Abstract
BACKGROUND AND PURPOSE: CT perfusion (CTP) is a more readily accessible method for evaluation of cerebral perfusion than single-photon emission CT (SPECT). We assessed whether there is any resting or drug-challenged CTP parameter correlating with cerebrovascular reserve (CVR) obtained by SPECT in Moyamoya patients.
MATERIALS AND METHODS: Normalized baseline CTP parameters and their percentage changes were calculated in 152 regions of interest (ROIs). On qualitative SPECT analysis, each ROI was classified in either the “impaired CVR” or “normal CVR” group. Quantitative CVR was calculated by using normalized SPECT values before and after acetazolamide administration. Baseline CTP parameters and their percentage changes were compared with qualitative and quantitative CVRs. Receiver operating characteristic (ROC) curve analysis defined the threshold values of CTP parameters that best predict impaired qualitative CVR.
RESULTS: The mean values of CTP parameters were significantly different between normal and impaired CVR groups. The percentage change of cerebral blood flow (pcCBF) was correlated most significantly with quantitative CVR (r = 0.89; P < .05). The correlation coefficients between the baseline CTP parameters and quantitative CVR were poor or not significant. The ROC-derived threshold values of pcCBF and mean transit time determined impaired CVR with a sensitivity of 94.4 and 85.2; specificity of 93.2 and 65.9; positive predictive value of 97.1 and 86.0; and negative predictive value of 87.2 and 64.4, respectively.
CONCLUSION: Baseline CTP parameters are not reliable for predicting impaired CVR. However, pcCBF correlated strongly with quantitative CVR; therefore, CTP evaluation for CVR in Moyamoya patients requires normalization and acetazolamide challenge.
Quantitative assessment of cerebrovascular reserve (CVR) to acetazolamide (ACZ) can determine the severity of hemodynamic impairment in patients with major cerebral artery occlusive disease.1 The risk of developing an ischemic event is known to be higher in patients with reduced CVR than in those with preserved CVR.2,3 Under physiologic conditions, ACZ increases cerebral blood flow (CBF); however, the degree of cerebral vessel dilation is lower in areas of reduced cerebral perfusion pressure, because the cerebral vessels are already dilated.4 As a result, ACZ-challenged single-photon emission CT (SPECT) increases the contrast in radioactivity levels between regions of adequate vascular reserve and those of inadequate reserve, and CVR obtained by using SPECT provides important information for hemodynamic status in patients with chronic cerebrovascular occlusive diseases.5 However, SPECT has to be performed usually in a 2-day setting due to tracer kinetics. Moreover, it provides less morphologic information than CT or MR imaging. On the other hand, CT perfusion (CTP) is a much more readily accessible imaging method for assessing the hemodynamic status in patients with cerebral steno-occlusive arterial disease and can provide both morphologic and hemodynamic information in a single investigation. Feasibility of CTP in the evaluation of patients with chronic cerebral ischemia has been attempted in several studies.6,7
Several hemodynamic parameters, such as cerebral blood volume (CBV), mean transit time (MTT), and CBF, can be acquired by CTP. Dilation of precapillary resistance vessels increases CBV; therefore, increase of CBV may be an initial indicator of a fall in cerebral perfusion pressure and reduction of CVR.3 In some studies, CBV or MTT has been measured to detect the presence of autoregulatory vasodilation, and MTT has been suggested to be more sensitive than CBV.6,8 Kaneko et al8 and Lythgoe et al9 showed that quantitative measurement of MTT by using a deconvolution algorithm can be a sensitive and reliable indicator of the cerebral perfusion reserve capacity, and it provides important information for the management of patients with occlusive cerebrovascular diseases.
In the present study, we compared the baseline and drug-challenged parameters obtained by ACZ-CTP with CVR obtained by SPECT to determine which CTP parameter best reflected CVR.
