Abstract
BACKGROUND AND PURPOSE: The Patlak model has been applied to first-pass perfusion CT (PCT) data to extract information on blood-brain barrier permeability (BBBP) to predict hemorrhagic transformation in patients with acute stroke. However, the Patlak model was originally described for the delayed steady-state phase of contrast circulation. The goal of this study was to assess whether the first pass or the delayed phase of a contrast bolus injection better respects the assumptions of the Patlak model for the assessment of BBBP in patients with acute stroke by using PCT.
MATERIALS AND METHODS: We retrospectively identified 125 consecutive patients (29 with acute hemispheric stroke and 96 without) who underwent a PCT study by using a prolonged acquisition time up to 3 minutes. The Patlak model was applied to calculate BBBP in ischemic and nonischemic brain tissue. Linear regression of the Patlak plot was performed separately for the first pass and for the delayed phase of the contrast bolus injection. Patlak linear regression models for the first pass and the delayed phase were compared in terms of their respective square root mean squared errors (√MSE) and correlation coefficients (R) by using generalized estimating equations with robust variance estimation.
RESULTS: BBBP values calculated from the first pass were significantly higher than those from the delayed phase, both in nonischemic brain tissue (2.81 mL × 100 g−1 × min−1 for the first pass versus 1.05 mL × 100 g−1 × min−1 for the delayed phase, P < .001) and in ischemic tissue (7.63 mL × 100 g−1 × min−1 for the first pass versus 1.31 mL × 100 g−1 × min−1 for the delayed phase, P < .001). Compared with regression models from the first pass, Patlak regression models obtained from the delayed data were of better quality, showing significantly lower √MSE and higher R.
CONCLUSION: Only the delayed phase of PCT acquisition respects the assumptions of linearity of the Patlak model in patients with and without stroke.
Hemorrhagic transformation (HT) is a serious complication of ischemic stroke, which can increase the risk of mortality up to 11 times.1 Combined data from 6 major stroke trials showed that severe hemorrhage with mass effect occurs in 4.8% of patients with stroke treated with tPA within 3 hours after symptom onset and in up to 6.4% in patients treated between 3 and 6 hours.2 Damage to the blood-brain barrier (BBB) is considered one of the contributing mechanisms to HT.3 Early detection of a damaged BBB could potentially be used to identify patients who are more likely to develop HT and might, therefore, represent a contraindication to acute reperfusion therapy.4 BBB damage manifested as parenchymal enhancement5 or CSF enhancement4 after contrast administration has been shown to be more frequently present in patients with acute stroke who develop HT. Direct measurement of BBB permeability (BBBP) by using perfusion CT (PCT) has also been attempted.6–14
A relatively simple and frequently applied model to calculate BBBP is the Patlak model.15,16 Applying this model to PCT data6,9–13 means using arterial and parenchymal contrast enhancement curves to calculate the rate of contrast transfer from an intravascular to an extravascular compartment, which is a measure of BBBP. In several of the referenced studies on PCT, the Patlak model was applied to the first pass of the contrast injection necessary for PCT.9,10,12 However, the original description of the Patlak model16 clearly states that it can be applied only after a steady-state phase of contrast transfer between the compartments has been reached and the graphic analysis of the Patlak model becomes linear. The Patlak model and its assumptions are described in the Appendix.
The purpose of this study was to assess whether the first pass or the delayed phase of a contrast bolus injection better respects the assumptions of linearity of the Patlak model for the assessment of BBBP in patients with acute stroke and patients without stroke by using PCT.
Materials and Methods
Design
Imaging data obtained as part of standard clinical stroke care at our institution were retrospectively reviewed with the approval of the institutional review board. At our institution, patients with suggestion of acute stroke and no history of significant renal insufficiency or contrast allergy routinely undergo a stroke CT survey including the following: noncontrast CT (NCT) of the brain, PCT at 2 cross-sectional positions, CT angiography (CTA) of the cervical and intracranial vessels, and postcontrast cerebral CT, performed in this chronologic sequence.
