Abstract
Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2 (COVID-19), came as a significant health care challenge for humans in 2019–20. Based on recent laboratory and epidemiological studies, a growing list of mutations in the virus has the potential to enhance its transmission or help it evade the immune response. To further compound the problems, there are considerable challenges to the availability of effective, affordable, safe vaccines on a mass scale. These impediments have led some to explore additional options available in traditional medicines, especially immune-boosting natural products. Saffron has been used for centuries to treat fever, bronchitis, cold and other immune, respiratory disorders. Herein, we discuss the potential role of saffron during and after COVID-19 infection, focusing on immunomodulation, respiratory, renal, and cardiovascular functions. As a nutraceutical or drug supplement, it can alleviate the magnitude of COVID-19 symptoms in patients. The anti-inflammatory, antioxidant, and other medicinal properties attributed to saffron bioactive compounds can help in both pre-and post-infection management strategies. The abnormalities associated with COVID-19 survivors include anxiety, depression, sleep disturbances, and post-traumatic stress disorder. Saffron can help manage these post-hospitalization abnormalities (sub-acute and chronic) too, owing to its anti-depressant property. It can help common people boost immunity and manage depression, stress and anxiety caused due to prolonged lockdown, isolation or quarantine.
Keywords: Saffron, Crocus sativus L., Coronavirus, COVID-19, Immunity, Stress, Depression, Herbal medicines, Pandemics, Lockdown
Saffron; Crocus sativus L.; Coronavirus; COVID-19; Immunity; Stress; Depression; Herbal medicines; Pandemics; Lockdown
1. Introduction
The respiratory pandemic COVID-19 (Coronavirus disease 2019) is the third epidemic of zoonotic origin to occur in the present century. COVID-19 was declared a pandemic by WHO on 12th March 2020, and its causative agent was named SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). Based on information shared on World Health Organization (WHO) and Centres for Disease Control and Prevention, USA (CDC, USA) portals, people suffering from SARS-CoV-2 may possess cough, congestion or runny nose, sore throat, fever or chills, difficulty in breathing or shortness of breath, headache, muscle or body aches, fatigue, loss of smell or taste, vomiting or nausea, diarrhea, and some patients may need hospitalization. The incubation period of COVID-19 ranges from 1 to 14 days, with an average of 5–6 days in most patients. Till 11th April, 2021 nearly 135.70 million people were infected worldwide, out of which about 2.93 million lost their lives (WHO, 2021) while the maximum number of deaths (approx. 5,75,205; 1.8%) were reported in the USA, and the maximum percentage of deaths was 19.5% in Yemen (COVID-19 Stats). Even though most infected individuals suffer only mild to moderate illness, the majority of older and co-morbid patients with diseases like hypertension, diabetes, asthma, etc., develop severe illness and die. The depressed immune function has increased susceptibility to novel coronavirus infection (Brahmbhatt, 2020).
Coronaviruses are enveloped, positive-sense, single-stranded RNA viruses of ~30 kb. Based on their genomic structure, these are divided into 4 genera (α, β, γ, and δ). Only two genera (α and β) infect mammals (Rabi et al., 2020). α coronaviruses (229E and NL63) cause common cold and croup (laryngotracheobronchitis), while β coronavirus examples are SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2 (Yuki et al., 2020). Although the vaccine development against SARS-CoV-2 is good news, there are some concerns about the safety, allergic reactions, free-access, affordability, and efficacy against new variants, and the possibility of generating a lethal mutant of the virus in the future. Further, thirty to forty percent of COVID-19 survivors have shown the symptoms of anxiety, depression, sleep disturbances, and post-traumatic stress disorder (Nalbandian et al., 2021). In a large-scale analysis of nearly sixty thousand COVID-19 survivors within 90 d after COVID-19 diagnosis, it has been estimated that the probability of occurrence of new psychiatric illnesses is 5.8% constituting predominantly anxiety disorder (4.7%), mood disorder (2%), insomnia (1.9%), dementia (1.6%) (Taquet et al., 2021). It has been further suggested that a multi-disciplinary collaboration is essential for physical and mental health care to survivors of acute COVID-19 (Nalbandian et al., 2021).
Given the issues discussed above, it is high time for people to boost their immune system. No other spice is as intriguing and splendid as saffron (Crocus sativus L.). It is associated with Greek gods, hanging gardens of Babylonia, Song of Solomon (Bible) for its essence and aroma. Medical practitioners like Hippocrates and Pliny have used it, and famous women like Cleopatra made its use in cosmetics (Husaini, 2010). Many in vitro and in vivo studies have confirmed the saffron as an antiviral, antioxidant, bronchodilator, anti-inflammatory, and strong immune booster acting on humoral as well as cellular immunity (Bhat et al., 2020; Kadri, 2014; Alam et al., 2020; Alshehri et al., 2017; Chiavaroli et al., 2017; Shahbazi and Bolhassani, 2016).
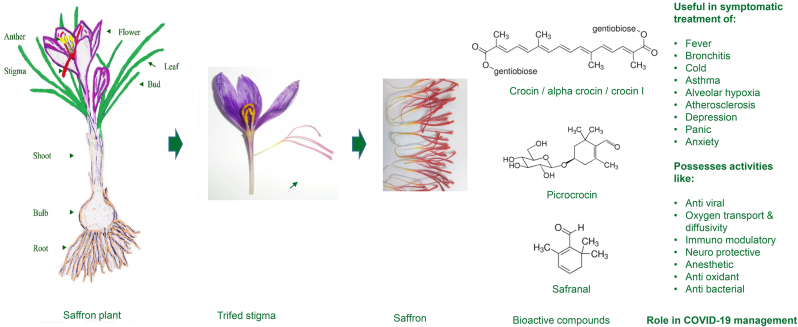
In this paper, we highlight the properties of saffron (Figure 1) that could help alleviate the symptoms of SARS-CoV-2 infection in light of its pathophysiology, making it a suitable candidate as a drug supplement. The purpose is not to present saffron as a solution to COVID-19, but only to explore its use in the integrated management of COVID-19. It needs to be evaluated for its potential role in the long-term physical and mental health management strategy of COVID-19 patients. The paper highlights its potential use in helping manage depression, stress, and anxiety caused due to prolonged lockdowns and isolation or quarantine of people during pandemics.
Figure 1.
Saffron stigma bioactive compounds with therapeutic value useful for COVID-19 management.
2. Pathophysiology and physiological immune response to SARS-CoV-2
The immune responses to viral infections are of two types, Innate Immune Response and Adaptive Immune Response. The major components involved in the innate immune response are Toll-like receptors (TLR) (Jiang et al., 2005), RIG-I-like receptors (retinoic acid-inducible protein 1 like) (RLR) (He et al., 2005), Nucleotide-binding and oligomerization Domain (NOD)-like receptors (NLR) (Yu et al., 2020), C-type lectin-like receptor (CLR) (Cui et al., 2019), Dendritic Cell (DC) (Ziebuhr et al., 2000). TLRs recognize pathogen-associated molecular patterns (PAMP) and target viral lipids, lipoproteins, proteins, and nucleic acids in cell membrane (TLR-2 and TLR-4), cytoplasm (TLR-3, TLR7/8), endosome, lysosome, and endocytolysosome. RLRs recognize viral RNA in the cytoplasm and cause induction of type 1 IFN and inflammatory cytokines to block viral replication (reviewed in Florindo et al., 2020). The major components of Adaptive Immune Response are the T cells and the Humoral immune response. In T-cell mediated response, CD4+ T cells promote the production of virus-specific antibodies by activating T-dependent B cells, which produce pro-inflammatory cytokines via NF-kB pathway. Cytotoxic CD8+ T cells infiltrate into the infected area and eliminate viral infected cells (Lauer et al., 2020). In the humoral immune response, activation of B cells by CD4+ T cells, memory, and antibodies secreting cells occur, targeting viral proteins through IgM, IgG, and IgA antibody production (Li et al., 2020; Bai et al., 2020).
