Abstract
Introduction
Corona virus disease (COVID-19) pandemic can cause myriad of ocular manifestations.
We report a case of unilateral multi focal central serous retinopathy, post COVID-19 infection in an Asian Indian female.
Case presentation
A 42-year-old female presented to us with unilateral blurring, in the right eye (OD), 12 days after COVID-19 infection. She had fever, chills, shortness of breath and cough with tiredness and was COVID- RT PCR positive. She was administered intravenous and oral antibiotics with injection heparin/remdesivir, during her 7 day stay at the hospital. She was also on steroid inhalers. She had no systemic history of note.
On ocular evaluation, her corrected distance visual acuity was 20/40 in OD and 20/20 in left eye (OS). Anterior segment was normal. Anterior vitreous was clear. Fundus examination of the OD showed central serous retinopathy (CSCR) with OS being normal.
Conclusion
CSCR can occur post COVID-19 due to steroid administration and physicians administering it should be aware of this and refer the patients to an ophthalmologist earlier.
Keywords: Corona virus disease-19 (COVID-19), Ophthalmic manifestations, Central serous chorioretinopathy, Spectral Domain Optical Coherence Tomography (SD-OCT), Inhalational steroids, Oral steroids
Introduction
Severe acute respiratory syndrome virus 2 (SARS-CoV2) infection resulted in a global pandemic of Coronavirus disease 2019 (COVID-19). Wuhan in China was the first place of the outbreak in December 2019. As of 23 March 2021, there have been 123,419,065 confirmed cases of COVID-19, including 2,719,163 deaths, reported to WHO. As of 19 March 2021, a total of 397,950,709 vaccine doses have been administered [1]. Conjunctival involvement, cotton wool spots (CWS) and retinal hemorrhages, central retinal artery/vein occlusion, ophthalmic artery occlusion, panuveitis, papillophlebitis, multifocal chorioretinitis and Adie’s syndrome are the ophthalmic manifestations associated with COVID-19 infection [2–6].
We describe an unique case of unilateral multifocal central serous retinopathy (CSCR) in a patient who had just recovered from COVID-19 and had been treated with inhalational and oral steroids.
Case presentation
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975 as revised in 1983. The patient’s written and informed consent was obtained and the study was approved by the hospital ethics committee.
A 42-year-old Asian Indian female presented to us with unilateral blurring, in the right eye (OD), 12 days after COVID-19 infection. Prior to presentation to us, she had fever, chills, shortness of breath and cough with generalized fatigue. Her physician noted that she was afebrile, oxygen saturation was 97% with few crepitations in her lungs and heart rate was 132/min. Complete blood count (CBC) was within normal limits, erythrocyte sedimentation rate (ESR) 35 mm /hr., random blood sugar (RBS)-118 mg/dl, COVID-19 rapid antigen test was negative.
Investigations done at the local hospital a day later showed upper respiratory swab for COVID-19 using real time polymerase chain reaction (RT-PCR) was positive and B-beta (Corona virus) CoV specific target gene and severe acute respiratory syndrome- corona virus 2 (SARS-CoV2) specific target gene were detected, WIDAL test negative, urine analysis was within normal limits. Chest X ray of the lung showed bilateral ground glass opacities (Fig. 1). Based on the records that were available with the patient, we in Table 1 show the medications administered during her stay at a local hospital.
Fig. 1.
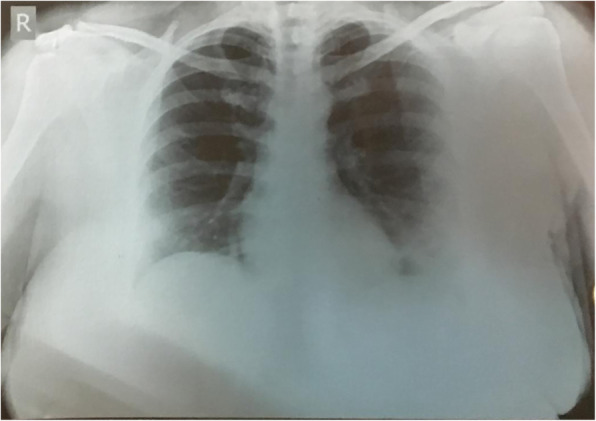
Chest X ray PA view of the lung showing bilateral ground glass opacities with left lung consolidation during her admission
Table 1.