We retrospectively identified a consecutive series of 130 patients admitted to the University of California, San Francisco, from January 2006 to June 2007, who met the following inclusion criteria: 1) admission to the emergency department with signs and symptoms suggesting hemispheric stroke within 12 hours after symptom onset; and 2) no evidence of intracerebral hemorrhage on the admission NCT. Patients’ charts were reviewed for demographic and clinical data.
Imaging Protocol
PCT studies were obtained on 16-section (95 patients) and 64-section (35 patients) CT scanners. Each PCT study involved successive gantry rotations performed in cine mode during intravenous administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of 1 image per second for the first 45 seconds. Additional gantry rotations were obtained at 60, 90, 120, 150, and 180 seconds. Acquisition parameters were 80 kilovolt (peak) and 100 mAs. Two successive PCT series at 2 different levels were performed following the noncontrast CT and before the CTA. At each PCT level, four 5-mm-thick sections (16-section CT scanners) or eight 5-mm-thick sections (64-section CT scanners) were assessed. The first PCT series was performed at the level of the third ventricle and the basal ganglia, and the second PCT series, above the lateral ventricles. For each PCT series, a 40-mL bolus of iohexol (300 mg/mL of iodine, Omnipaque; Amersham Health, Princeton, NJ) was administered into an antecubital vein by using a power injector at an injection rate of 5 mL per second for all patients. CT scanning was initiated 7 seconds after the start of the injection of the contrast bolus. Because there is no published description on how BBBP measurements from the second series are influenced by the contrast bolus of the first series, the second PCT series was not used for the purpose of this study.
Image Postprocessing
PCT data were analyzed using PCT software developed by Philips Medical Systems (Cleveland, Ohio). This software relies on the central volume principle, which is the most accurate for low injection rates of iodinated contrast material.17 The software obtains mathematic descriptions of the time-attenuation curves for each pixel, by applying curve fitting by least mean squares, after correcting for motion and noise reduction through an anisotropic edge-preserving spatial filter. A closed-form (noniterative) deconvolution is then applied to calculate the mean transit time (MTT) map.18 The deconvolution operation requires a reference arterial input function (most often within the anterior cerebral artery) automatically selected by the PCT software within a region of interest drawn by the user. The cerebral blood volume (CBV) map is calculated from the area under the time-attenuation curves.19 The PCT infarct core and salvageable brain tissue are automatically calculated by the software by using MTT and CBV reported in the literature as the most accurate (PCT salvageable brain tissue: MTT > 145% of the contralateral side values plus CBV ≥ 2.0 mL × 100 g−1; PCT infarct core: MTT > 145% of the contralateral side values plus CBV < 2.0 mL × 100 g−1).20
BBBP measurements were extracted from PCT data by using a second prototype software developed by Philips Medical Systems. This software is based on the Patlak model,16 which is described in detail in the Appendix. Applying the Patlak model to PCT involves performing linear regression by using data calculated from the PCT datasets. The slope of these regression lines was used as an indicator of BBBP. The prototype software used for this study allowed us to perform linear regression separately for PCT data from the first pass and from the delayed phase of the contrast injection. The cutoff point between the first pass and the delayed phase was automatically detected by the software.
Two variables measuring the quality of the linear fit were used to quantify how well the assumptions of the Patlak model were met by data extracted from the first pass and the delayed phase of the PCT acquisition. One of these variables is the square root mean squared error (√MSE), which is a measure of variability of data points around a straight line: A value close to 0 indicates a smaller spread of data points around the line, corresponding to a better fit. The other variable is the correlation coefficient (R), which measures the strength of a linear relationship: an R value closer to 1 indicates stronger linearity.
