetabolic axillary lymph nodes ipsilateral to the vaccinated arm.
The avid nodes may be single or multiple, as in Fig 5 . Hypermetabolic nodes may also be seen in the subpectoral region, as in Fig 6 . On the CT component, the hypermetabolic nodes may be enlarged, as in Fig 7 , or may be within normal size limits.
Figure 5.
A 67-year-old woman with a history of metastatic right-sided breast cancer diagnosed 2 years previously presented for a restaging examination. (a) Coronal fused PET/CT and (b) coronal PET only and (c) contrast-enhanced axial CT images demonstrated intense uptake in non-enlarged left axillary nodes (arrows). The patient received the COVID-19 vaccine in the left arm 2-weeks earlier.
Figure 6.
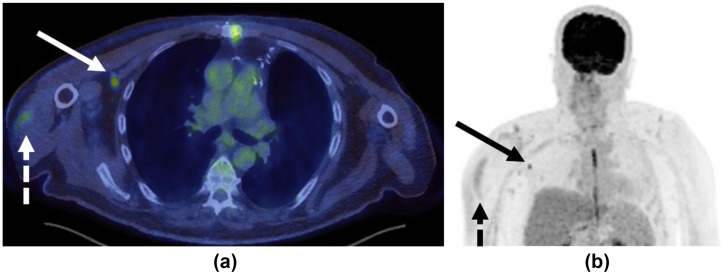
A 67-year-old man with known history of colorectal malignancy had routine surveillance 18F-FDG PET/CT imaging 9 days post-COVID-19 vaccination in the right arm. (a) Axial fused integrated positron emission tomography with computed tomography (PET/CT) and (b) maximum intensity projection (MIP) images demonstrated mild increased uptake at site of injection in the right deltoid (dashed arrows) and moderate increased uptake (SUVmax 4.6) in a solitary right subpectoral lymph node (solid arrows).
Figure 7.
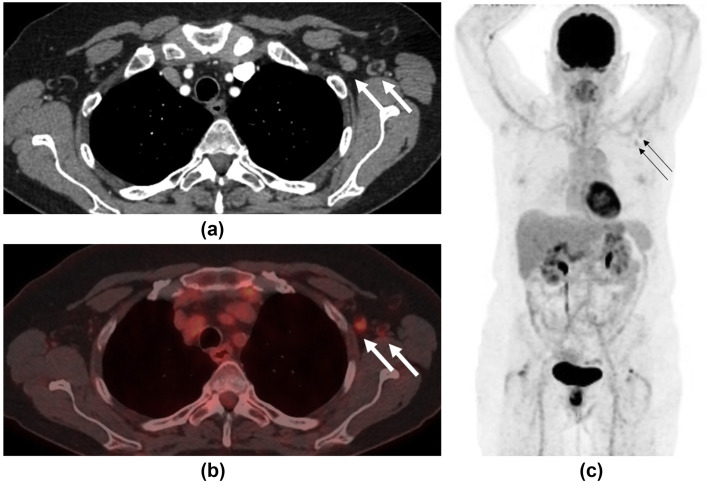
A 62-year-old woman with a history of bronchial neuroendocrine tumour presented for surveillance contrast-enhanced CT imaging 5 days after receiving her COVID-19 vaccination in her left arm. (a) This demonstrated new left axillary lymphadenopathy with nodes measuring up to 1.3 cm in short axis diameter (arrows). Further imaging with 18F-FDG PET/CT was performed 19-days post-vaccination to assess for disease recurrence. (b) Axial fused PET/CT and (c) MIP demonstrated faint increased uptake in left axillary lymph nodes (arrows) with an SUVmax of 2.6. The largest node had reduced in size to 4 mm, suggesting the initial lymph node enlargement was due to reactive hyperplasia.
An important finding that indicates the hypermetabolic lymph node is likely to be vaccine related is the synchronous finding of hypermetabolic inflammation in the ipsilateral deltoid muscle, corresponding to the site of injection, as seen in Figure 6, Figure 8 . In Fig 7, the FDG-uptake within the axillary lymph node persisted for almost 3 weeks. Other early data have shown this to persist beyond 5 weeks post-injection,9 and to last longer after the second vaccine dose.10
Figure 8.
