Abstract
BACKGROUND AND PURPOSE: Our aim was to investigate the presence of brain gray matter (GM) abnormalities in patients with different forms of essential tremor (ET).
MATERIALS AND METHODS: We used optimized voxel-based morphometry (VBM) and manually traced single region-of-interest analysis in 50 patients with familial ET and in 32 healthy subjects. Thirty patients with ET had tremor of the arms (a-ET), whereas the remaining 20 patients had both arm and head tremor (h-ET).
RESULTS: VBM showed marked atrophy of the cerebellar vermis in the patients with h-ET with respect to healthy subjects (Pcorrected < .001). Patients with a-ET showed a trend toward a vermal GM volume loss that did not reach a significant difference with respect to healthy controls (Puncorrected < .01). The region-of-interest analysis showed a reduction of the cerebellar volume (CV) in the h-ET group (98.2 ± 13.6 mm3) compared with healthy controls (110.5 ± 15.5 mm3, P < .012) as well as in the entire vermal area (790.3 ± 94.5 mm2, 898.6 ± 170.6 mm2, P < .04 in h-ET and control groups, respectively).
CONCLUSIONS: Atrophy of the cerebellar vermis detected in patients with h-ET strongly supports the evidence for the involvement of the cerebellum in the pathophysiology of ET. The lack of a significant CV loss observed in patients with a-ET suggests that a-ET and h-ET might represent distinct subtypes of the same disease.
Essential tremor (ET) is a slowly progressive disorder usually occurring with a pattern that suggests autosomal dominant inheritance, characterized by a postural and kinetic tremor most commonly affecting the forearms and hands. The exact pathophysiology of ET is still unknown. Several lines of evidence from physiologic and neuroimaging studies point toward a major role of the cerebellum in this disease. In patients with ET, positron-emission tomography studies have demonstrated a bilateral increase in olivary glucose use1 and a bilateral increase in cerebellar and thalamic blood flow2; and MR spectroscopy has revealed a reduced cerebellar N-acetylaspartate, consistent with neuronal loss or dysfunction in the cerebellar cortex.3 On the other hand, a recent voxel-based morphometry (VBM) study failed to find gray or white matter abnormalities in the cerebellum of patients with intentional or postural ET, which raises doubt about the role that the cerebellum plays in ET.4
However, recent neuropathologic studies in patients with ET have demonstrated brain stem Lewy bodies in some patients and cerebellar pathology in others, further supporting the evidence that the cerebellum is of importance for the pathophysiology of this disease.5,6 The most recognized clinical feature of ET is a kinetic and postural tremor of the arms. This tremor may also affect other regions of the body (such as the head, face, tongue, legs), and tremor may occur in both head and arms in 34%–53% of patients.7 Albeit rarely, isolated head tremor has been found in 1%–10% of patients,7 suggesting that ET may be a composite of several phenotypes. In the current study, we used VBM to investigate morphologic volume-related abnormalities in the brains of patients with familial ET. Because patients with only postural with or without kinetic arm tremor (a-ET) and patients with both arm and head tremor (h-ET) may be distinct clinical subtypes of ET,8 we investigated these patients separately in comparison with healthy subjects.
Materials and Methods
Patients
From January 2003 to February 2007, fifty consecutive patients with a diagnosis of familial ET based on the criteria of the Movement Disorder Society on Tremor9 and 32 healthy controls (ET group: 26 women (52%); mean age, 65.2 ± 14.3 years; control group: 16 women (50%); mean age, 66.2 ± 8.1 years) were included in our study. Forty-three patients had definite ET, and 7 had probable ET. All patients with ET included in the current study were from unrelated families and had at least a first-degree relative with ET. Tremor was quantified by using the Fahn-Tolosa-Martin Tremor Rating Scale, Part A (Fahn-TRS-A),10 and by the Bain scale.11 Tremorogenic drug use, metabolic disorders, and hyperthyroidism were excluded in all patients. Patients were taking the following medications at the time of the study: propranolol antagonists (35 patients), primidone (9 patients), and clonazepam (12 patients). Subjects with a history of other neuropsychiatric disorders or alcohol or drug abuse were excluded. All subjects were recruited from the Neurology Unit of the University “Magna Graecia” of Catanzaro. Demographic and clinical characteristics are shown in Table 1. All subjects gave their written informed consent to participate in the study, which was approved by the local ethics committee.
Table 1:
Characteristics of the patients with ET and control subjects*
| Characteristic | Controls | a-ET | h-ET | P Value |
|---|---|---|---|---|
| Sex (M/F) | 16/16 | 18/12 | 6/14 | .11† |
| Age, yr | 66.2 (8.1) | 61.5 (16.5) | 70.6 (7.6) | .03‡ |
| Age at onset, yr | 43.0 (18.9) | 49.9 (16.9) | .18§ | |
| Duration of ET, yr | 18.6 (15.1) | 20.4 (14.7) | .70§ | |
| Fahn-TRS-A scale | 8.50 (2–17) | 12.50 (5–16) | .003‖ | |
| Bain scale | 34.0 (25–65) | 41.50 (30–66) | .011‖ |
Note:—ET indicates essential tremor; a-ET, arm tremor; h-ET, arm and head tremor; Fahn-TRS-A, Fahn-Tolosa-Martin Tremor Rating Scale, Part A.