Shows medications administered to the patient while she was admitted at the local hospital
| Route | Drug | Dose/duration |
|---|---|---|
| Intravenous | Cefoperazone+ Sulbactam | 1000 mg + 1000 mg for 4 days |
| Subcutaneous | Heparin | 5000 units every 8 h for 3 days |
| Intravenous | Remdesivir | 200 mg loading dose, then 100 mg a day for 4 days |
| Oral | Dexamethasone | 6 mg daily for 7 days |
| Oral | Azithromycin | 500 mg for 7 days |
| Oral | Doxycycline | 100 mg twice daily for 7 days |
| Oral | Montelukast and Levocetrizine combination | (10 mg) and (5 mg) for 7 days |
| Oral | Vitamin C | 1 g for 7 days |
| Oral | Pantoprazole | 40 mg once daily for 7 days |
| Oral | Ivermectin | 12 mg once daily for 7 days |
| Inhalation | Oxygen | 2 litres/minute for 7 days |
| Inhalation | Formoterol fumarate dehydrate and budesonide 200 combination | (6 mcg) and 9200mcg) twice daily, which was continued even after discharge |
| Inhalation | Salbutamol rotacaps | four times daily |
She had no systemic history of note
At the time of discharge she was switched to oral steroids (methylprednisolone) 16 mg once daily till her presentation to us
Legends: mg milligram; mcg microgram
On ophthalmic examination, her corrected distance visual acuity was 20/40 in OD and 20/20 in the left eye (OS). The intraocular pressure was 15 and 18 mmHg in OD/OS respectively. Examination of the anterior segment was normal in both eyes (BE). Fundus evaluation, OD showed absent foveal reflex with serous elevation of the retina with ring reflex at the macula. The OS was within normal limits.
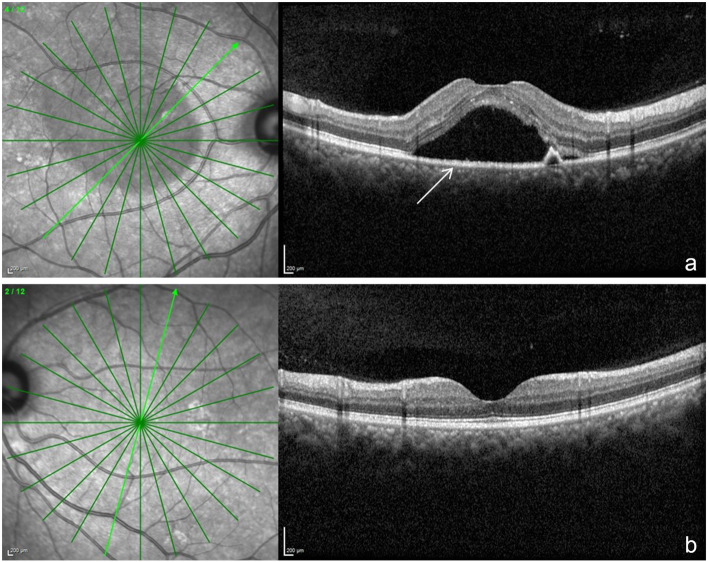
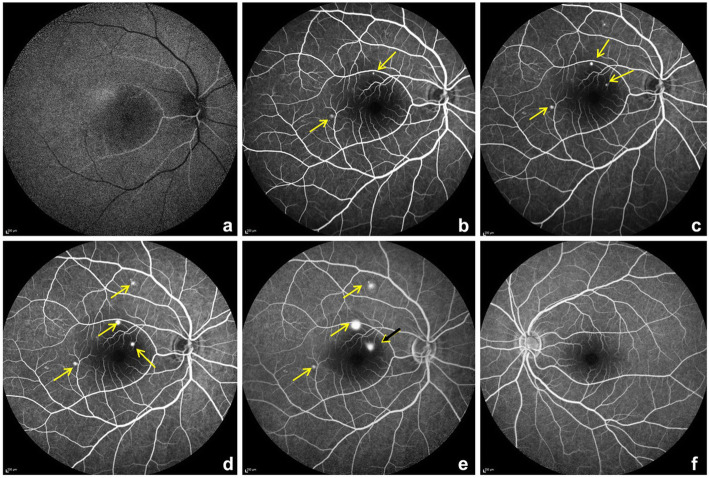
A spectral domain optical coherence tomography (SD-OCT) scan on the Spectralis™ (Heidelberg Engineering, Heidelberg, Germany) of the OD showed hyper-reflective dots in the posterior vitreous, altered foveal contour with serous detachment in the macula and with pigment epithelial detachment (Fig. 2 a). OS was normal (Fig. 2 b). Fundus fluoroscein angiography (Fig. 3a-e) showed an arm-retina time of 18 s with multiple hyperfluoroscent spots seen in the macula which increased in size and intensity in later films in an inkblot pattern characteristic of central serous retinopathy in OD. One of the lesion showed a mixed “smoke stack” and “ink blot” appearance (Fig 3e).