The host response to SARS-CoV-2 begins with the initial physiological immune response followed by the pathogenic hyperinflammatory phase. The physiological host response encompasses viral entry, infection, and the early immune phase. It involves the entry of SARS-CoV-2 into alveolar epithelial cells by binding to angiotensin-converting enzyme 2 (ACE2) through surface spike (S) protein-mediated by transmembrane serine protease 2 (TMPRSS2) (Hoffmann et al., 2020). It is then followed by active replication and viral release, causing pyroptosis. Viral-mediated cell death causes the release of various damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). These are recognized by pattern-recognition receptors on alveolar macrophages and endothelial cells. PAMPs are recognized in the extracellular space by Toll-like receptors (TLRs), causing induction of pro-inflammatory cytokine transcription factors such as NF-κβ, while DAMPs are recognized intracellularly by nucleotide-binding domain leucine-rich repeat (NLR) proteins causing activation of inflammasomes and conversion of pro-IL-1β to active-IL-1β (Huang et al., 2020; Schnappauf et al., 2019; Soy et al., 2020). The DAMP/PAMPs recognition causes the release of pro-inflammatory cytokine and chemokine, activation of inflammasomes, and the recruitment of monocytes, macrophages, and virus-specific T cells to eliminate the infected cells (Bohn et al., 2020).
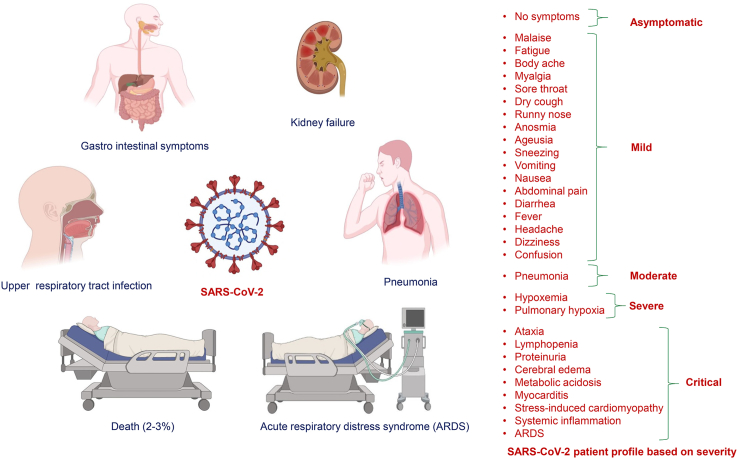
A more severe pathological phase follows the physiological phase in about 20 percent of infectious cases (Parasher, 2020). It is marked by an increased secretion of pro-inflammatory cytokines and chemokines, like interleukins (IL-1, IL-6, IL-8, IL-12, and IL-120), tumour necrosis factors (TNF-α, IFN-β and IFN-λ), C-X-C motif chemokine ligand 10 (CXCL10), macrophage inflammatory protein-1α (MIP-1α), monocyte chemo-attractant protein-1 (MCP-1) and interferon gamma-induced protein 10 (IP-10) (Huang et al., 2020; Zhou et al., 2020a; Qin et al., 2020). The cytokine surge serves as a chemo-attractant for neutrophils, CD4 helper T cells, and CD8 cytotoxic T cells, causing excessive infiltration of immune cells in the lungs. CD4 helper T cells activate B cells for producing virus-specific antibodies, while CD8 T cells kill the virus-infected cells.
In addition to ACE2, SARS-CoV-2 can also bind to dendritic-cell specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) and DC-SIGN-related protein (DC-SIGNR, L-SIGN) (Jeffers et al., 2004; Marzi et al., 2004; Yang et al., 2004). DC-SIGN is highly expressed on dendritic cells and macrophages. Dendritic cells (DCs) are involved in phagocytosis of virus in the lungs. After phagocytosis, DCs migrate to lymphoid organs and activate antigen-specific T cells, which move into the lungs and destroy virus-infected alveolar cells. DCs, macrophages, pathological cytotoxic T cells derived from CD4 helper T cells help fight the virus, but eventually cause lung inflammation and injury [Fang et al., 2012; Small et al., 2001]. The host cells undergo apoptosis releasing new viral particles. The cycle of infection and apoptosis continues, leading to the loss of pneumocytes which are involved in the gas exchange between the alveoli and blood. This causes diffuse alveolar damage, and eventually results in an acute respiratory distress syndrome (Bohn et al., 2020). This may then progress to activation of a procoagulant response, multiple organ failure and death, especially in old aged, immune-compromised, or with underlying pathology (Gautam et al., 2020) (Figure 2).
Figure 2.
SARS-CoV-2 symptoms and pathophysiology based on disease severity.
While uncontrolled immunity causes pulmonary tissue damage and reduced lung capacity, immune insufficiency or misdirection increases viral replication and tissue damage. Therefore, the induction of a balanced host immune response against SARS-CoV-2 is critical for controlling its infection (Florindo et al., 2020). Some herbs are traditionally known to contain components that stabilize the functioning of innate immunity (macrophages, neutrophils, and dendritic cells) as well as acquired immunity (T cells and B cells).
3. A brief description of saffron medicinal properties
The healing properties of saffron are recorded in Materia Medica by Pedanio Dioscorides, a Greek medical practitioner of the first century A.D. Physicians like Hippocrates and Pliny have used it in cases of excessive drunkenness, loss of male potency, and as an aphrodisiac. Modern medicine has acknowledged several therapeutic effects and pharmaceutical applications of saffron.
Medicinal properties of saffron are attributed to the presence of volatile as well as non-volatile aroma yielding compounds. The red stigmas of Crocus sativus accumulate different bioactive compounds amongst which safranal, crocin, campherol, picrocrocin, crocetin, α- and β-carotenes are of prime importance. The ability to synthesize these compounds is not common across species. Picrocrocin and crocin have been detected only in saffron (Crocus species), Buddleja (Liao et al., 1999), and Gardenia (Pfister et al., 1996).
Research on the physicochemical and biochemical properties of saffron along with the bioactivity of its compounds, has confirmed its role in pharmacognosy (Table 1). A vast number of papers have been published focusing on cancer, antioxidant properties, sedative effect, neuronal injury, etc. (reviewed in Premkumar and Ramesh, 2010; Licón et al., 2010; Mokhtari-Zaer et al., 2020). Saffron and its constituents are considered an efficient treatment for coronary artery diseases, neurodegenerative disorders, bronchitis, asthma, diabetes, fever, and colds (Boskabady and Farkhondeh, 2016). It is a promising natural medicine in treating metabolic syndrome (Razavi and Hosseinzadeh, 2017). It is a potent natural antioxidant used in folk medicine to treat cold, scarlet fever, and asthma (Boskabady and Farkhondeh, 2016; Boskabady et al., 2019). Several in vivo studies have confirmed the antioxidant and anti-inflammatory role of ethanol or aqueous extracts of saffron, safranal, and crocin (Hosseinzadeh and Younesi, 2002; Hosseinzadeh and Ghenaati, 2006). Chatterjee et al. (2005) reported crocin to be a more potent antioxidant agent than α tocopherol. Reduction of blood bilirubin level and decreased blood cholesterol and triglycerides after using crocetin and crocin have also been reported (Nair et al., 1991). The anticancer properties of saffron have also been reported (Duke et al., 1987; Abdullaev and Espinosa-Aguirre, 2004).
Table 1.