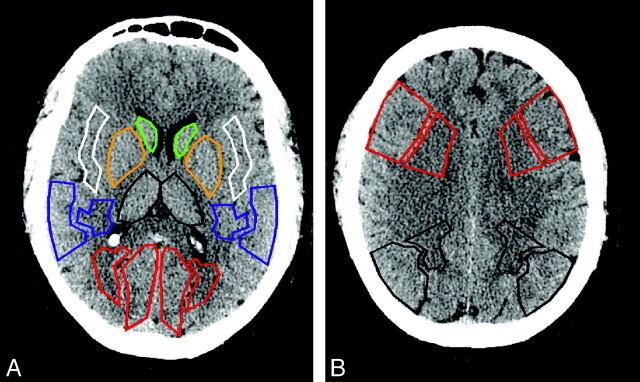
Slopes, √MSE, and Rs were measured in regions of interest corresponding to vascular territories drawn on each PCT section. For the purpose of the statistical analysis, regions of interest located within nonischemic brain parenchyma (in patients both with and without stroke) were distinguished from regions of interest in ischemic brain parenchyma (only present in patients with stroke). Regions of interest in the ischemic hemisphere in patients with stroke (ischemic regions of interest) matched the areas delineated as abnormal (infarct core or salvageable brain tissue) by the software by using the thresholds listed previously. Regions of interest located within bilateral hemispheres in patients without stroke and in the nonischemic hemisphere in patients with stroke (nonischemic regions of interest) were drawn on an anatomic basis (cortical gray matter, white matter, basal ganglia), as represented in Fig 1.
Fig 1.
Graphic illustration of the regions of interest drawn on PCT sections in patients without stroke (as well as on the nonischemic hemisphere of patients with stroke).
Statistical Analysis
Slopes, √MSEs, and Rs extracted from the first pass and delayed phase of PCT acquisitions in ischemic and nonischemic regions of interest were compared by using generalized estimating equation models with robust variance estimation, with fixed effects for patients, type of CT scanner, and type of regions of interest. Because the distribution of the parameters was not normal but skewed, rather than reporting simple means, we described estimated mean values of the slopes (obtained by log transformation of the data), √MSE, and Rs from fitting clustered log values. For all values, 95% confidence intervals (CIs) were also calculated.
Results
Patients and Imaging Studies
One hundred thirty patients matched our inclusion criteria. Twenty-nine of these patients showed an acute ischemic hemispheric stroke on their PCT studies, and 101 did not have evidence of any abnormalities (patients without stroke). Final diagnoses in the nonstroke group were the following: transient ischemic attacks in 12, vertigo in 9, migraine in 7, other neurologic disorders in 9, adverse drug reactions in 3, other non-neurologic disorders in 12, and undetermined in 49. Five patients were excluded from the nonstroke group because of PCT data that could not be analyzed due to motion artifacts or improper timing of contrast bolus injection. The patient characteristics of both groups are summarized in Table 1. In patients with stroke, the median time from symptom onset to PCT was 3 hours (range, 1.5–12 hours). The radiation dose for the prolonged PCT acquisition protocol (cine mode for 45 seconds, followed by additional gantry rotations at 60, 90, 120, 150, and 180 seconds) was only 10% (0.25 mSv) greater than the radiation dose of the conventional PCT protocol (cine mode for 45 seconds with no additional rotations).
Table 1:
Patient characteristics
| Stroke | Nonstroke | |
|---|---|---|
| No. of patients | 29 | 96 |
| No. of men (%) | 13 (45%) | 42 (44%) |
| Median age (yr) | Median = 72 | Median = 55 |
| Interquartile range = 66–82 | Interquartile range = 45–70 | |
| Range = 52–93 | Range = 17–91 | |
| Time from stroke to PCT (hr) | Median = 3 | Median = 3.5 |
| Interquartile range = 2–6 | Interquartile range = 2–7 | |
| Range = 1.5–12 | Range = 1–12 | |
| Stroke location: | ||
| ACA and MCA territories | 3 | |
| MCA territory | 24 | |
| ACA territory | 2 | |
| PCA territory | 0 |
Note:—ACA indicates anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; PCT, perfusion CT.
The Patlak analyses for the 29 patients with stroke and 96 patients without stroke were performed in a total number of 290 ischemic regions of interest and 1560 nonischemic regions of interest.