Fig. 2.
a shows a spectral domain optical coherence tomography scan across the macula of the OD. The white arrow points to a doom shaped elevation which is the serous retinopathy. Also in the scan is a smaller doom which represents retinal pigment epithelial detachment. b shows the normal scan of OS
Fig. 3.
a-e fundus fluoroscein angiography (FFA) of the OD from early phases a,b to later phases c-e. The yellow arrows point to a pinpoint leak initially and increasing in size in later phases. The black arrow with yellow arrow head adjacent to optic disc shows a mixed inkblot and smoke stack pattern. f- shows normal left eye
At the time of ocular presentation, investigations showed serum Ferritin 87.90 U/L (females 11–307), Procalcitonin 0.032(< 0.5), mild leucocytosis 11,600 (4500–11,000), ESR-35 (< 20 mm/hr), CRP 10.7 mg/l(< 3), Immunoglobulin (Ig) G antibodies to nucleocapsid antigen of SARS-CoV2 were positive 4.41 (< 1.0 non reactive).
Based on the clinical and imaging findings, a diagnosis of unilateral OD multifocal central serous chorioretinopathy (CSCR) was made. In consultation with chest physician the inhalational and oral steroids were stopped.
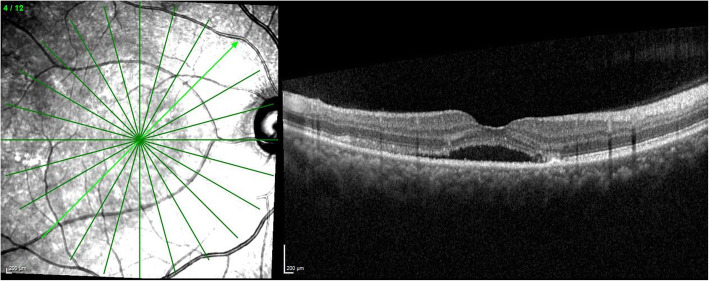
One month later, there was an improvement in her vision to 20/25 in OD, OCT showed reduction of the sub-retinal fluid and the hyper-reflective material and resolution of the pigment epithelial detachment (Fig. 4)
Fig. 4.
shows a spectral domain optical coherence tomography scan across the macula of the OD with reduction of the sub-retinal fluid and the pigment epithelial detachment a month later
We would like to report for the first time a case of a unilateral multifocal CSCR in an Asian Indian post COVID-19 treatment.