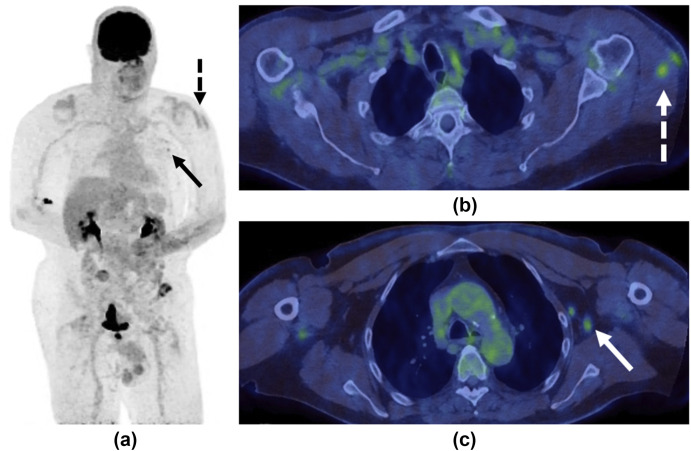
A 69-year-old man presented with episodes of visual loss in the right eye. 18F-FDG PET/CT was performed to investigate possible vasculitis. (a) MIP, (b,c) fused PET/CT and (d) CT only images demonstrated faint uptake at the site of injection in the left deltoid muscle (dashed arrows) and moderately avid (SUVmax 4.3) but non-enlarged left-sided axillary lymph nodes (solid arrows). On further questioning the patient revealed that she had received the COVID-19 vaccination in the left arm 14 days earlier.
Magnetic resonance imaging
COVID-19 vaccine-related axillary lymphadenopathy is detected much less commonly on magnetic resonance imaging (MRI) than on ultrasound and CT due to the lower number of MRI studies performed. It may be detected on imaging of the breasts, thorax, or musculoskeletal MRI of the upper limbs. Findings are again non-specific, including axillary lymph nodes with a thickened cortex, which may or not be enlarged. There may be increased signal on T2 and short-tau inversion recovery (STIR) sequences in the ipsilateral deltoid muscle and overlying skin corresponding to oedema secondary to local inflammation at the injection site. This would increase confidence that any lymphadenopathy was vaccine related.
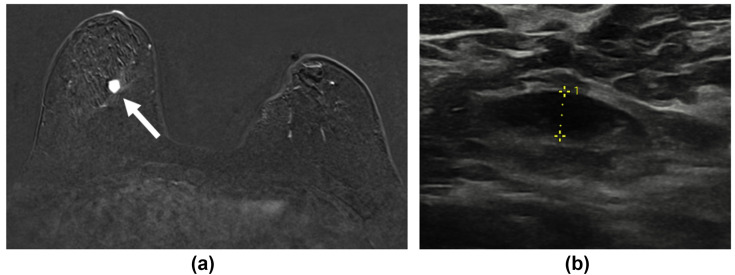
It is likely that axillary lymphadenopathy will be seen more frequently on breast MRI compared to mammography as it provides greater visualisation of the axillary node basin. In Fig 9 , the initial breast MRI, which detected a new right breast malignancy, showed no lymphadenopathy; however, the follow-up ultrasound showed an enlarged ipsilateral axillary node (Fig 9b) concerning for nodal metastases. The patient had an ipsilateral COVID-19 vaccine 3 days earlier in the right arm. Subsequent biopsy of the lymph node demonstrated reactive hyperplasia only.
Figure 9.
A 57-year-old woman on the high-risk screening pathway due to BRCA mutation was recalled to assessment clinic following the finding of a new opacity in the lower right breast on screening mammograms. She had a history of contralateral breast cancer treated with wide local excision 6-years previously. (a) Contrast-enhanced subtracted MRI image demonstrated an enhancing lesion in the central lower aspect of the right breast (white arrow), but no enlarged axillary lymph nodes. (b) Grey-scale ultrasound performed 2-weeks later demonstrated an enlarged right axillary lymph node. Both the index lesion and the lymph node were biopsied. The breast mass was confirmed to be a new malignancy; however, the lymph node was confirmed as reactive change only.