BBBP Measurements and Parameters Describing the Quality of the Linear Regression According to the Patlak Model
Compared with BBBP values from the delayed steady-state phase, BBBP values calculated from the first pass were 5.8 (95% CI, 3.3–10.1) times higher in ischemic regions of interest and 2.7 (95% CI, 2.3–3.1) times higher in nonischemic regions of interest (Table 2 and Fig 2). CIs for the first-pass BBBP measurements were considerably larger than those from the delayed phase. For both the delayed phase and the first pass, BBBP values were significantly higher (P < .05) in ischemic regions of interest compared with nonischemic regions of interest.
Table 2:
Estimated means and 95% CIs of BBBP measurements according to the Patlak model and the parameters describing the quality of the linear regression*
| First-Pass | Delayed Phase | P Value | |
|---|---|---|---|
| Ischemic ROIs (N = 290) | |||
| BBBP (95% CI) | 7.63 (4.78–12.19) | 1.31 (1.03–1.68) | <.001 |
| √MSE × 103 (95% CI) | 0.59 (0.36–0.96) | 0.089 (0.073–0.11) | <.001 |
| R (95% CI) | 0.53 (0.46–0.61) | 0.69 (0.59–0.78) | <.001 |
| Nonischemic ROIs (N = 1560) | |||
| BBBP (95% CI) | 2.81 (2.42–3.27) | 1.05 (0.97–1.14) | <.001 |
| √MSE × 103 (95% CI) | 0.18 (0.14–0.24) | 0.041 (0.035–0.048) | <.001 |
| R(95% CI) | 0.57 (0.52–0.61) | 0.85 (0.81–0.88) | <.001 |
Note:—ROI = regions of interest; BBBP, blood-brain barrier permeability; √MSE, squareroot mean squared errors.
A √MSE close to 0 indicates a smaller spread of data points around the line, corresponding to a better fit. An R close to 1 indicates a stronger linearity.
Fig 2.
Graphic illustration of the calculation of BBBP by using the Patlak model. A, The Patlak plots are constructed from the arterial and parenchymal time-enhacement curves (the recorded values used to create the illustrated Patlak plot are reported in Table 3). The first-pass component of the Patlak plots is clearly not linear, especially within the tissue at risk (shown in green in B), whereas the delayed phase respects the linear steady-state assumption of the Patlak model. B, BBBP maps at the level of the basal ganglia are shown. They are extracted from the Patlak plots and calculated pixel by pixel. The first-pass BBBP map is noisier than the delayed-phase BBBP map because the quality of the linear fit is less and results in BBBP values that are overestimated compared with the delayed-phase BBBP values. This is true again especially within the tissue at risk (shown in green). The PCT infarct core (shown in red) and at-risk brain tissue (shown in green) are automatically calculated by the software by using the MTT and CBV thresholds reported in the literature as the most accurate (PCT salvageable brain tissue: MTT > 145% of the contralateral side values plus CBV ≥ 2.0 mL × 100 g−1; PCT infarct core: MTT > 145% of the contralateral side values plus CBV < 2.0 mL × 100 g−1).20
The quality of the linear fit as part of the application of the Patlak model to the PCT data was significantly better for the delayed phase compared with the first pass, with significantly lower √MSE values and higher Rs (Table 2). √MSE values from the delayed phase were 6.6 (95% CI, 4.1–10.6) times lower than corresponding values from the first pass in ischemic regions of interest and 4.5 (95% CI, 3.4–5.9) times lower in nonischemic regions of interest. Rs from the delayed phase were 1.29 (95% CI, 1.11–1.47) times higher than corresponding values from the first pass in ischemic regions of interest and 1.50 (95% CI, 1.36–1.63) times higher in nonischemic regions of interest.
Discussion
The Patlak model15,16 is a relatively simple and frequently applied model to calculate BBBP and can be used to extract BBBP measurements from PCT data.6,9–13 The Patlak model assumes a unidirectional transfer of tracer from a reversible compartment (artery) to an irreversible compartment (brain parenchyma). It allows the calculation of BBBP values from the slope of the graphic analysis of the Patlak model, the Patlak plot, provided that the plot is linear.