Published data show that SARS-CoV-2 binds to the host cells via the angiotensin converting-enzyme (ACE) 2 receptor [7]. Endothelial cells become vulnerable when the ACE 2 receptors are expressed and binding of SARS-CoV-2 may cause systemic endothelial dysfunction. All major organs like the lungs, heart, veins, and arteries have higher density ACE 2 receptors. Endothelial dysfunction leads to vasoconstriction, ischemia, tissue edema, and a procoagulant state secondary to endothelial alterations including endothelitis [7].
The exact pathophysiology of ocular transmission of the virus remains incompletely understood, although there is preliminary evidence of SARS-CoV-2 being detected in ocular secretions. The ocular tropism of the virus and its potential to cause localized ocular disease are worth considering [8].
Steroids may be necessary to manage the post COVID-19 systemic manifestations including the cytokine storm.
Our patient was administered oral and inhalational steroids during her stay at the local hospital. CSCR occurs or is aggravated by administration of corticosteroids irrespective of the route of administration. Steroids when used topically for skin conditions, intra-articular, intravenous, intramuscular, oral, epidural, intranasal and inhalation are all associated with CSCR [9, 10].
It is postulated that the blood retinal barrier may be damaged, with damage to retinal pigment epithelial pump and hyperpermeability of choriocapillaries leading to CSCR.
CSCR can develop secondary to exogenous corticosteroids several years later.
There may be a temporal correlation between the use of a corticosteroid nasal spray and the development of CSCR. Posterior sub capsular cataract can occur after nasal/inhaled steroids.
Cessation of inhalational steroids can lead to resolution of CSCR, which also happened partially in our patient [11].
The choroid has extensive choriocapillaries whose role is to supply oxygen and nutrients to the outer retina which has no vascular network. Glucocorticoids possibly enhance the fibroblasts proliferation with compromised capillary function leading to their fragility. Corticosteroids may affect choroid, Bruch’s membrane, or the retinal pigment epithelium [12].
Postulates on the mechanism of CSCR include vascular auto-regulation via increased transcription of adrenergic receptors or potentiation of vascular reactivity, effects from steroid-induced systemic hypertension, or a prothrombotic effect. Inhibition of collagen synthesis in Bruch’s membrane may be another mechanism. The barrier function of retinal pigment epithelium (RPE) may be compromised due to impaired ion and water transport. The role of the RPE in CSCR pathogenesis remains poorly understood. Increased tissue hydrostatic pressure in the choroid can cause the barrier function of the RPE to be compromised and lead to areas of fluid accumulation between the retina and the RPE which can also lead to pigment epithelial detachment(s) which also represent a form of RPE decompensation [13]
Some refer to the pinpoint areas of leakage seen in acute CSCR as “micro-rips” or “blowouts”.
CSCR is a self-limiting condition; various modalities of treatment have been described in the literature for the recurrent cases. One month after stopping steroids (inhalation and oral) there was a significant improvement in patient’s visual acuity and SD-OCT findings. Subsequently she was lost to follow up.
Conclusion
We hereby describe a patient with unilateral serous chorioretinopathy post COVID-19 infection, who developed ophthalmic manifestations 12 days after she was discharged from the hospital, after having tested negative for COVID-19. Patients with COVID-19 should be warned about possible ophthalmic sequelae even after their systemic recovery. Physicians treating COVID-19 should be aware of these important sequelae and refer the patient to an ophthalmologist for timely intervention.