CT
Due to the high volume of CT studies performed, it is likely that axillary lymphadenopathy will be detected with increasing frequency during this mass-vaccination period. Findings suggestive of COVID-19 vaccine-related lymphadenopathy include enlarged or borderline enlarged axillary lymph nodes (Fig 10 ) and a thickened cortex. There may be subtle surrounding fat stranding (Fig 10). CT is, however, less sensitive at detecting COVID-19-related axillary lymphadenopathy than PET/CT, as shown in Fig 6, increased FDG uptake may be seen in non-enlarged lymph nodes that appear normal on CT.
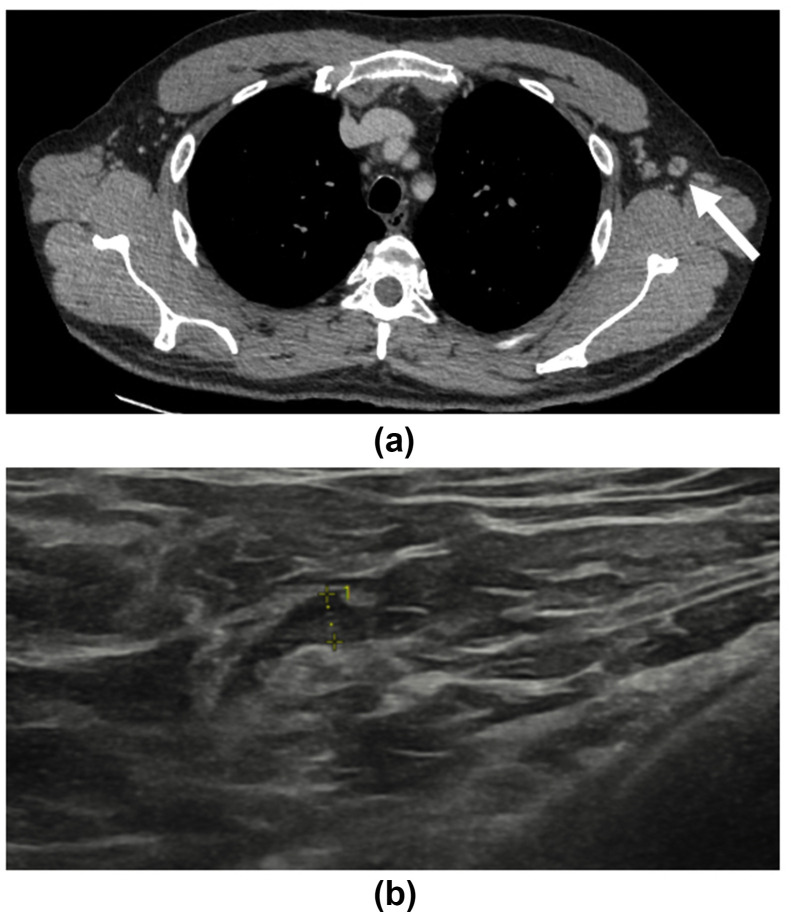
Figure 10.
A 51-year-old man with a history of left-sided chromophobe renal cell carcinoma who underwent left nephrectomy 3 years earlier presented for surveillance CT imaging. (a) This demonstrated minor interval enlargement of a left axillary lymph node measuring 9.7 mm in short axis, with a preserved fatty hilum (arrow). Minor surrounding fat stranding is noted. The patient had received the COVID-19 vaccination just 3-days earlier. (b) Follow-up grey-scale ultrasound images taken 5 weeks after vaccination demonstrated a left axillary lymph node, which had significantly improved in appearance with a thickened cortex measuring 2.9 mm in keeping with benign reactive hyperplasia.
Discussion
The present review is a multi-modality pictorial illustration of unilateral axillary lymphadenopathy in patients who had received their COVID-19 vaccination in the ipsilateral arm several days to several weeks prior to imaging. This is an increasingly common finding as more of the population receives their COVID-19 vaccine.
The differential for unilateral axillary lymphadenopathy is wide and includes breast cancer as the most common malignant differential, as well as a range of benign causes such as infection, inflammation, or trauma to the ipsilateral breast, arm and chest wall, as well as recent vaccination. A particular diagnostic challenge is encountered when this finding is detected in patients with a history of breast cancer due to the concern of metastatic disease.