Our analysis shows that only Patlak plots calculated from the delayed phase of PCT acquisition (and not from the first pass) are linear and verify the assumptions of the Patlak model. The Patlak model can thus be applied to PCT only if a delayed phase (3 minutes in our protocol) is obtained. The selection of an appropriate relatively low temporal resolution allows adequate reconstruction of the linear part of the Patlak plots, while minimizing the additional radiation dose to the patients (10% in our protocol).
The absence of linearity of Patlak plots constructed from first-pass data indicates that first-pass data do not respect the Patlak model assumptions. BBBP measurements extracted from the first-pass model by using the Patlak model are thus inaccurate. This observation has important repercussions, because BBBP measurements obtained from first pass were significantly higher (overestimated) compared with BBBP measurements from the delayed phase. It challenges the results and conclusions of all previous studies that used the Patlak model and applied it to first-pass PCT data.9,10,12
The graphs in Fig 2 demonstrate the nonlinearity of the Patlak plots calculated from first-pass PCT data, primarily in the tissue at risk. The enhancement in the tissue at risk is clearly delayed compared with the arterial enhancement, reflecting the fact that the tissue at risk receives its blood supplies from collaterals.21 The intensity of the enhancement in the tissue at risk, on the other hand, is preserved or even increased.20 In tissue at risk, the delay in enhancement together with the relatively high enhancement causes the Patlak plot to rise steeply during the first pass and to recede just as steeply at the end of the first-pass. A linear regression can be performed but is meaningless because the steep line fitted to the first-pass data results from half of the points going down instead of up and merely reflects a delay in enhancement. Permeability maps extracted from first-pass PCT data are thus actually images of delayed increased contrast enhancement (as typically encountered in the tissue at risk) rather than true images of BBBP. The same observations apply to Patlak plots extracted from first-pass PCT data in the infarct core, but the changes are not as dramatic, for there is hardly any blood arriving either through the occluded vessel or through collaterals.21 As a result, BBBP values measured from the first pass and from the delayed phase do not differ as much in the infarct core as they do in the tissue at risk.
We acknowledge the following limitations to this study:
The goal of the study was to evaluate how the Patlak model could be applied to PCT data. We did not assess other models that have been applied to PCT data to calculate BBBP, such as the distributed parameter model.7,22 Future studies are needed to determine whether our conclusions regarding the delayed acquisition required to apply the Patlak model to PCT data also hold true for these alternative models.
Brain perfusion is very different in ischemic brain (low CBF, high MTT) and in nonischemic brain (high CBF, low MTT). In previous studies addressing different topics such as reproducibility or quantitative accuracy of PCT results, ischemic brain has been considered separately from nonischemic brain, on the basis of the concept that the same variation in PCT measurements can result in different relative errors when applied to different PCT values. Following this standard approach, we compared first-pass and delayed phase both in ischemic regions of interest (with low brain perfusion) and nonischemic (with preserved perfusion).
Finally, we limited our analysis to the first PCT bolus, and we did not include data from the second PCT bolus performed routinely as part of our stroke CT protocol. This was motivated by the lack of research on the impact of the first bolus of contrast on the measurements of BBBP values from the second bolus. Saturation of the parenchymal compartment by the contrast from the first bolus could theoretically decrease the amount of contrast extravasation during the second bolus and the corresponding BBBP measurements. Such theoretic considerations will need to be verified by additional appropriate studies.
In conclusion, only the delayed phase of the PCT acquisition (and not the first-pass) respects the assumptions of the Patlak model. BBBP measurements extracted from first-pass PCT data overestimate BBBP values obtained from the delayed phase. Further research is required to investigate the relevance of delayed-phase PCT BBBP values in terms of predictive value for hemorrhagic transformation in patients with acute stroke; however, it can be concluded that first-pass data from PCT studies should not be used to calculate BBBP in patients with acute stroke.