Materials and Methods
Study Patients
Between June 2005 and December 2007, 53 Moyamoya patients presenting with transient ischemic attack were referred to our institute through the neurologic clinics for evaluation of cerebral vascular and perfusion status via digital subtraction angiography (DSA) and CTP. The inclusion criteria of patients in this study were as follows: underwent both ACZ-CTP and SPECT, bilateral Moyamoya disease diagnosed by DSA as proposed by Suzuki and Kodama,10 normal finding of posterior circulation on DSA, no evidence of hemorrhage on CT scans, and no notable renal insufficiency or allergy to the contrast agent. Pediatric patients were excluded from the study. To avoid partial volume of arterial input function (AIF), we excluded patients with bilateral advanced angiographic stage; therefore, we included patients who had at least one hemisphere showing angiographic stage I or II with reasonable antegrade flow.10 Then, we semiquantitatively assessed summary parameters directly obtained from a time-concentration curve in all of the study patients. To minimize the effect of bolus delay and dispersion, we excluded patients in whom both hemispheres showed bolus arrival time difference relative to cerebellum being more than 1.7 seconds, which was estimated based on the mean values of bolus arrival time difference in stage I and II hemispheres from our pool of 53 Moyamoya patients. Finally, 19 adult patients with ischemic Moyamoya diseases were enrolled in this study. Among the 19 patients, there were 7 men (age range, 32.0–53.0 years; mean age, 39.7 years) and 12 women (age range, 27.0–66.0 years; mean age, 43.9 years). Our institutional review board approved this study, and written informed consent was obtained from every participant in accordance with the guidelines of the institutional review board at our institution.
CT Imaging Protocol
The imaging protocol consisted of nonenhanced CT and CTP before and after ACZ injection. CTP studies were performed in the transverse plane by using a 64-channel multidetector CT scanner (Brilliance 64 Channel CT; Philips Medical Systems, Cleveland, Ohio). CTP consisted of a 60-second series with 30 gantry rotations performed in a cine mode during the intravenous administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of one image per 2 seconds, resulting in a series of 30 images for each assessed section. After nonenhanced CT scan of the whole brain, at the level of the basal ganglia (BG) covering all 3 of the vascular territories, a 4-cm-thick slab oriented in the transverse plane was defined, and the slab was divided into 8 adjacent 5-mm-thick sections per location to maximize the signal intensity-to-noise ratio without increasing the dose to the patient. A 50-mL bolus of nonionic contrast media (Omnipaque, iodine 300 mg/mL; Amersham Health, Princeton, NJ) was administered into an antecubital vein by using a power injector with an injection rate of 4.5 mL/s. The acquisition parameters were 80 kVp and 120 mAs. CT scanning was initiated 2 seconds after the start of the injection. The gantry angle was parallel to and above the orbital roof to avoid radiation exposure to the lens. CT scans were studied once before intravenous infusion of 1000 mg of ACZ (Diamox; Wyeth, Marietta, Pa) and again 20 minutes after the infusion of ACZ. Both at-rest and ACZ-CTP studies were obtained during the same session with patients remaining in the supine position.
CTP Data Processing
CTP data were analyzed by using brain perfusion software (Extended Brilliance Workstation v 3.0; Philips Medical Systems). The software relies on the central volume principle to calculate perfusion parameters from the time-concentration curve. It has been reported that this principle is the most accurate for low injection rates of the iodinated contrast agent.11 The software first performs a motion correction, and noise reduction was then done by using an anisotropic, edge-preserving spatial filter. Summary parameters were obtained directly from the time-attenuation curve. Among them, we semiquantitatively assessed the bolus arrival time difference relative to cerebellum to exclude cases with severe bolus delay. The software applies curve fitting by a least-mean-squares method to obtain mathematical descriptions of the time-attenuation curves, and the MTT map was calculated by a closed-form (noniterative) deconvolution operation from the time-concentration curve of a particular voxel and the AIF.11 An AIF was selected by placing a small circular region of interest (ROI) within the earliest appearing and most densely enhancing artery (usually one of the middle cerebral arteries [MCAs] ipsilateral to the less affected hemisphere, angiographic stage I or II, and bolus arrival time difference <1.7 seconds). A venous function was selected by placing a circular ROI within a superior sagittal sinus. For each voxel, the CBV map was calculated from the areas under the time-concentration curves. CBF map for each voxel was finally calculated according to the following equation, which combines CBV and MTT value: CBF = CBV/MTT.12
Ethylcysteinate Dimer SPECT Protocol
Brain perfusion was scintigraphically studied with ethylcysteinate dimer (Tc99m-ECD; Neurolite; BMS Imaging, Billeria, Mass) and SPECT by using a 2-day protocol with a mean time of 3.0 ± 1.1 days between the 2 studies. Each study required 30 minutes after intravenous injection of approximately 925 MBq of the ligand. In the ACZ studies, Tc99m-ECD was injected 20 minutes after ACZ injection. For acquisition, a triple-head gamma camera (Multispect 3; Siemens, Erlangen, Germany) equipped with high-resolution collimators was used. The projection images were reconstructed by filtered back projection followed by a 3D butterworth filter. For uniform attenuation correction, Chang first-order method was used.