Active ingredients of saffron and the rationale of their use as nutraceuticals.
| Component/active ingredient | Medicinal role | Reference |
|---|---|---|
| Saffron extract |
|
Hosseinzadeh and Noraei (2009); Hosseinzadeh et al. (2012); Akhondzadeh et al. (2010); Christodoulou et al. (2014); Abdullaev (2002); Khorasani et al. (2008); Lequerc (1973); Hosseinzadeh et al. (2012); Garc-Olmo et al. (1999); Kianbakht and Ghazavi (2011); Khavari et al. (2015); Xu et al. (2007) |
| Picrocrocin |
|
Lequerc (1973); Khavari et al. (2015); Baluchnejadmojarad et al. (2019); Boroushaki et al. (2007); Alayunt et al. (2019); Ahmad et al. (2005); Patel and Bhutani (2014) |
| Carotenoids |
|
Kanakis et al. (2009); Das et al. (2010); Molnar et al. (2000); Bakshi et al. (2016); Mozaffari et al. (2019); Patel and Bhutani (2014); Yarijani et al. (2017) |
4. Saffron in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) management
Coronavirus is known to act more severely on individuals with weak immunity. The major pathway of cell destruction in COVID-19 patients is mostly immune-mediated apoptosis. Therefore a robust immune system can help reduce the severity of the viral infection and subsequent disease response. Since ACE2 receptor is found in multiple organs viz. oral and nasal mucosa, lungs, stomach, intestine, bladder, heart, and kidney (Zhou et al., 2020b; Xu et al., 2020; Donoghue et al., 2000), cell-mediated immunity causes damage through cytokine storm (Williams and Chambers 2014; Cameron et al., 2008). It could be helpful if inflammation is suppressed during this severe stage (Shi et al., 2020). Immunity-boosting medicinal plants can help during the early non-severe stage, while herbs with anti-inflammatory and anti-thrombotic properties can help during a later or severe stage (Gautam et al., 2020). The potential role of saffron and its constituents and the possible action mechanism is described under relevant heads in the following sub-sections.
4.1. Ethnobotanical use in epidemics
Saffron has been included by Unani medicine among the drugs used during the epidemic. Stamen of saffron can be used as a fumigant for sanitizing the environment due to the antimicrobial activity of its volatile oils. Ibn-Rushd (1126–1198 CE), a great scholar born in Spain commonly known by the name Averroes, describes a medicine that he claims a savior during an epidemic as ‘whoever has used this formulation during an epidemic remained protected from it.’ The composition of this medicine is as follows: Two parts of saffron along with Aleovera L. and Commiphora myrrha Nees Engl. one part each (Nikhat and Fazil 2020). Both traditional and experimental evidence suggests the possible therapeutic effect of saffron and its constituents on various aspects of health, which can be helpful in the management of SARS-CoV-2 pandemic as well (Table 2). Ancient Iranian physician, Avicenna, has stated that saffron oil can facilitate breath and strengthen the respiratory organs. Four cumulative concentrations of a hydro-ethanolic extract of saffron and its constituent safranal were tested on guinea pig tracheal smooth muscle, and the effect was found to be comparable to that of theophylline (Boskabady and Aslani, 2006; Boskabady et al., 2019; Mokhtari-Zaer et al., 2015). Even saffron petal extract (SPE) has been found useful through photochemical analysis, revealing the presence of flavonoids, anthocyanins, and tannins. The SPE was injected intra-peritoneally to rats for 14 days, and the results showed an increase in the number of white blood cells and antibody response without any alteration in hematological parameters (Babaei et al., 2014).
Table 2.
Saffron as a drug-adjuvant or supplement in Covid-19 pandemic management.
| Role | Remarks | Reference |
|---|---|---|
| Immunomodulation |
|
Mokhtari-Zaer et al. (2020); Vijayabhargava and Asad (2011); Boskabady et al. (2020); Ghazavi et al. (2009); Escribano et al. (1999); Bani et al. (2011); Yang et al. (2012); Ding et al. (2015); Singh et al. (2020) |
| Respiratory function |
|
Bayrami and Boskabady (2012); Frank (1961); Di Luccio and Gainer (1980); Giaccio (2004); Xiong et al. (2015); Yang et al. (2012); Ding et al. (2015) |
| Renal function |
|
Kianbakht and Ghazavi (2011); Yarijani et al. (2017) |
| Cardio-vascular |
|
Goyal et al. (2010); Gainer et al. (1993); Bharti et al. (2012) |
| Anxiety and depression |
|
Akhondzadeh et al. (2004); Basti et al. (2007); Hosseinzadeh et al. (2012); Halataei et al. (2011); Hosseinzadeh and Noraei (2009); De Monte et al. (2014) |
4.2. As immunity booster
Currently, immunomodulatory drugs that target interleukins, like tocilizumab (an IgG1 monoclonal antibody against IL-6 receptor) are reported to be beneficial in moderate-to-severe cases of SARS-CoV-2 infection. Bioactive constituents of saffron can affect both cellular and humoral immunity functions, which can be quite beneficial (Table 2). Immunomodulation by these saffron components can help as a management strategy against SARS-CoV-2. Its immunomodulatory activity may involve direct targeting of Toll-like receptors (TLRs), attributed to nuclear factor (NF-κB), activator protein 1 (AP-1), and downstream signaling pathways (reviewed in Zeinali et al., 2019; Boskabady et al., 2020). A randomized, double-blind placebo-controlled clinical trial has been conducted to determine the immunomodulatory effects of saffron. It was observed that saffron increases the IgG level and decreases the IgM level compared with baseline and placebo. Furthermore, it increases the percentage of monocytes in comparison to placebo. Hence, the sub-chronic daily use of 100 mg saffron was suggested to have temporary immune-modulatory activities without any adverse effects (Kianbakht and Ghazavi, 2011). Saffron has been shown to enhance IFN-γ to IL-4 ratio in human lymphocytes and thereby affect Th1 and Th2 balance in them (Boskabady et al., 2011). These properties could aid in modulating the immune response during SARS-CoV-2 infection. A study on sensitized guinea-pigs has shown that the total and differential count of white blood cells (WBC) gets affected positively by the saffron extract and safranal (Bayrami and Boskabady, 2012). Azithromycin is a preferred antibiotic in SARS-CoV-2 management due to its anti-inflammatory action, and the prevention of secondary bacterial infection (Parasher 2020). Saffron has been shown to reduce inflammation by inhibiting cyclooxygenase enzyme activity (Rahmani et al., 2017). This property can help tackle excessive lung inflammation in SARS-CoV-2 patients due to the release of pro-inflammatory cytokines and chemokines.
4.3. In respiratory problems
In traditional medicine, saffron has been used to treat fever, bronchitis, cold, pertussis, asthma, and respiratory function improvement. Safranal, a major component of saffron, might be useful in treating respiratory disorders, mostly chronic bronchitis. It has significant therapeutic effects on lung pathology and tracheal hyper-responsiveness (Boskabady et al., 2012, 2014). Saffron can be useful in SARS-CoV-2 management as it has been shown to inhibit the release of inflammatory cytokine, and production of nitric oxide and nitrite (Boskabady et al., 2014), endotheline-1, and total protein secretion (Gholamnezhad et al., 2013), and the recruitment of inflammatory cells to the lungs in sensitized guinea pigs (Mahmoudabady et al., 2013). Additionally, it sedates coughing through an anesthetic effect on the vagal nerves of the alveoli (Giaccio, 2004). Byrami et al. (2013) demonstrated the preventive effects of the saffron extract on tracheal responsiveness and plasma levels of IL-4, IFN-γ, total nitric oxide, and nitrite in sensitized guinea-pigs. The saffron extract was reported to show a preventive effect on tracheal responses, serum levels of inflammatory mediators and also showed increased Th1/Th2 balance. The SARS-CoV-2 infection causes loss of pneumocytes, which are involved in the gas exchange between the alveoli and blood. In an earlier study, crocetin has shown the most remarkable effect due to its ability to increase the speed of oxygen transport and diffusivity in vivo as well as in vitro (Frank, 1961). It can be useful in hemorrhages, alveolar hypoxia, atherosclerosis, tumors and may help SARS-CoV-2 patients.
Bukhari et al. (2015) evaluated the antioxidant potential of saffron in normal human bronchial epithelial cells (NHBE) and the anti-inflammatory potential of safranal in a murine model of asthma. In bronchial epithelial cells, safranal significantly reduced oxidative stress via iNOS reduction and prevented apoptosis in these cells, which could be useful in reducing the immune-mediated cell destruction in COVID-19 patients. This safranal mediated iNOS inhibition attenuated asthmatic features in the murine model of allergic asthma (Table 2).