National Institute for Health and Care Excellence (NICE) guidance for assessment of enlarged axillary nodes advocates a “watch and wait” approach, with referral to a specialist if persistent at 6 weeks. This guidance has not changed in light of the COVID-19 pandemic. Public Health England Screening issued advice in February stating that the scheduling of mammograms should not be postponed following COVID-19 vaccination. The guidance states that lymphadenopathy noted at screening must always be recalled for assessment even if the patient reports recent vaccination.11
This approach differs from guidance in the United States. There, the Society of Breast Imaging (SBI) released guidance in late January 2021 advising that women with unilateral axillary adenopathy who received a COVID-19 vaccination in the ipsilateral arm in the preceding 4 weeks should receive a short-term follow-up examination 4–12 weeks following the second vaccine dose. They suggest information on vaccine status (including date and laterality) is obtained prior to imaging. They also suggest that screening examinations are performed prior to the first COVID-19 vaccination or 4–6 weeks following the second dose.12 The Canadian Society of Breast Imaging also endorsed the SBI recommendations.
In February, further guidance from a multidisciplinary panel of experts was published in the journal Radiology. This also endorsed collecting vaccine information in every pre-imaging questionnaire and scheduling imaging either before or 6 weeks after the second vaccine dose, unless it is being performed for an urgent indication. In patients with clinically evident axillary adenopathy in the setting of recent ipsilateral vaccination, they suggest observing for at least 6 weeks before referring for imaging evaluation. They advise short-interval follow-up imaging with ultrasound with or without biopsy at least 6 weeks later if there is a higher risk of metastatic adenopathy, such as in those patients with breast, head and neck, upper extremity/trunk melanoma, or lymphoma.
More recently, in March 2021, Journal of the American College of Radiology proposed using “BIRADS 2 Benign” assessment with clinical follow-up for isolated unilateral adenopathy after recent COVID-19 vaccination in the ipsilateral arm.13 This less conservative approach will inevitably result in fewer follow-up examinations.
At the Royal Free Hospital, information is collected on COVID-19 vaccination history as part of the patient questionnaire prior to imaging. This is critical information, which aids interpretation of imaging findings, avoids unnecessary biopsies, and thereby reduces patient anxiety.
NICE guideline recommendations of follow-up imaging at 4–6 weeks in women with axillary adenopathy and a recent ipsilateral COVID-19 vaccine history are suitable for otherwise healthy individuals; however, until more data on the duration of radiologically evident axillary lymphadenopathy are obtained and follow-up protocols determined, management should be guided by clinical context, with a low threshold for short-term follow-up imaging and/or tissue diagnosis in high-risk patients, such as those with a history of ipsilateral breast cancer, melanoma, or lymphoma.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.UK government regulatory approval decision statement. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19 Available at: (accessed 27/02/2021)
- 2.UK government regulatory approval decision statement. https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca Available at: (accessed 27/02/2021)
- 3.Washington T., Bryan A., Clemow C. Adenopathy following COVID-19 vaccination. Radiology. 2021 Feb 24 doi: 10.1148/radiol.2021210236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Özütemiz C., Krystosek L.A., Church A.L., et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021 Feb 24 doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J., Powderly W.G., Opal S.M. Infectious diseases. Amsterdam. Elsevier Health Sci. 2017;2(4):145. [Google Scholar]
- 6.Studdiford J., Lamb K., Horvath K., et al. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28:1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 7.Panagiotidis E., Exarhos D., Housianakou I., et al. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1) Eur Radiol. 2010;20:1251–1253. doi: 10.1007/s00330-010-1719-5. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds C.E., Zuckerman S.P., Conant E.F. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021 Feb 5 doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 9.Shirone N., Shinkai T., Yamane T., et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26:248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 10.Baden L.R., Sahly El H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England Advice for women following COVID-19 vaccination Available at: https://www.england.nhs.uk/south/wp-content/uploads/sites/6/2021/03/20210308_CascadeCovidVaccine-v01.00-002.pdf (accessed 20/4/2021)
- 12.Grimm L., Destounis S., Dogan B., et al. Society of Breast Imaging Patient Care and Delivery Committee. SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf Available at: (Accessed 20/4/2021)
- 13.Lehman C.D., D'Alessandro H.A., Mendoza D.P., et al. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol. 2021 Mar 4;S1546–1440(21) doi: 10.1016/j.jacr.2021.03.001. 00212-X. [DOI] [PMC free article] [PubMed] [Google Scholar]