Data Analysis
On qualitative SPECT analysis, all of the baseline and ACZ-challenged SPECT scans were scored for relative perfusion abnormalities by using a 10-level color scale. Based on the consensus of 2 physicians, the relative perfusion changes were compared between the baseline and ACZ studies. Cases with a 10% (one color change) or more reduction of perfusion in each vascular territory in the ACZ study, compared with the baseline study, were defined as having an “impaired CVR group,” whereas cases that did not show a 10% reduction in perfusion were defined as having a “normal CVR group.”13
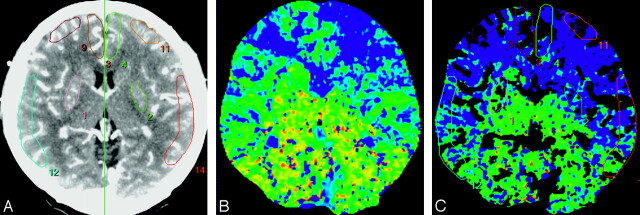
For quantitative analysis, an experienced neuroradiologist and a physician of nuclear medicine consensually drew 8 standardized elliptical mirrored ROIs manually on the BG section level of the reference CT image (Fig 1A) and SPECT over the cortical gray matter of the expected territory of the anterior cerebral artery (ACA), the MCA, and the external borderzone (EBZ), as well as the BG bilaterally. The placements of ROIs in EBZ were performed according to the method described previously.14,15 Mirrored ROIs were drawn in the bilateral cerebellar hemispheres for normalization of hemodynamic parameters. For quantitative analysis of CTP parameters, large cortical vessels were automatically excluded (Fig 1C). From each of ROIs, absolute values of the CBF, CBV, and MTT were calculated. For the ACZ-CTP study, a section at the same level as the one selected for the baseline study was selected. For normalization of each hemodynamic parameter, the ratio of values of hemodynamic parameters obtained from ROIs on each vascular territory to those obtained from ROIs on the ipsilateral cerebellar hemisphere was calculated. For evaluation of percent change (PC) of CTP parameters, PC was calculated as follows: PC (%) = (NVACZ − NVBaseline) ÷ NVBaseline × 100, where NVBaseline and NVACZ represent normalized values of the hemodynamic parameters before and after intravenous injection of ACZ, respectively. Normalized baseline CTP parameters and their PCs were calculated in 152 ROIs. For quantitative SPECT analysis, the ratio of the quantitative radioactivity count obtained from the ROI in each vascular territory to the count obtained from ROI in the ipsilateral cerebellar hemisphere was calculated as the normalized value.4 For the ACZ-challenged SPECT study, a section at the same level as the one selected in the baseline study was selected, and the normalized value was calculated by using the same methods as in the baseline study. Quantitative CVR was calculated by using normalized SPECT values before and after ACZ administration, similar to the calculation method with PCs of CTP parameters. Baseline CTP parameters and their PCs were compared with qualitative and quantitative CVRs obtained by SPECT in all of the ROIs.
Fig 1.
ROIs drawn in a reference CT image (A), MTT map (B), and vessel-removed MTT map (C). The ROIs were placed on cortical regions in the MCA territory, EBZ, ACA territory, and BG.