4.4. Antiviral role
Antiviral drugs like remdesivir and favipiravir have shown some efficacy in SARS-CoV-2 management (Parasher 2020). There exists strong scientific evidence for the antiviral effects of saffron (Table 2). Antiviral (anti-HSV-1 and anti-HIV-1) effect of saffron has been tested, and it was found that the saffron extract shows mild activity while crocin and picrocrocin indicated significant anti-HSV-1 and anti-HIV-1 activities. Both crocin and picrocrocin were found to be effective for inhibiting the virus entry as well as its replication. Further, it has been suggested that crocin and picrocrocin are promising anti-HSV and anti-HIV agents for herbal therapy against viral infections (Soleymani et al., 2018). Crocin and picrocrocin prevented the virus from entering the Vero cell, which disrupted the virus entry mechanism.
Recently, in silico analysis for pharmacokinetic, toxicological, and ADMET (absorption, distribution, metabolism, excretion, and toxicity) parameters of saffron bioactive molecules indicated that crocetin has a high drug score against SARS-CoV-2 (Kordzadeh et al., 2020). It was elucidated that crocin and crocetin possess a high binding affinity towards the main protease (PDB ID: 6M03) of SARS-CoV-2, and crocetin shows translocation through lipid bilayer as a drug molecule.
4.5. Depression and anxiety management
There are several long-term residual effects of SARS-CoV-2 infection. According to a post-hospitalisation COVID-19 study, four in five patients with COVID-19 have persistent symptoms and continue to experience negative impacts on their physical and mental health, as well as ability to work (UKRI). The long-term effects include fatigue, dyspnea, cognitive problems, depression, sleep abnormalities, and deterioration in the quality of life (Carfi et al., 2020; Tenforde et al., 2020; Huang et al., 2021; Chopra et al., 2020). These long-term effects can be categorized into: (1) subacute abnormalities present from 1–3 months beyond acute COVID-19; and (2) chronic abnormalities persisting beyond 3 months of the onset of acute COVID-19 (Greenhalgh et al., 2020; Shah et al., 2021). Saffron can be a suitable candidate for the management of anxiety, depression, neuropsychiatric disorders and the other long-term effects including subacute and chronic abnormalities of SARS-CoV-2 infection. This can be examplified by many earlier safety studies of saffron where double-blind randomized trials focused on the patients with neuropsychiatric problems (Table 3). It has been reported to be more effective than placebo or almost equivalent to the therapeutic doses of fluoxetine and imipramine (Kafi et al., 2018; Gohari et al., 2013; Qadir et al., 2020) (Tables 2 and 3). Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) anti-depressant, helpful as a drug for people with depression, anxiety, panic, or obsessive-compulsive symptoms (Meltzer et al., 1979). Imipramine is a tricyclic anti-depressant (TCA) used mainly in treating depression, anxiety, panic disorder, and bedwetting (Post et al., 1974).
Table 3.
Clinical studies on safety and dosage of saffron in patients suffering from neuro-psychiatric disorders/diseases.
| Constituent | Dosage/day | Duration of dosage (weeks) | Neuro-psychiatric disorder/disease | Remarks | References |
|---|---|---|---|---|---|
| Saffron capsule | 30 mg/day, BID | 4 | Patients with depression | No significant differences in the frequency of adverse effects between saffron and placebo groups | Mansoori et al., (2011); Shahmansouri et al., (2014); Noorbala et al., (2005); Akhondzadeh et al., (2005); Ghajar et al., (2017); Mazidi et al., (2016); Akhondzadeh-Basti et al. (2007); Lopresti et al. (2018); Esalatmanesh et al., (2017); Tabeshpour et al., (2017); Kashani et al., (2017); Akhondzadeh et al., (2010a); Akhondzadeh et al., (2010b); Mousavi et al., (2015) |
| Saffron Capsule | 30 mg/day, BID | 6 | Patients with depression | No significant differences in the frequency of adverse effects between saffron and fluoxetine groups | |
| Hydroalcoholic extract of saffron | 30 mg/day, BID | 6 | Patients with depression | No significant differences in the frequency of adverse effects between saffron and fluoxetine groups | |
| Saffron capsule | 30 mg/day, TDS | 6 | Patients with depression | Dry mouth and sedation was significantly more frequent in the Imipramine group than saffron group | |
| Saffron capsule | 30 mg/day, BID | 6 | Patients with depression | No significant differences in the frequency of adverse effects between saffron and citalopram groups | |
| Saffron Capsule | 50 mg/day, BID | 12 | Patients with anxiety and depression | Side effects were rare | |
| Saffron Capsule | 30 mg/day, BID | 8 | Depressed outpatients | No significant differences in the frequency of adverse effects between saffron and fluoxetine groups | |
| Saffron extract | 14 mg/day, BID | 8 | Teenagers with anxiety or depressive symptoms | Headache occurred more frequently in the placebo group than in the saffron group | |
| Saffron | 30 mg/day, BID | 10 | Patients with obsessive-compulsive disorder | No significant differences in the frequency of adverse effects between saffron and fluvoxamine groups | |
| Saffron | 30 mg/day, BID | 8 | Women with postpartum depression | No significant differences in the frequency of adverse effects between saffron and placebo groups | |
| Saffron | 15 mg/day, BID | 6 | Women with postpartum depression | No significant differences in the frequency of adverse effects between saffron and fluoxetine groups | |
| Saffron capsule | 30 mg/day, BID | 16 | Patients having Alzheimer's disease | No significant differences in the frequency of adverse effects between saffron and placebo groups | |
| Saffron capsule | 30 mg/day, BID | 22 | Patients having Alzheimer's disease | Vomiting occurred significantly more frequently in the donepezil group than in the saffron groups | |
| Saffron aqueous extract and crocin | 15 mg/day, BID | 12 | Patients having Schizophrenia | No toxic effect on thyroid, liver, kidney, and hematologic systems |
Human monoamine oxidase (hMAO) enzymes hMAO-A and hMAO-B are two important targets for treating neuropsychiatric and neurodegenerative diseases. hMAO-A isoform regulates serotonin, epinephrine, and norepinephrine metabolism, and hMAO-B metabolizes benzylamine and phenethylamine, while both isoforms are involved in the metabolism of dopamine. In an interesting study, the artificially designed chemical derivatives of safranal were shown to modulate the activities of these enzymes, and one such derivative was exceptionally potent and highly promising hMAO-B inhibitor (De Monte et al., 2014).
5. Potential as a drug adjuvant
Twenty-one percent (21%) of the trials on COVID-19 management have focussed on non-vaccine approaches like immunomodulatory (18%) and dietary supplementation (3%). There are many immune modulator based treatments in development like AT-100 (recombinant human surfactant protein D, rhSP-D reduces inflammation and modulates lung immune response), BPI-002 (small molecule as a potent T cell co-stimulator), 7HP-349 (small-molecule integrin activator as an oral adjuvant), Brilacidin (defensin mimetic candidate), PRTX-007 (oral small molecules which activate toll-like receptor 7) (Florindo et al., 2020).
Saffron is power-packed with B vitamins, vitamin C, carotenoids, and phytochemicals, which boost immunity. Saffron constituents are patented in several polyherbal drug formulations used for the treatment of cardiovascular and central nervous system diseases as well as for boosting immune function and depression treatment (Mohajeri et al., 2020). Clinical trials show that if saffron extract is administered @ 20–200 mg/day for ten days to several weeks, it is effective in patients suffering from Central Nervous System (CNS) diseases like Alzheimer's and psychological disorders like depression (Abdullaev, 2002; Abdullaev and Espinosa-Aguirre, 2004; Bakshi et al., 2010; Bathaie and Mousavi, 2010; Rezaee and Hosseinzadeh, 2013; Lopresti and Drummond, 2014; Farokhnia et al., 2014; Bhandari, 2015; Pitsikas, 2015). The most common effective doses of saffron (30–50 mg/day) used in clinical studies are considered safe and are noticeably lower than the toxic doses (>5 g/day) (Mehri et al., 2020). Doses higher than 10 g/day induce abortion and lead to vomiting, vertigo, dizziness, hematuria, bleeding in the uterus and gastrointestinal mucosa (Schmidt et al., 2007).