Data are given as mean values (SD) or median values (range) when appropriate.
χ2 test.
One-way ANOVA, followed by unpaired t test, corrected according to Bonferroni.
Unpaired test.
Mann-Whitney U test.
MR Imaging
Brain MR imaging was performed according to our routine protocol by a 1.5T unit (Signa NV/I; GE Healthcare, Milwaukee, Wis). We used a 3D T1-weighted spoiled gradient-recalled sequence with the following parameters: voxel size, 1.2 × 0.94 × 0.94 mm3; TR, 15.2 ms; TE, 6.7 ms; flip angle, 15°; 115 sections; matrix size, 256 × 256; FOV, 24 cm. With the subject supine, cushions were carefully packed around the head to limit motion. A neuroradiologist, who was blinded to the study, detected neither abnormal nor unusual findings in any of the screened images (including the patient and control groups).
VBM
VBM analysis was performed by an optimized protocol12 by using SPM2 software (www.fil.ion.ucl.ac.uk/spm). This procedure has been previously described in detail.12 Briefly, a customized gray matter (GM) template is generated and subsequently used to normalize all of the structural images in native space to the stereotaxic Montréal Neurological Institute (MNI) space. All images (of both patients and controls) were first spatially normalized (16-parameter affine) by using the standard MNI template in SPM2. Then each normalized image was segmented into GM and white matter (WM) and CSF. The segmented GM images were averaged and smoothed (isotropic kernel; full width at half maximum [FWHM], 8 mm) to obtain a customized GM template. Next, each original MR image in native space was segmented into GM, WM, and CSF; then the GM images were spatially normalized to the customized GM template in MNI space. The deformation parameters obtained from this step were then applied to the corresponding original images in native space. The normalized anatomic T1 images were segmented again into the 3 tissue classes. Finally, all GM images were modulated13 and smoothed with a 10-mm FWHM Gaussian kernel before statistical analysis.
Volumetric-Based Region-of-Interest Analysis
In addition, to validate the VBM analysis, we performed a region-of-interest volumetric analysis of the cerebellum following the protocol of Hagemann et al.14 Whole-brain 3D T1-weighted images were analyzed on a UNIX workstation (Sun Microsystems, Santa Clara, Calif) by using MRreg software (www.erg.ion.ucl.ac.uk/MRreg.html). Details on this method, which provides a high accuracy for the assessment of cerebellar measurements, are extensively described elsewhere.15 Briefly, before volumetric analysis and to facilitate the measurements in the coronal and midsagittal planes, we realigned each volume scan in 3D space according to internal cerebellar structures within the same software. We measured total intracranial volume (TIV), total brain volume (TBV), and cerebellar volume (CV). To define regions of interest in individual sections, we used 2 methods, both available in the volume-measurement tool of MRreg: the manual tracing that we used to measure TIV and the region growing, a semiautomated method that allowed us to obtain total brain and cerebellar volumes. These volumes were estimated by use of the Cavalieri principle.16 Therefore, measurements were performed in every tenth, third, and second section for TIV, TBV and CV, respectively. The cerebral volume (CrV) was defined as TBV − CV.
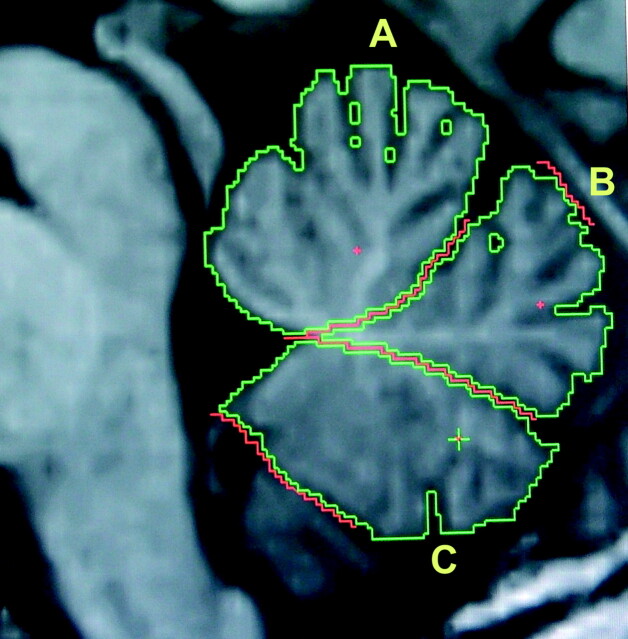
In addition, we measured the vermal area on the midsagittal images, because the VBM analysis highlighted greater involvement of this region in h-ET than in controls. The area of the vermis was divided into 3 lobules (anterior, posterior superior, and posterior inferior) by drawing a straight line from the most posterior point of the fourth ventricle to the beginning of the primary fissure or prepyramidal fissure, respectively (Fig 1). Following the suggestions of Hagemann et al,14 we did not measure the different lobular volumes because this would not have been possible in all subjects due to the lack of contrast between the different structures. In fact, planimetry of midsagittal vermal areas has the advantage of giving data on a plane, which usually is used by radiologists for commenting on the state of the vermis. To assess intrarater reliability, our primary rater (D.M.) blindly reanalyzed scans for the 20 randomly selected study participants. To assess inter-rater reliability; another rater (P.L.) independently performed region-of-interest analyses. The intra- and inter-rater reliability differences between region-of-interest volumetric measurements were assessed with the Spearman correlation coefficient.