Statistical Analysis
We tested the differences in the values of CTP parameters and their PCs between normal and impaired CVR groups on qualitative analysis with Student t test of means, with significance set at P < .05. Then, we tested the differences in the values of CTP parameters and their PCs between the 2 groups in each vascular territory. Pearson correlation coefficient was calculated to evaluate the relationship between the values of CTP parameters and their PCs and the quantitative values of CVR in all of the ROIs. Receiver operating characteristic (ROC) curves provided a visual comparison of each CTP parameter and the accuracy of their PC in defining impaired CVR. Threshold values were assigned for each CTP parameter and their PCs by using the ROC-curve analysis to maximize the sensitivity and specificity of the threshold value.
Results
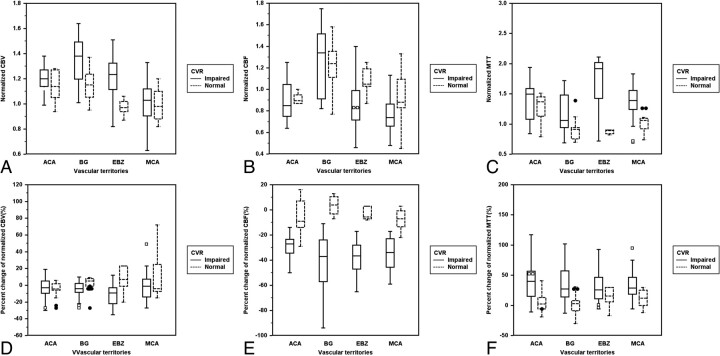
There were no adverse effects observed in our patients due to injection of contrast material or ACZ. As for qualitative CVR, 45 ROIs showed normal CVR, and 107 ROIs showed impaired CVR. In the comparison of CTP parameters with qualitative CVR, the mean values of all of the baseline CTP parameters and their PCs were significantly different in EBZ between the normal and impaired CVR groups. The PC of CBV (pcCBV) was significantly different in EBZ between the normal and impaired CVR groups, and the PC of CBF (pcCBF) and the PC of MTT (pcMTT) were significantly different in all of the vascular territories (Table 1 and Fig 2). For baseline CTP parameters, the mean values of CBV and MTT in the impaired CVR group were significantly higher than those in normal CVR groups in both EBZ and BG, and the mean values of CBF were significantly lower than those in normal CVR groups only in EBZ (Table 1 and Fig 2).
Table 1:
The differences of the mean values of CT perfusion parameters in each vascular territory between the normal and impaired cerebrovascular reserve groups
| Variable | MCA | EBZ | ACA | BG |
|---|---|---|---|---|
| CBV | ||||
| nCVR | 0.99 | 0.97* | 1.14 | 1.15* |
| iCVR | 1.02 | 1.22* | 1.21 | 1.36* |
| CBF | ||||
| nCVR | 0.94 | 1.07* | 0.90 | 1.24 |
| iCVR | 0.77 | 0.86* | 0.90 | 1.28 |
| MTT | ||||
| nCVR | 1.15 | 0.91* | 1.29 | 0.92* |
| iCVR | 1.39 | 1.67* | 1.39 | 1.16* |
| pcCBV (%) | ||||
| nCVR | 12.6 | 6.5* | −6.5 | 17.3 |
| iCVR | −2.2 | −11.4* | −3.9 | −6.1 |
| pcCBF (%) | ||||
| nCVR | −7.4* | −3.3* | −5.9* | 3.7* |
| iCVR | −35.1* | −38.4* | −30.0* | −44.3* |
| pcMTT (%) | ||||
| nCVR | 11.6* | 13.3* | 2.2* | 12.7* |
| iCVR | 33.4* | 35.6* | 43.1* | 34.6* |
Note:—CBV indicates cerebral blood volume; CBF, cerebral blood flow; MTT, mean transit time; pcCBV, percentage of change of CBV; pcCBF, percentage of change of CBF; pcMTT, percentage of change of MTT; nCVR, normal cerebrovascular reserve group; iCVR, impaired cerebrovascular reserve group; MCA, middle cerebral artery; EBZ, anterior external borderzone; ACA, anterior cerebral artery; BG, basal ganglia.
Values are statistically significant using the Student t test (P < .05).
Fig 2.