More than 109.20 million people have so far recovered from COVID-19 infection, out of which 32.76–43.68 million (30–40 %) survivors may be suffering the symptoms of anxiety, depression, sleep disturbances, and post-traumatic stress disorder. The development of a saffron-based formulation and its commercialization can help provide these people an over-the-counter medication for effective management of the long-term adverse effects of COVID-19. One example of a patented commercial saffron nutraceutical product (for macular degeneration) is ‘Saffron, 2020’. It combines saffron with vital eye health nutrients such as lutein and zeaxanthin, antioxidant vitamins (A, B2, C, and E), resveratrol, and zinc (Saffron 2020). Microarray studies on this product depict that its efficacy is not only because of the antioxidant action of crocins but also due to the activation of multiple pathways (Marco et al., 2019).
6. Conclusion
In the absence of effective allopathic drugs and the mass availability of effective, safe, and affordable vaccines for 7.8 billion people, World Health Organisation (WHO) guidelines on social distancing, wearing masks, frequent handwashing are an essential management strategy. Given the rising number of cases, the emergence of new mutants of the novel coronavirus, and repeated lockdowns in several parts of the world, there is a lot of confusion and distress among the common people. Even though 80.4 % (109.20 million) of the infected people recovered, 30–40 % (32.76–43.68 million) of those who recovered may suffer from anxiety, depression, sleep disturbances, and post-traumatic stress disorder for up to even six months. Saffron is a natural product that could help alleviate the symptoms of severe acute respiratory syndrome coronavirus 2 (COVID-19) patients and manage the post-covid long-term sub-acute and chronic abnormalities associated with COVID-19 patients. Saffron can be used to manage stress and anxiety during prolonged lockdown, isolation, and quarantine. Its efficacy in depression management is comparable with drugs like fluoxetine, imipramine, citalopram. It is a potential adjuvant in the form of an immunity-supplement and anti-depressant in future drug formulations. Detailed research on dosage, method of administration, efficacy, etc., needs to be undertaken to explore the potential of saffron in managing the health issues arising because of the COVID-19 pandemic.
Declarations
Author contribution statement
AMH (1st author) conceived, conceptualized, analysed, presented and wrote the paper, with significant inputs from KNJ (2nd) and GAW (3rd) in the preparation of tables and literature review.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are highly thankful to the National Mission for Himalayan Studies (NMHS) for providing fellowship to KNJ for her research activities. The first author is grateful to Er. Kireet Kumar and his team for support and cooperation in conducting saffron research studies.
References
- Abdullaev F.I. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp. Biol. Med. 2002;227(1):20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- Abdullaev F.I., Espinosa-Aguirre J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004;28(6):426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Ahmad A.S., Ansari M.A., Ahmad M., Saleem S., Yousuf S., Hoda M.N., Islam F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005;81(4):805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S., Fallah-Pour H., Afkham K., Jamshidi A.H., Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial. BMC Compl. Altern Med. 2004;4(1):12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S., Sabet M.S., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacol. 2010;207(4):637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S., Sabet M.S., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S. Saffron in the treatment of patients with mild-to-moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Therapeut. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S., Tahmacebi-Pour N., Noorbala A.A., Amini H., Fallah-Pour H., Jamshidi A.H. Crocus sativus L. in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh-Basti A., Moshiri E., Noorbala A.A., Jamshidi A.H., Abbasi S.H., Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31:439–442. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Alam M.A., Quamri M.A., Sofi G., Ayman U., Ansari S., Ahad M. Understanding COVID-19 in the light of epidemic disease described in Unani medicine. Drug Metab. Personal. Ther. 2020;1 doi: 10.1515/dmdi-2020-0136. (ahead-of-print) [DOI] [PubMed] [Google Scholar]
- Alayunt Ö.N., Aksoy L., Karafakioğlu Y.S., Sevimli S. Assessment of anti-inflammatory and antioxidant properties of safranal on CCI4-induced oxidative stress and inflammation in rats. An. Acad. Bras. de Ciênc. 2019;91(2) doi: 10.1590/0001-3765201920181235. [DOI] [PubMed] [Google Scholar]
- Alshehri Z.S., AL-Bishri W.M., Hussein R.H., EL-Halwagy M.E. Saffron (Crocus sativus) ameliorates tnbs-induced colitis in rats via downregulation of inflammatory cytokines tnf-α and il-10, caspases-3 gene expression and oxidative stress in experimental rats. Int. J. Life Sci. Pharm. Res. (IJLPR) 2017;7:1–9. [Google Scholar]
- Babaei A., Arshami J., Haghparast A., Mesgaran M.D. Effects of saffron (Crocus sativus) petal ethanolic extract on hematology, antibody response, and spleen histology in rats. Avicenna J. Phytomed. 2014;4(2):103. [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. Jama. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi H.A., Hakkim F.L., Sam S. Molecular mechanism of crocin induced caspase mediated MCF-7 cell death: in vivo toxicity profiling and ex vivo macrophage activation. Asian Pac. J. Cancer Prev. APJCP. 2016;17(3):1499–1506. doi: 10.7314/apjcp.2016.17.3.1499. [DOI] [PubMed] [Google Scholar]
- Bakshi H., Sam S., Rozati R., Sultan P., Islam T., Rathore B., Saxena R.C. DNA fragmentation and cell cycle arrest: a hallmark of apoptosis induced by crocin from Kashmiri saffron in a human pancreatic cancer cell line. Asian Pac. J. Cancer Prev. APJCP. 2010;11(3):675–679. [PubMed] [Google Scholar]
- Baluchnejadmojarad T., Mohamadi-Zarch S.M., Roghani M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β-induced rat model of Alzheimer’s disease: underlying mechanisms. Metab. Brain Dis. 2019;34(6):1747–1759. doi: 10.1007/s11011-019-00481-6. [DOI] [PubMed] [Google Scholar]
- Bani S., Pandey A., Agnihotri V.K., Pathania V., Singh B. Selective Th2 upregulation by Crocus sativus: a neutraceutical spice. Evid. base Compl. Alternative Med. 2011;2011 doi: 10.1155/2011/639862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basti A.A., Moshiri E., Noorbala A.A., Jamshidi A.H., Abbasi S.H., Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31(2):439–442. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Bathaie S.Z., Mousavi S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010;50(8):761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- Bayrami G., Boskabady M.H. The potential effect of the extract of Crocus sativus and safranal on the total and differential white blood cells of ovalbumin-sensitized Guinea pigs. Res. Pharm Sci. 2012;7(4):249. [PMC free article] [PubMed] [Google Scholar]
- Bhandari P.R. Crocus sativus L. (saffron) for cancer chemoprevention: a mini review. J. Tradit Compl. Med. 2015;5(2):81–87. doi: 10.1016/j.jtcme.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S., Golechha M., Kumari S., Siddiqui K.M., Arya D.S. Akt/GSK-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia–reperfusion injury in rats. Eur. J. Nutr. 2012;51(6):719–727. doi: 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- Bhat S.A., Rather S.A., Iqbal A., Qureshi H.A., Islam N. Immunomodulators for curtailing COVID-19: a positive approach. J. Drug Deliv. Therapeut. 2020;10(3-s):286–294. [Google Scholar]