Appendix
The model described by Patlak et al15,16 is a theoretic model of blood-brain exchange. It is a multicompartmental model that assumes the unidirectional transfer of a tracer from a reversible (arterial) compartment to an irreversible tissue compartment (in this case, the brain parenchyma) for a certain period of time. Transfer of tracer is assumed to be unidirectional when a steady-state phase is reached between reversible compartments (intravascular space and the blood-brain barrier complex). Such a steady-state phase can only occur after the initial rapid changes in tracer concentration have subsided, so the arterial concentration decreases slowly enough for the tissue compartment to follow.
The graphic representation of the Patlak model is called the Patlak plot (Fig 2). When the Patlak plot is linear, unidirectional transfer is said to be present. The slope of the plot indicates the rate of transfer between the reversible and the irreversible compartments. When applied to PCT, the Patlak model uses iodinated contrast as the tracer and takes advantage of the Hounsfield attenuation to be directly proportional to the iodinated contrast concentration. Enhancement within the arterial input function (reversible compartment) and within the parenchyma (irreversible compartment) with time is used to construct the Patlak plot. The plot is described by the following equation:
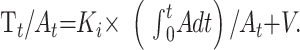 |
1) |
In this equation, Tt is the tissue enhancement at time t; At is the arterial enhancement at time t; Ki is the rate constant of net contrast transfer; and V is the distribution volume, which is typically considered to be equal to the CBV in the considered region of interest. The ratio of Tt to At, which is plotted on the y-axis, is called “apparent distribution volume.” The ratio of the integral of A to At, which is plotted on the x-axis, is called “normalized plasma integral.” The slope of a regression line fit to the linear part of the Patlak plot is an approximation of Ki (the rate of transfer) at that time. This value represents the amount of accumulated tracer in relation to the amount of tracer that has been available in plasma and is a measurement of BBBP expressed in mL × 100 g−1 × min−1. The y-axis intercept is equal to the CBV. An example of a Patlak plot can be seen in Fig 2. Arterial and parenchymal time-enhancement values used to calculate this Patlak plot, as well as the different steps of the calculation, are reported in Table 3.
Table 3:
Arterial and parenchymal time-enhancement curves used to calculate the Patlak plot in Fig 2, as well as the different steps of the calculation
| Time (sec) | Arterial Enhancement (HU) |
Integral of Arterial Enhancement (HU) |
X-Patlak (sec) |
Tissue-at-Risk Enhancement (HU) |
Y-Patlak Tissue at Risk (mL × 100 g−1) |