Clustered box-and-whisker graphs show the differences in the mean values of baseline and drug-challenged CT perfusion parameters between the normal and impaired cerebrovascular reserve groups in each vascular territory. For analysis of baseline CTP parameters (A to C), the mean values of CBV (A) and MTT (C) in the impaired CVR group were significantly higher than those in normal CVR group in EBZ and BG, and the mean values of CBF (B) were significantly lower than those in normal CVR group only in EBZ. For analysis of the percentage changes of baseline CTP parameters (D to F), pcCBV (D) was significantly different between the normal and impaired CVR groups only in EBZ, and pcCBF (E) and pcMTT (F) were significantly different in all of the vascular territories.
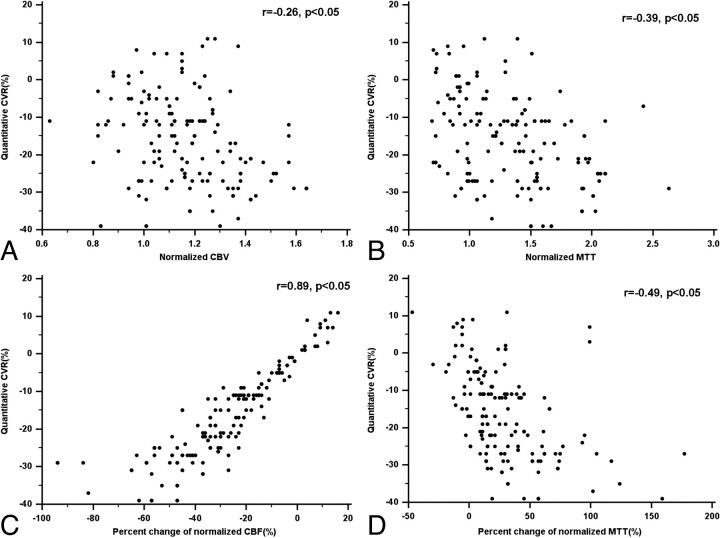
For quantitative analysis, there was a excellent correlation between the pcCBF and quantitative CVR in all of the vascular territories (r = 0.85–0.92; P < .05). There was a fair to good negative correlation between the pcMTT and the quantitative CVR in all of the vascular territories (r = −0.42 to −0.69; P < .05; Table 2 and Fig 3). For baseline CTP parameters, there was a fair negative correlation between the MTT and quantitative CVR in MCA, EBZ, and BG (r = −0.56 to −0.43; P < .05). There was a good negative correlation between the CBV (r = −0.65; P < .05) and quantitative CVR only in EBZ and no significant correlation between the CBF and quantitative CVR in all of the vascular territories (Table 2 and Fig 3).
Table 2:
The correlation coefficient between the CT perfusion parameters and quantitative cerebrovascular reserve obtained by SPECT
| Variable | MCA | EBZ | ACA | BG |
|---|---|---|---|---|
| CBV vs CVR | −0.04 | −0.65* | −0.15 | −0.40 |
| CBF vs CVR | 0.39 | 0.14 | −0.08 | 0.04 |
| MTT vs CVR | −0.43* | −0.56* | −0.07 | −0.45* |
| pcCBV vs CVR | 0.32 | 0.33 | −0.26 | 0.41 |
| pcCBF vs CVR | 0.91* | 0.92* | 0.85* | 0.88* |
| pcMTT vs CVR | −0.45* | −0.52* | −0.69* | −0.42* |
Note:—CBV indicates cerebral blood volume; CBF, cerebral blood flow; MTT, mean transit time; pcCBV, percentage of change of CBV; pcCBF, percentage of change of CBF; pcMTT, percentage of change of MTT; CVR, cerebrovascular reserve; MCA, middle cerebral artery; EBZ, anterior external borderzone; ACA, anterior cerebral artery; BG, basal ganglia.
Values are statistically significant using the Pearson correlation coefficient (P < .05).
Fig 3.
Scatter plots show the correlation between the CT perfusion parameters and the quantitative cerebrovascular reserve by using SPECT on the 152 ROIs in all of the vascular territories.