- Bohn M.K., Hall A., Sepiashvili L., Jung B., Steele S., Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. 2020;35:288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroushaki M.T., Mofidpour H., Sadeghnia H.R. 2007. Protective Effect of Safranal against Hexachlorobutadiene-Induced Nephrotoxicity in Rat. [Google Scholar]
- Boskabady M.A., Aslani M.R. Relaxant effect of Crocus sativus (saffron) on Guinea-pig tracheal chains and its possible mechanisms. J. Pharm. Pharmacol. 2006;58(10):1385–1390. doi: 10.1211/jpp.58.10.0012. [DOI] [PubMed] [Google Scholar]
- Boskabady M.H., Farkhondeh T. Anti-inflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytother Res. 2016;30(7):1072–1094. doi: 10.1002/ptr.5622. [DOI] [PubMed] [Google Scholar]
- Boskabady M.H., Byrami G., Feizpour A. The effect of safranal, a constituent of Crocus sativus (saffron), on tracheal responsiveness, serum levels of cytokines, total NO and nitrite in sensitized Guinea pigs. Pharmacol. Rep. 2014;66(1):56–61. doi: 10.1016/j.pharep.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Boskabady M.H., Gholamnezhad Z., Ghorani V., Saadat S. The effect of crocus sativus (saffron) on the respiratory system: traditional and experimental evidence. Sci. Spice. Culin. Herb. Latest Lab. Pre-Clin. Clin. Stud. 2019;1:30–54. [Google Scholar]
- Boskabady M.H., Gholamnezhad Z., Khazdair M.R., Tavakol-Afshari J. Saffron. Woodhead Publishing; 2020. Anti-inflammatory and immunomodulatory effects of saffron and its derivatives; pp. 405–421. [Google Scholar]
- Boskabady M.H., Seyedhosseini Tamijani S.M., Rafatpanah H., Rezaei A., Alavinejad A. The effect of Crocus sativus extract on human lymphocytes' cytokines and T helper 2/T helper 1 balance. J. Med. Food. 2011;14(12):1538–1545. doi: 10.1089/jmf.2011.1697. [DOI] [PubMed] [Google Scholar]
- Boskabady M.H., Tabatabaee A., Byrami G. The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized Guinea-pigs. Phytomedicine. 2012;19(10):904–911. doi: 10.1016/j.phymed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt R.V. Herbal medicines in management and prevention of COVID-19. J. Pharmacogn. Phytochem. 2020;9(3):1221–1223. [Google Scholar]
- Bukhari S.I., Pattnaik B., Rayees S., Kaul S., Dhar M.K. Safranal of Crocus sativus L. inhibits inducible nitric oxide synthase and attenuates asthma in a mouse model of asthma. Phytother Res. 2015;29(4):617–627. doi: 10.1002/ptr.5315. [DOI] [PubMed] [Google Scholar]
- Byrami G., Boskabady M.H., Jalali S., Farkhondeh T. The effect of the extract of Crocus sativus on tracheal responsiveness and plasma levels of IL-4, IFN-γ, total NO and nitrite in ovalbumin sensitized Guinea-pigs. J. Ethnopharmacol. 2013;147(2):530–535. doi: 10.1016/j.jep.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13e9. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfi A., Bernabei R., Landi F., Against Gemelli. COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Poduval T.B., Tilak J.C., Devasagayam T.P. A modified, economic, sensitive method for measuring total antioxidant capacities of human plasma and natural compounds using Indian saffron (Crocus sativus) Clin. Chim. Acta. 2005;352(1-2):155–163. doi: 10.1016/j.cccn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Chiavaroli A., Recinella L., Ferrante C., Locatelli M., Carradori S., Macchione N., Zengin G., Leporini L., Leone S., Martinotti S., Brunetti L., Vacca M., Menghini L., Orlando G. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeost. Agents. 2017;31(3):531–541. [PubMed] [Google Scholar]
- Chopra V., Flanders S.A., O’Malley M. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou E., Kadoglou N., Kakazanis Z., Stasinopoulou M., Moustardas P., Balafas V., Valsami G. AAPS Annual Meeting and Exposition. 2014. The impact of saffron administration on atherosclerosis development in an experimental animal model. [Google Scholar]
- COVID-19 Stats Realtime coronavirus statistics with charts. https://epidemic-stats.com/
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I., Das S., Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: a histopathological study. Acta Histochem. 2010;112(4):317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- De Monte C., Carradori S., Chimenti P., Secci D., Mannina L., Alcaro F., Petzer A., N'Da C.I., Gidaro M.C., Costa G., Alcaro S., Petzer J.P. New insights into the biological properties of Crocus sativus L.: chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014;82:164–171. doi: 10.1016/j.ejmech.2014.05.048. [DOI] [PubMed] [Google Scholar]
- Di Luccio R.C., Gainer J.L. Increasing alveolar oxygen transport. Aviat. Space Environ. Med. 1980;51(1):18–20. [PubMed] [Google Scholar]
- Ding J., Su J., Zhang L., Ma J. Crocetin activates Foxp3 through TIPE2 in asthma-associated Treg cells. Cell. Physiol. Biochem. 2015;37(6):2425–2433. doi: 10.1159/000438595. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Duke J.A., Bogenschutz-Godwin M.J., duCellier J., Duke P.K. CRC, Press. Inc.; Boca, Raton, Florida: 1987. Handbook of Medicinal Herbs. [Google Scholar]
- Esalatmanesh S., Biuseh M., Noorbala A.A., Mostafavi S.A., Rezaei F., Mesgarpour B. Comparison of saffron and fluvoxamine in the treatment of mild-to-moderate obsessive-compulsive disorder: a double blind, randomized clinical trial. Iran. J. Psychiatry. 2017;12:154–162. [PMC free article] [PubMed] [Google Scholar]
- Escribano J., Dıaz-Guerra M.J.M., Riese H.H., Ontañón J., Garcıa-Olmo D., Garcıa-Olmo D.C., Fernández J.A. In vitro activation of macrophages by a novel proteoglycan isolated from corms of Crocus sativus L. Canc. Lett. 1999;144(1):107–114. doi: 10.1016/s0304-3835(99)00211-6. [DOI] [PubMed] [Google Scholar]
- Fang M., Siciliano N.A., Hersperger A.R., Roscoe F., Hu A., Ma X., Shamsedeen A.R., Eisenlohr L.C., Sigal L.J. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc. Natl. Acad. Sci. 2012;109:9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M., ShafieeSabet M., Iranpour N., Gougol A., Yekehtaz H., Alimardani R. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: a double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014;29(4):351–359. doi: 10.1002/hup.2412. [DOI] [PubMed] [Google Scholar]
- Florindo H.F., Kleiner R., Vaskovich-Koubi D. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15:630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. Purpura resulting from artificial abortion. Deut Med. Wochschr. 1961;86:1618–1620. doi: 10.1055/s-0028-1112983. [DOI] [PubMed] [Google Scholar]
- Gainer J.L., Rudolph D.B., Caraway D.L. The effect of crocetin on hemorrhagic shock in rats. Circ. Shock. 1993;41(1):1–7. [PubMed] [Google Scholar]
- Garc-Olmo D.C., Riese H.H., Escribano J., Onta N.J., Fernandez J.A., Atiénzar M., Garcí-Olmo D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr. Canc. 1999;35(2):120–126. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- Gautam S., Gautamb A., Chhetric S., Bhattaraid U. Immunity against COVID-19: potential role of Ayush Kwath. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar A., Neishabouri S.M., Velayati N., Jahangard L., Matinnia N., Haghighi M. Crocus sativus L. versus citalopram in the treatment of major depressive disorder with anxious distress: a double-blind, controlled clinical trial. Pharmacopsychiatry. 2017;50:152–160. doi: 10.1055/s-0042-116159. [DOI] [PubMed] [Google Scholar]
- Ghazavi A., Mosayebi G., Salehi H., Abtahi H. Effect of ethanol extract of saffron (Crocus sativus L.) on the inhibition of experimental autoimmune encephalomyelitis in C57bl/6 mice. Pakistan J. Biol. Sci. 2009;12(9):690–695. doi: 10.3923/pjbs.2009.690.695. [DOI] [PubMed] [Google Scholar]
- Gholamnezhad Z., Koushyar H., Byrami G., Boskabady M.H. The extract of Crocus sativus and its constituent safranal, affect serum levels of endothelin and total protein in sensitized Guinea pigs. Iran. J. Basic. Med. Sci. 2013;16(9):1022. [PMC free article] [PubMed] [Google Scholar]
- Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004;44(3):155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- Gohari A.R., Saeidnia S., Mahmoodabadi M.K. An overview on saffron, phytochemicals, and medicinal properties. Pharm. Rev. 2013;7(13):61. doi: 10.4103/0973-7847.112850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S.N., Arora S., Sharma A.K., Joshi S., Ray R., Bhatia J. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17(3-4):227–232. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. Management of post-acute COVID-19 in primary care. Br. Med. J. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- Halataei B.A.S., Khosravi M., Arbabian S., Sahraei H., Golmanesh L., Zardooz H. Saffron (Crocus sativus) aqueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother Res. 2011;25(12):1833–1838. doi: 10.1002/ptr.3495. [DOI] [PubMed] [Google Scholar]
- He Y., Zhou Y., Siddiqui P., Niu J., Jiang S. Identification of immunodominant epitopes on the membrane protein of the severe acute respiratory syndrome-associated coronavirus. J. Clin. Microbiol. 2005;43(8):3718–3726. doi: 10.1128/JCM.43.8.3718-3726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H., Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in Guinea pigs. Fitoterapia. 2006;77(6):446–448. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Noraei N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23(6):768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Younesi H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2(1):7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H., Sadeghnia H.R., Ghaeni F.A., Motamedshariaty V.S., Mohajeri S.A. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26(3):381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husaini A.M. Husaini. Saffron, Global Science Books, Ltd; UK: 2010. Foreword; p. ii. [Google Scholar]
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Jr., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., He Y., Liu S. SARS vaccine development. Emerg. Infect. Dis. 2005;11(7):1016. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri Z.H.M. Immunomodulatory effects of Crocus sativus L. (petals) and Boragoofficinalis L. (whole plant) aqueous extracts in albino mice. Al-Mustansiriyah J. Sci. 2014;25(1):7–16. [Google Scholar]
- Kafi M., Kamili A.N., Husaini A.M., Ozturk M., Altay V. Global Perspectives on Underutilized Crops. Springer; Cham: 2018. An expensive spice saffron (Crocus sativus L.): a case study from Kashmir, Iran, and Turkey; pp. 109–149. [Google Scholar]
- Kanakis C.D., Tarantilis P.A., Pappas C., Bariyanga J., Tajmir-Riahi H.A., Polissiou M.G. An overview of structural features of DNA and RNA complexes with saffron compounds: models and antioxidant activity. J. Photochem. Photobiol. B Biol. 2009;95(3):204–212. doi: 10.1016/j.jphotobiol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Kashani L., Eslatmanesh S., Saedi N., Niroomand N., Ebrahimi M., Hosseinian M. Comparison of saffron versus fluoxetine in treatment of mild-to-moderate postpartum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry. 2017;50:64–68. doi: 10.1055/s-0042-115306. [DOI] [PubMed] [Google Scholar]
- Khavari A., Bolhassani A., Alizadeh F., Bathaie S.Z., Balaram P., Agi E., Vahabpour R. Chemo-immunotherapy using saffron and its ingredients followed by E7-NT (gp96) DNA vaccine generates different anti-tumor effects against tumors expressing the E7 protein of human papillomavirus. Arch. Virol. 2015;160(2):499–508. doi: 10.1007/s00705-014-2250-9. [DOI] [PubMed] [Google Scholar]
- Khorasani G., Hosseinimehr S.J., Zamani P., Ghasemi M., Ahmadi A. The effect of saffron (Crocus sativus) extract for healing of second-degree burn wounds in rats. Keio J. Med. 2008;57(4):190–195. doi: 10.2302/kjm.57.190. [DOI] [PubMed] [Google Scholar]
- Kianbakht S., Ghazavi A. Immunomodulatory effects of saffron: a randomized double-blind placebo-controlled clinical trial. Phytother Res. 2011;25(12):1801–1805. doi: 10.1002/ptr.3484. [DOI] [PubMed] [Google Scholar]
- Kordzadeh A., Saadatabadi A.R., Hadi A. Investigation on penetration of saffron components through lipid bilayer bound to spike protein of SARS-CoV-2 using steered molecular dynamics simulation. Heliyon. 2020;6(2020) doi: 10.1016/j.heliyon.2020.e05681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lequerc H. Masson & C/ie; Paris: 1973. Precis de Phytotherapie; p. 1973. [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Xing X. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y.H., Houghton P.J., Hoult J.R.S. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J. Nat. Prod. 1999;62(9):1241–1245. doi: 10.1021/np990092+. [DOI] [PubMed] [Google Scholar]
- Licón C., Carmona M., Llorens S., Berruga M.I., Alonso G.L. Potential healthy effects of saffron spice (Crocus sativus L. stigmas) consumption. Funct. Plant Sci. Biotechnol. 2010;4:64–73. [Google Scholar]
- Lopresti A.L., Drummond P.D. Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying anti-depressant mechanisms of action. Hum. Psychopharmacol. Clin. Exp. 2014;29(6):517–527. doi: 10.1002/hup.2434. [DOI] [PubMed] [Google Scholar]
- Lopresti A.L., Drummond P.D., Inarejos-García A.M., Prodanov M. affron®, a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2018;232:349–357. doi: 10.1016/j.jad.2018.02.070. [DOI] [PubMed] [Google Scholar]
- Mahmoudabady M., Neamati A., Vosooghi S., Aghababa H. Hydroalcoholic extract of Crocus sativus effects on bronchial inflammatory cells in ovalbumin sensitized rats. Avicenna J. Phytomed. 2013;3(4):356. [PMC free article] [PubMed] [Google Scholar]
- Mansoori P., Akhondzadeh S., Raisi F., Ghaeli P., Jamshidi A., Nasehi A. A randomized, double-blind, placebo-controlled study of safety of the adjunctive saffron on sexual dysfunction induced by a selective serotonin reuptake inhibitor. J. Med. Plant. 2011;1:121–130. [Google Scholar]
- Marco S.D., Carnicelli V., Franceschini N., Di Paolo M., Piccardi M., Bisti S., Falsini B. Saffron: a multitask neuroprotective agent for retinal degenerative diseases. Antioxidants. 2019;8(7):224. doi: 10.3390/antiox8070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A., Gramberg T., Simmons G., Moller P., Rennekamp A.J., Krumbiegel M., Geier M., Eisemann J., Turza N., Saunier B., Steinkasserer A., Becker S., Bates P., Hofmann H., Pohlmann S. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78(21):12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidi M., Shemshian M., Mousavi S.H., Norouzy A., Kermani T., Moghiman T., Sadeghi A., Mokhber N., Ghayour-Mobarhan M., Ferns G.A. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med. 2016;13(2):195–199. doi: 10.1515/jcim-2015-0043. [DOI] [PubMed] [Google Scholar]
- Mehri S., Razavi B.-M., Hosseinzadeh H. Safety and toxicity of saffron. In: Koocheki Alireza, Khajeh-Hosseini Mohammad., editors. Saffron, Woodhead Publishing Series in Food Science, Technology and Nutrition. 2020. pp. 517–530. [Google Scholar]
- Meltzer H.Y., Young M., Metz J., Fang V.S., Schyve P.M., Arora R.C. Extrapyramidal side effects and increased serum prolactin following fluoxetine, a new anti-depressant. J. Neural. Transm. 1979;45(2):165–175. doi: 10.1007/BF01250091. [DOI] [PubMed] [Google Scholar]
- Mohajeri S.A., Hedayati N., Bemani-Naeini M. Saffron. Woodhead Publishing; 2020. Available saffron formulations and product patents; pp. 493–515. [Google Scholar]
- Mokhtari-Zaer A., Khazdair M.R., Boskabady M.H. Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: possible mechanisms. Avicenna J. Phytomed. 2015;(5):365. [PMC free article] [PubMed] [Google Scholar]
- Mokhtari-Zaer A., Saadat S., Ghorani V., Memarzia A., Boskabady M.H. Saffron. Academic Press; 2020. The effects of saffron (crocus sativus) and its constituents on immune system: experimental and clinical evidence; pp. 193–217. [Google Scholar]
- Molnar J., Szabo D., Pusztai R., Mucsi I., Berek L., Ocsovszki I. Membrane associated antitumor effects of crocine-, ginsenoside-and cannabinoid derivates. Anticancer Res. 2000;20(2A):861–867. [PubMed] [Google Scholar]