Infarcted Tissue Enhancement (HU) |
Y-Patlak Infarcted Tissue (mL × 100 g−1) |
|---|---|---|---|---|---|---|---|
| At | ∫At | ∫At/At | At riskt | (at riskt/At) Converted in mL × 100 g−1 | inft | (inft/At) Converted in mL × 100 g−1 | |
| 9 | 30.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 108.9 | 78.1 | 0.7 | 0.3 | 0.4 | 0 | 0.0 |
| 11 | 399.4 | 446.7 | 1.1 | 1.3 | 0.5 | 0.3 | 0.1 |
| 12 | 821.2 | 1237.1 | 1.5 | 3.3 | 0.7 | 0.8 | 0.2 |
| 13 | 1256 | 2462.3 | 2.0 | 6.4 | 0.8 | 0.7 | 0.1 |
| 14 | 1678.8 | 4110.3 | 2.4 | 11 | 1.1 | 1.3 | 0.1 |
| 15 | 2123.9 | 6203.4 | 2.9 | 17.6 | 1.3 | 3.6 | 0.3 |
| 16 | 2507 | 8679.6 | 3.5 | 26.1 | 1.7 | 7.7 | 0.5 |
| 17 | 2648.6 | 11297.4 | 4.3 | 36 | 2.2 | 12.6 | 0.8 |
| 18 | 2237.3 | 13503.9 | 6.0 | 41.2 | 3.0 | 14.5 | 1.0 |
| 19 | 1972.7 | 15445.8 | 7.8 | 49.8 | 4.1 | 15.4 | 1.3 |
| 20 | 1673.2 | 17088.2 | 10.2 | 57.8 | 5.6 | 15.5 | 1.5 |
| 21 | 1374.5 | 18431.9 | 13.4 | 64.7 | 7.6 | 15.4 | 1.8 |
| 22 | 1075.5 | 19476.6 | 18.1 | 70.1 | 10.6 | 15.6 | 2.3 |
| 23 | 801.6 | 20247.4 | 25.3 | 73.9 | 14.9 | 15.1 | 3.1 |
| 24 | 595.6 | 20812.2 | 34.9 | 75.6 | 20.6 | 13.9 | 3.8 |
| 25 | 458.4 | 21239.8 | 46.3 | 75.5 | 26.7 | 13.6 | 4.8 |
| 26 | 351.7 | 21560.7 | 61.3 | 73.6 | 33.9 | 14.3 | 6.6 |
| 27 | 264.1 | 21794 | 82.5 | 70.3 | 43.1 | 15.2 | 9.3 |
| 28 | 216.7 | 21979.9 | 101.4 | 65.9 | 49.2 | 15.2 | 11.4 |
| 29 | 205.6 | 22154.7 | 107.8 | 60.6 | 47.7 | 13.6 | 10.7 |
| 30 | 212.5 | 22336.4 | 105.1 | 55.3 | 42.1 | 12.2 | 9.3 |
| 31 | 223 | 22528.6 | 101.0 | 50.2 | 36.5 | 12.3 | 8.9 |
| 32 | 237 | 22734.8 | 95.9 | 45.2 | 30.9 | 13.3 | 9.1 |
| 33 | 265.3 | 22969.3 | 86.6 | 40.9 | 25.0 | 13.9 | 8.5 |
| 34 | 297.3 | 23235.8 | 78.2 | 37.3 | 20.3 | 13.7 | 7.5 |
| 35 | 325.5 | 23530.5 | 72.3 | 34.2 | 17.0 | 12.4 | 6.2 |
| 36 | 357.4 | 23857.1 | 66.8 | 31.4 | 14.2 | 10.8 | 4.9 |
| 37 | 389.2 | 24215.5 | 62.2 | 29.1 | 12.1 | 10.1 | 4.2 |
| 38 | 403 | 24587.7 | 61.0 | 27.3 | 11.0 | 9.9 | 4.0 |
| 39 | 391.7 | 24948.6 | 63.7 | 26 | 10.7 | 8.7 | 3.6 |
| 40 | 380.5 | 25298.3 | 66.5 | 25.1 | 10.7 | 7.2 | 3.1 |
| 41 | 394.3 | 25661.8 | 65.1 | 24.6 | 10.1 | 7.4 | 3.0 |
| 42 | 415.2 | 26046.2 | 62.7 | 24.4 | 9.5 | 9.4 | 3.7 |
| 43 | 421.8 | 26437.2 | 62.7 | 24.6 | 9.4 | 11.5 | 4.4 |
| 44 | 410.6 | 26817 | 65.3 | 25.1 | 9.9 | 13.1 | 5.2 |
| 45 | 381.7 | 27167.9 | 71.2 | 25.5 | 10.8 | 13.4 | 5.7 |
| 60 | 275 | 27412.1 | 99.7 | 20.7 | 12.2 | 10.7 | 6.3 |
| 90 | 178.3 | 27559.6 | 154.6 | 15.2 | 13.8 | 8 | 7.3 |
| 120 | 129.4 | 27658.2 | 213.7 | 11.8 | 14.8 | 6.6 | 8.3 |
| 150 | 103.6 | 27731 | 267.7 | 9.8 | 15.3 | 5.7 | 8.9 |
| 180 | 93 | 27793.2 | 298.9 | 8.8 | 15.3 | 5.1 | 8.9 |
Note:—At indicates the arterial enhancement at time t; ∫At, integral of the arterial enhancement at time t; inft, infarcted tissue enhancement at time t.