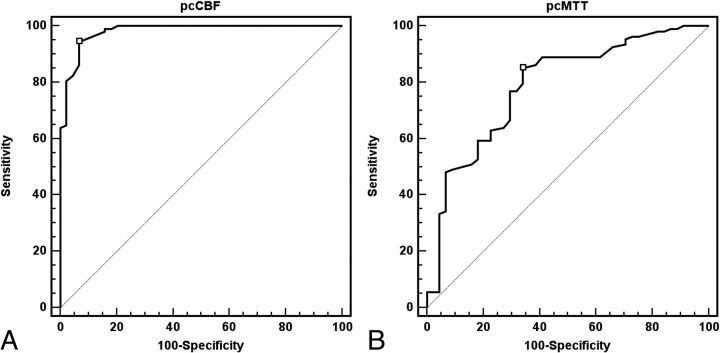
For defining impaired CVR with pcCBF, statistical analysis yielded a threshold value of −16% with a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 94.4%, 93.2%, 97.1%, and 87.2%, respectively (Fig 4). For defining impaired CVR with pcMTT, statistical analysis yielded a threshold value of 10%, with a sensitivity, specificity, PPV, and NPV of 85.2%, 65.9%, 86.0%, and 64.4%, respectively (Fig 4). In the ROC curves analysis, the most accurate CTP parameter in defining the impaired CVR was the pcCBF, as evidenced by the greatest area under the ROC curve at 0.98 (95% confidence interval = 0.94–0.99).
Fig 4.
ROC curve analysis for each CT perfusion parameter's ability to correctly identify the impaired cerebrovascular reserve. With optimal threshold value (−16%) of pcCBF, the sensitivity, specificity, PPV, and NPV for defining the impaired cerebrovascular reserve are 94.4%, 93.2%, 97.1%, and 87.2%, respectively (the area under the ROC curves = 0.98; A). For defining impaired CVR with pcMTT, statistical analysis gave a threshold value of 10%, with a sensitivity, specificity, PPV, and NPV of 85.2%, 65.9%, 86.0%, and 64.4%, respectively (the area under the ROC curves = 0.79; B).
Discussion
In this study, we found that the pcCBF among CTP parameters correlated significantly with quantitative CVR obtained by SPECT, indicating that pcCBF is a reliable parameter of vascular reserve in patients with Moyamoya disease in spite of the limitation of the deconvolution algorithm in Moyamoya disease. Although we found a correlation between baseline CTP parameters, such as CBV and MTT and quantitative CVR, this correlation was weaker than the correlation between pcCBF obtained by ACZ-CTP and quantitative CVR.
According to previous reports,16–19 baseline CTP parameters could reflect CVR. Kikuchi et al16 and Yamauchi et al17 reported that the measurement of baseline CBV and MTT by CTP can be used as an index of impaired CVR. By using a PET study, Nariai et al18 showed that variations of the CBF response to ACZ are accounted for by changes of CBV and that CBV is significantly and independently associated with CBF response during ACZ administration. However, there are different underlying physiologic mechanisms between CTP and PET imaging; therefore, the data obtained with the 2 methods may not exactly be interchangeable. In the present study, we found that the value of CBV was variable compared with qualitative and quantitative CVRs measured by SPECT and that the relationship between CBV and autoregulatory vasodilation was not linear. This result can be explained by the fact that the small penetrating arterioles at which level the autoregulatory changes occur represent only a small fraction of total CBV; therefore, the degree of autoregulatory vasodilation that leads to an increase of CBV may be variable.
In previous studies6,20 on the correlation between MTT and CVR, MTT has been suggested to be more sensitive than CBV for the identification of autoregulatory hemodynamic compromise. By using dynamic susceptibility contrast-enhanced MR imaging, Kikuchi et al16 showed that the regions with severely decreased perfusion reserve showed significantly higher MTT than those with moderately decreased or normal perfusion reserves and that CVR impairment could be evaluated with MTT. However, our present study showed a poor or fair correlation between baseline MTT value and quantitative CVR. MTT and CBF calculations are highly dependent on the choice of AIF. In Moyamoya disease, the presence of steno-occlusion of main cerebral arteries and collateral vessels always leads to the delay and dispersion of the bolus of the contrast agent. Therefore, accurate calculation of MTT and CBF values can be limited. The MTT value could be overestimated, and the CBF value could be underestimated. Although we excluded Moyamoya patients with bilateral advanced angiographic stage from this study, the weak correlation between the baseline CTP parameters and CVR could partly be attributed to delay and dispersion of the contrast bolus.