Acknowledgements
Dr. Nikhita Reddy, Department of Retina, Narayana Nethralaya, Bengaluru, India
Abbreviations
- SARS-CoV2
Severe Acute Respiratory Syndrome Corona Virus 2
- COVID-19
Coronavirus disease 2019
- CWS
Cotton wool spots
- OD
Right eye
- CBC
Complete blood count
- ESR
Erythrocyte sedimentation rate
- RBS
Random blood sugar
- RT-PCR
Real time polymerase chain reaction
- OS
Left eye
- SD-OCT
Spectral domain optical coherence tomography
- CSCR
Central serous chorioretinopathy
- Ig
Immunoglobulin
- ACE
Angiotensin converting-enzyme
- RPE
Retinal pigment epithelium
Authors’ contributions
Design: S S, B R. Acquisition of data: SS, PBG, DM. Analysis and intrepretation: SS, PBG,PM, AK. Manuscript writing: SS, PBG, DM, PM, AK, RS. Manuscript editing: SS, PBG, PM,AK, RS. Intellectual content: SS, PBG, PM, AK, RS. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Available on request
Declarations
Ethics approval and consent to participate
The study was approved by the hospital ethics committee.
The study was approved by the Narayana Nethralaya Ethics committee, Vide approval number EC reference NO C/2020/09/09 (virtual). All tenets of the Helsinki declaration were adhered to.
Patient's written and informed consent was obtained for inclusion in the study.
Consent for publication
The patient’s written and informed consent was obtained.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation (WHO), COVID-19 dashboard. https://covid19.who.int/. Accessed 24 Mar 2021.
- 2.Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867. doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, López Vázquez Á, Ferro-Osuna M. Papillophlebitis in a COVID-19 patient: inflammation and hypercoagulable state [published online ahead of printJul 30] Eur J Ophthalmol. 2020;2020:1120672120947591. doi: 10.1177/1120672120947591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortiz-Seller A, Martínez Costa L, Hernández-Pons A, Valls Pascual E, Solves Alemany A, Albert-Fort M. (2020) Ophthalmic and neuro-ophthalmic manifestations of coronavirus disease 2019 (COVID-19). Ocul Immunol Inflamm 16; 28: 1285-1289. doi: 10.1080/09273948.2020.1817497. Epub 2020 Oct 6 [DOI] [PubMed]
- 5.Sheth JU, Narayanan R, Goyal J, Goyal V (2020) Retinal vein occlusion in COVID-19: a novel entity. Indian J Ophthalmol 68:2291–2293. https://doi.org/10.4103/ijo.IJO238020 [DOI] [PMC free article] [PubMed]
- 6.Sanjay S, Srinivasan P, Jayadev C, Mahendradas P, Gupta A, Kawali A, Shetty R. Post COVID-19 Ophthalmic Manifestations in an Asian Indian Male. Ocul Immunol Inflamm. 2021: 1–6. doi: 10.1080/09273948.2020.1870147. Epub ahead of print) [DOI] [PubMed]
- 7.To KF. Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) J Pathol. 2004;203(3):740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho D, Low R, Tong L, Gupta V, Veeraraghavan A, Agrawal R. (2020) COVID-19 and the ocular surface: a review of transmission and manifestations. Ocul Immunol Inflamm;28:726–734. doi: 10.1080/09273948.2020.1772313. Epub 2020 Jun 16. PMID: 32543262, 5 [DOI] [PubMed]
- 9.Haimovici R, Gragoudas ES, Duker JS, Sjaarda RN, Eliott D. (1997) Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology. Oct;104(10):1653–1660. doi: 10.1016/s0161-6420(97)30082-7. [DOI] [PubMed]
- 10.Tittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, Freund B, Guyer DR, Slakter JS, Sorenson JA. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63–68. doi: 10.1016/s0002-9394(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsuka AS, Khanamiri HN, Lam QN, El-Annan J. Intranasal corticosteroids and central serous Chorioretinopathy: a report and review of the literature. Hawaii J Med Public Health. 2019;78(5):151–154. [PMC free article] [PubMed] [Google Scholar]
- 12.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47(5):431–448. doi: 10.1016/S0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103–126. doi: 10.1016/j.survophthal.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on request