Footnotes
This work was supported by Philips Medical Systems. Scott Pohlman and Sunny Virmani are employees of Philips Medical Systems. Thomas Schneider was an employee of Philips Medical Systems at the time of this study. Max Wintermark receives funding from the National Center for Research Resources, Grant KL2 RR024130, GE Healthcare, Philips Medical Systems, and Boston Scientific; he is a consultant for Concentric. The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institutes of Health, or the other sponsors.
References
- 1.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke 2001;32:1330–35 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Donnan G, Fieschi C, et al, for the ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS—ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–74 [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol 2003;28:229–44 [DOI] [PubMed] [Google Scholar]
- 4.Latour LL, Kang DW, Ezzeddine MA, et al. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 2004;56:468–77 [DOI] [PubMed] [Google Scholar]
- 5.Kim EY, Na DG, Kim SS, et al. Prediction of hemorrhagic transformation in acute ischemic stroke: role of diffusion-weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol 2005;26:1050–55 [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolini A, Gasparetto B, Furlan M, et al. Functional perfusion and blood-brain barrier permeability images in the diagnosis of cerebral tumors by angio CT. Comput Med Imaging Graph 1994;18:145–50 [DOI] [PubMed] [Google Scholar]
- 7.Bisdas S, Hartel M, Cheong LH, et al. Detection of early vessel leakiness in acute ischemic stroke using computed tomography perfusion may indicate hemorrhagic transformation. Acta Radiol 2007;48:341–44 [DOI] [PubMed] [Google Scholar]
- 8.Bisdas S, Hartel M, Cheong LH, et al. Prediction of subsequent hemorrhage in acute ischemic stroke using permeability CT imaging and a distributed parameter tracer kinetic model. J Neuroradiol 2007;34:101–08 [DOI] [PubMed] [Google Scholar]
- 9.Cianfoni A, Cha S, Bradley WG, et al. Quantitative measurement of blood-brain barrier permeability using perfusion-CT in extra-axial brain tumors. J Neuroradiol 2006;33:164–68 [DOI] [PubMed] [Google Scholar]
- 10.Goh V, Halligan S, Bartram CI. Quantitative tumor perfusion assessment with multidetector CT: are measurements from two commercial software packages interchangeable? Radiology 2007;242:777–82 [DOI] [PubMed] [Google Scholar]
- 11.Leggett DA, Miles KA, Kelley BB. Blood-brain barrier and blood volume imaging of cerebral glioma using functional CT: a pictorial review. Eur J Radiol 1999;30:185–90 [DOI] [PubMed] [Google Scholar]
- 12.Lin K, Kazmi KS, Law M, et al. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. AJNR Am J Neuroradiol 2007;28:1292–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung WT, Lee TY, Del Maestro RF, et al. In vivo CT measurement of blood-brain transfer constant of iopamidol in human brain tumors. J Neurooncol 1992;14:177–87 [DOI] [PubMed] [Google Scholar]
- 14.Bisdas S, Yang X, Lim CC, et al. Delineation and segmentation of cerebral tumors by mapping blood-brain barrier disruption with dynamic contrast-enhanced CT and tracer kinetics modeling: a feasibility study. Eur Radiol 2008;18:143–51 [DOI] [PubMed] [Google Scholar]
- 15.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data: generalizations. J Cereb Blood Flow Metab 1985;5:584–90 [DOI] [PubMed] [Google Scholar]
- 16.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983;3:1–7 [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Maeder P, Thiran JP, et al. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 2001;11:1220–30 [DOI] [PubMed] [Google Scholar]
- 18.Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol 1983;18:94–99 [DOI] [PubMed] [Google Scholar]
- 19.Ladurner G, Zilkha E, Iliff D, et al. Measurement of regional cerebral blood volume by computerized axial tomography. J Neurol Neurosurg Psychiatry 1976;39:152–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979–85 [DOI] [PubMed] [Google Scholar]
- 21.Tan JC, Dillon WP, Liu S, et al. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007;61:533–43 [DOI] [PubMed] [Google Scholar]
- 22.Johnson JA, Wilson TA. A model for capillary exchange. Am J Physiol 1966;210:1299–303 [DOI] [PubMed] [Google Scholar]