The percentage change of CBF has been used to evaluate hemodynamic impairment. Patients with no robust increase of CBF after ACZ administration are considered to have an increased risk of stroke and may benefit from interventions designed to increase blood flow.21 Despite the limitation of the deconvolution algorithm in Moyamoya patients, our CTP results showed that additional ACZ-challenged study can provide important clinical information for evaluating the extent of CVR impairment.
There are several limitations with regard to the quantification of CTP in Moyamoya patients. The presence of collateral vessels introduces delay and dispersion to the contrast bolus. These effects are not accounted for in the kinetic model used in the quantification of perfusion. The main problem is the impossibility of measuring the true AIF, which is estimated from major cerebral vessels.22 Togao et al23 used a deconvolution method with AIF to evaluate cerebral hemodynamics in Moyamoya disease and described the limitation of this method in Moyamoya patients. As shown by Calamante et al,24 the use of summary parameters as an alternative to the deconvolution analysis also presents some difficulties in Moyamoya disease. Delay and dispersion of the bolus can also affect many of the summary parameters, such as time to peak, and none of the summary parameters give a direct measure of CBF. For improvement of this limitation, Calamante et al24 proposed local AIF with independent component analysis; however, this method should be validated for clinical application. Contrary to the previous studies on perfusion MR in Moyamoya disease, our present study has specific features in study design and hypothesis. To avoid partial volume of AIF and reduce the effect of bolus delay and dispersion, we strictly included study patients who had at least one hemisphere showing angiographic stage I or II with reasonable antegrade flow and mild bolus delay on the summary parameter; bolus arrival time difference relative to cerebellum was less than 1.7 seconds, which was estimated based on the mean value in stage I and II hemispheres from our pool of 53 Moyamoya patients. Our study was focused more on the percentage changes of baseline CTP parameters during ACZ injection than baseline parameters. We expected that the percentage change of the CTP parameter would be less affected from delay and dispersion of the contrast bolus than baseline parameters. Unlike baseline CTP parameters, their percentage changes (pcCBF and pcMTT) in this study correlated significantly with CVR obtained from SPECT. However, further studies to validate our hypothesis, study design, and results should be performed with a larger population.
Conclusions
CTP is a much more readily accessible method for the evaluation of cerebral perfusion than SPECT. The baseline CTP parameters, including CBV and MTT, correlate weakly with CVR obtained from SPECT, which is attributable to the limitation of the deconvolution algorithm in Moyamoya patients. However, the percentage change of normalized baseline CBF correlated strongly with quantitative CVR. Therefore, CTP evaluation for hemodynamic status in Moyamoya patients requires normalization and ACZ challenge. Our preliminary results in Moyamoya patients should be validated with larger population studies.
Acknowledgments
We acknowledge Scott Pohlman of the Philips Medical Systems for his cooperation and valuable advice.
Footnotes
This work was supported by the 2007 grant from Ajou University School of Medicine.
References
- 1.Endo H, Inoue T, Ogasawara K, et al. Quantitative assessment of cerebral hemodynamics using perfusion-weighted MRI in patients with major cerebral artery occlusive disease: comparison with positron emission tomography. Stroke 2006;37:388–92 [DOI] [PubMed] [Google Scholar]
- 2.Kuroda S, Houkin K, Kamiyama H, et al. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 2001;32:2110–16 [DOI] [PubMed] [Google Scholar]
- 3.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002;33:1857–62 [DOI] [PubMed] [Google Scholar]