- Mousavi B., Bathaie S.Z., Fadai F., Ashtari Z., Ali Beigi N., Farhang S. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015;5:413–419. [PMC free article] [PubMed] [Google Scholar]
- Mozaffari S., Yasuj S.R., Motaghinejad M., Motevalian M., Kheiri R. Crocin acting as a neuroprotective agent against methamphetamine-induced neurodegeneration via CREB-BDNF signaling pathway. Iran. J. Pharm. Res.: IJPR. 2019;18(2):745. doi: 10.22037/ijpr.2019.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S.C., Pannikar B., Panikkar K.R. Antitumour activity of saffron (Crocus sativus) Canc. lett. 1991;57(2):109–114. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A. Post-acute COVID-19 syndrome. Nat. Med. 2021 doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikhat S., Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci. Total Environ. 2020:138859. doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbala A.A., Akhondzadeh S., Tahmacebi-Pour N., Jamshidi A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild-to-moderate depression: a double-blind, randomized pilot trial. J. Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med. J. 2020:1–9. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N.K., Bhutani K.K. Suppressive effects of Mimosa pudica (L.) constituents on the production of LPS-induced pro-inflammatory mediators. EXCLI J. 2014;13:1011. [PMC free article] [PubMed] [Google Scholar]
- Pfister S., Meyer P., Steck A., Pfander H. Isolation and structure elucidation of carotenoid− glycosyl esters in gardenia fruits (gardenia jasminoidesellis) and saffron (crocus sativuslinne) J. Agric. Food Chem. 1996;44(9):2612–2615. [Google Scholar]
- Pitsikas N. The effect of Crocus sativus L. and its constituents on memory: basic studies and clinical applications. Evid. Based Compl. Altern. Med. 2015 doi: 10.1155/2015/926284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post M.L., Kennard O., Horn A.S. Possible pharmacological and theoretical implications of X-ray structure of the tricyclic anti-depressant imipramine. Nature. 1974;252(5483):493–495. doi: 10.1038/252493a0. [DOI] [PubMed] [Google Scholar]
- Premkumar K., Ramesh A. Anticancer, antimutagenic and antioxidant potential of saffron: an overview of current awareness and future perspectives. Funct. Plant Sci. Technol. 2010;4:91–97. [Google Scholar]
- Qadir S., Bashir S., John R. Saffron. Academic Press; 2020. Saffron—immunity system; pp. 177–192. [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and Coronavirus disease 2019: what we know so far. Pathogens. 2020;9 doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani A.H., Khan A.A., Aldebasi Y.H. Saffron (Crocus sativus) and its active ingredients: role in the prevention and treatment of disease. Phcog. J. 2017;9(6) [Google Scholar]
- Razavi B.M., Hosseinzadeh H. Saffron: a promising natural medicine in the treatment of metabolic syndrome. J. Sci. Food Agric. 2017;97(6):1679–1685. doi: 10.1002/jsfa.8134. [DOI] [PubMed] [Google Scholar]
- Rezaee R., Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran. J. Basic Med. Sci. 2013;16(1):12–26. [PMC free article] [PubMed] [Google Scholar]
- Saffron . 2020. By Persavita: the Best Saffron Supplement for Macular Degeneration.https://www.persavita.com/ca/ [Google Scholar]
- Schmidt M., Betti G., Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien. Med. Wochenschr. 2007;157:315–319. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- Schnappauf O., Chae J.J., Kastner D.L., Aksentijevich I. The pyrin inflammasome in health and disease. Front. Immunol. 2019;10:1745. doi: 10.3389/fimmu.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of COVID-19: summary of NICE, SIGN, and RCGP rapid guideline. Br. Med. J. 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- Shahbazi S., Bolhassani A. Immunostimulants: types and functions. J. Med. Microbiol. Infect. Dis. 2016;4(3):45–51. [Google Scholar]
- Shahmansouri N., Farokhnia M., Abbasi S.H., Kassaian S.E., Noorbala Tafti A.A., Gougol A. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild-to-moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014;155:216–222. doi: 10.1016/j.jad.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451e4. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Haileselassie Y., Brim H., Ashktorab H., Habtezion A. Tu1284 protective effect of saffron in mouse colitis models through immune modulation. Gastroenterology. 2020;158(6):S-1043. doi: 10.1007/s10620-021-07163-3. [DOI] [PubMed] [Google Scholar]
- Small B.A., Dressel S.A., Lawrence C.W., Drake D.R., 3rd, Stoler M.H., Enelow R.I., Braciale T.J. CD8(+) T cell-mediated injury in vivo progresses in the absence of effector T cells. J. Exp. Med. 2001;194:1835–1846. doi: 10.1084/jem.194.12.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymani S., Zabihollahi R., Shahbazi S., Bolhassani A. Antiviral effects of saffron and its major ingredients. Curr. Drug Deliv. 2018;15(5):698–704. doi: 10.2174/1567201814666171129210654. [DOI] [PubMed] [Google Scholar]
- Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeshpour J., Sobhani F., Sadjadi S.A., Hosseinzadeh H., Mohajeri S.A., Rajabi O. A double-blind, randomized, placebo-controlled trial of saffron stigma (Crocus sativus L.) in mothers suffering from mild-to-moderate postpartum depression. Phytomedicine. 2017;36:145–152. doi: 10.1016/j.phymed.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatr. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde M.W. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morb. Mortal. Wkly. Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKRI Four in five patients with COVID-19 have persistent symptoms. https://www.ukri.org/news/four-in-five-patients-with-covid-19-have-persistent-symptoms/ URL:
- Vijayabhargava K., Asad M. Effect of stigmas of Crocus sativus L. (saffron) on cell mediated and humoral immunity. Nat. Prod. J. 2011;1(2):151–155. [Google Scholar]
- WHO . 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ Available at. [Google Scholar]
- Williams A.E., Chambers R.C. The mercurial nature of neutrophils: still an enigma in ARDS? Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L217e30. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Wang J., Yu H., Zhang X., Miao C. Anti-asthma potential of crocin and its effect on MAPK signaling pathway in a murine model of allergic airway disease. Immunopharmacol. Immunotoxicol. 2015;37(3):236–243. doi: 10.3109/08923973.2015.1021356. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;8:12. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Gong Z., Yu W., Gao L., He S., Qian Z. Increased expression ratio of Bcl-2/Bax is associated with crocin-mediated apoptosis in bovine aortic endothelial cells. Basic Clin. Pharmacol. Toxicol. 2007;100(1):31–35. doi: 10.1111/j.1742-7843.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Yang L., Shen X., Cheng W., Zhao B., Ali K.H., Ji H. Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2012;674(2-3):391–396. doi: 10.1016/j.ejphar.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Yarijani Z.M., Pourmotabbed A., Pourmotabbed T., Najafi H. Crocin has anti-inflammatory and protective effects in ischemia-reperfusion induced renal injuries. Iran. J. Basic Med. Sci. 2017;20(7):753. doi: 10.22038/IJBMS.2017.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Du L., Ojcius D.M., Pan C., Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microb. Infect. 2020;22(2):74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215(2020):108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinali M., Zirak M.R., Rezaee S.A., Karimi G., Hosseinzadeh H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: a review. Iran. J. Basic Med. Sci. 2019;22(4):334. doi: 10.22038/ijbms.2019.34365.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270e3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wnag D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81(4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.