- 4.Schroeder T. Cerebrovascular reactivity to acetazolamide in carotid artery disease: enhancement of side-to-side CBF asymmetry indicates critically reduced perfusion pressure. Neurol Res 1986;8:231–36 [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto S, Watanabe M, Uematsu T, et al. Correlation of angiographic circulation time and cerebrovascular reserve by acetazolamide-challenged single photon emission CT. AJNR Am J Neuroradiol 2004;25:242–47 [PMC free article] [PubMed] [Google Scholar]
- 6.Chen A, Shyr MH, Chen TY, et al. Dynamic CT perfusion imaging with acetazolamide challenge for evaluation of patients with unilateral cerebrovascular steno-occlusive disease. AJNR Am J Neuroradiol 2006;27:1876–81 [PMC free article] [PubMed] [Google Scholar]
- 7.Eastwood JD, Alexander MJ, Petrella JR, et al. Dynamic CT perfusion imaging with acetazolamide challenge for the preprocedural evaluation of a patient with symptomatic middle cerebral artery occlusive disease. AJNR Am J Neuroradiol 2002;23:285–87 [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko K, Kuwabara Y, Mihara F, et al. Validation of the CBF, CBV, and MTT values by perfusion MRI in chronic occlusive cerebrovascular disease: a comparison with 15O-PET. Acad Radiol 2004;11:489–97 [DOI] [PubMed] [Google Scholar]
- 9.Lythgoe DJ, Ostergaard L, William SC, et al. Quantitative perfusion imaging in carotid artery stenosis using dynamic susceptibility contrast-enhanced magnetic resonance imaging. Magn Reson Imaging 2000;18:1–11 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki J, Kodama N. Moyamoya disease: a review. Stroke 1983;14:104–10 [DOI] [PubMed] [Google Scholar]
- 11.Wintermark M, Maeder P, Thiran JP, et al. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 2001;11:1220–30 [DOI] [PubMed] [Google Scholar]
- 12.Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol 1983;8:94–99 [DOI] [PubMed] [Google Scholar]
- 13.Ozgur HT, Kent Walsh T, Masaryk A, et al. Correlation of cerebrovascular reserve as measured by acetazolamide-challenged SPECT with angiographic flow patterns and intra- or extracranial arterial stenosis. AJNR Am J Neuroradiol 2001;22:928–36 [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Zwan A, Hillen B, Tulleken CAF, et al. Variability of the territories of the major cerebral arteries. J Neurosurg 1992;77:927–40 [DOI] [PubMed] [Google Scholar]
- 15.Hupperts RMM, Lodder J, Heuts-van Raak, et al. Borderzone brain infarcts on CT taking into account the variability in vascular supply areas. Cerebrovasc Dis 1996;6:294–300 [Google Scholar]
- 16.Kikuchi K, Murase K, Miki H, et al. Quantitative evaluation of mean transit times obtained with dynamic susceptibility contrast-enhanced MR imaging and with 133Xe SPECT in occlusive cerebrovascular disease. Am J Roentgenol 2002;179:229–35 [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi H, Okazawa H, Sugimoto K, et al. The effect of deafferentation on cerebral blood flow response to acetazolamide. AJNR Am J Neuroradiol 2004;25:92–96 [PMC free article] [PubMed] [Google Scholar]
- 18.Nariai T, Suzuki R, Hirakawa K, et al. Vascular reserve in chronic cerebral ischemia measured by the acetazolamide challenge test: comparison with positron-emission tomography. AJNR Am J Neuroradiol 1995;16:563–70 [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi H, Okazawa H, Kishibe Y, et al. Reduced blood flow response to acetazolamide reflects pre-existing vasodilation and decreased oxygen metabolism in major cerebral arterial occlusive disease. Eur J Nucl Med 2002;29:1349–56 [DOI] [PubMed] [Google Scholar]
- 20.Derdeyn CP, Grubb RL Jr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology 1999;53:251–59 [DOI] [PubMed] [Google Scholar]
- 21.Webster MW, Makaroun MS, Steed DL, et al. Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg 1995;21:338–45 [DOI] [PubMed] [Google Scholar]
- 22.Calamante F, Ganesan V, Kirkham FJ, et al. MR perfusion imaging in Moyamoya syndrome: potential implications for clinical evaluation of occlusive cerebrovascular disease. Stroke 2001;32:2810–16 [DOI] [PubMed] [Google Scholar]
- 23.Togao O, Mihara F, Yoshiura T, et al. Cerebral hemodynamics in Moyamoya disease: correlation between perfusion-weighted MR imaging and cerebral angiography. AJNR Am J Neuroradiol 2006;27:391–97 [PMC free article] [PubMed] [Google Scholar]
- 24.Calamante F, Mørup M, Hansen LK. Defining a local arterial input function for perfusion MRI using independent component analysis. Magn Reson Med 2004;52:789–97 [DOI] [PubMed] [Google Scholar]