Fig 1.
Sample manual region-of-interest tracings assessing planimetric measurements of the vermal-functional areas by using the MRreg software.14 Data are shown for a control subject with no atrophy. A, The anterior lobule. B, The posterior superior lobule. C, The posterior inferior lobule. A + B + C = midsagittal vermal area.
Statistical Analysis
The normalized, segmented, modulated, and smoothed GM volume maps were statistically analyzed by using the general linear model based on the Gaussian random field theory.17 The analyses between groups were conducted as analyses of covariance (ANCOVA). TIV, derived from the sum of GM, WM, and CSF, and total GM volume, was entered in the models (ANCOVA) to account for the possible effects of these variables. Age and sex for each subject were also entered into the design matrix as nuisance regressors (confounding covariates). To avoid edge effects close to the tissue borders, we excluded all voxels with a GM P value < .1. Different statistical analyses were performed to investigate the following: 1) the overall network of regions involved in the entire ET group, and 2) specific regions for each ET subgroup versus healthy controls, by definition of pair-wise linear contrasts. All statistical parametric analyses were thresholded at P < .001 corrected for multiple comparisons at the cluster level (Pcorrected < .001). To confirm the accuracy of spatial localization of the identified brain regions at an individual level, we verified the position of the maximal group differences on the normalized whole-brain image of each single subject. More specifically, for each individual, we identified the cerebellar regions on the basis of their image intensity and the location of the sulci and verified that the maximal difference was located in the structure identified in the group analysis. Checking the data in this way allowed us to confirm that there was a complete overlap of each individual in the regions of significant patient-control differences. To assess whether clinical variables were associated with the identified GM reduction, we furthermore carried out linear regression analysis for the whole ET group, while taking into account the presence or absence of head tremor. For this purpose, we extracted (by using the volume-of-interest function of SPM2) the raw GM volume values in those brain regions that showed a significant GM reduction in patients compared with controls. These raw values were fed into separate regression analyses (performed with the Statistical Package for the Social Sciences, Volume 12.0.1 [SPSS, Chicago, Ill]). The coordinates of voxels exhibiting the greatest group-specific effects were transferred from MNI space to Talairach space by using a nonlinear transform approach.18
For region-of-interest analysis, we used 1-way t tests and ANCOVAs with age, sex, and TIV as the covariates to identify group differences. A significance level of P < .05 was established. We did not correct for the number of the multiple comparisons in these analysis, given our stated intention of merely generating hypotheses for future investigation.
The χ2 square test was used to compare sex distributions among groups. One-way analysis of variance (ANOVA) followed by unpaired t tests, corrected according to Bonferroni, was performed for comparison of the age at examination. The differences in the mean of age at onset and disease duration between ET groups were assessed by using the unpaired t test, whereas for Fahn-TRS-A and Bain scales, the Mann-Whitney U test was used. Finally, to assess the correlation between GM values and the considered variables, we calculated the Pearson correlation test. All statistical analyses had a 2-tailed α level of < .05 for defining significance.
Results
Clinical Findings
Fifty patients with ET were grouped according to the presence or absence of head tremor. Thirty (24 definite and 6 probable) of the 50 patients with ET had a-ET (12 women [40%]; mean age, 61.5 ± 16.5 years; mean age at onset, 43.0 ± 18.9 years), whereas the remaining 20 patients (19 definite and 1 probable) had h-ET (14 women [70%]; mean age, 70.6 ± 7.6 years; mean age at onset, 49.9 ± 16.9 years). Although the age at onset and the disease duration were not significantly different between each ET group, patients with h-ET were older than patients with a-ET (P = .028, corrected according to Bonferroni) (Table 1). The sex distribution of patients with h-ET was different from that of the other groups, but this difference did not reach statistical significance (χ2 = 4.35, P = .11). Patients with h-ET scored higher on the Fahn-TRS-A scale (12.50; range, 5–16) and Bain scale (41.50; range, 30–66) than patients in a-ET group (Fahn-TRS-A scale, 8.50; range, 2–17; Bain scale, 34.0; range, 25–65; P = .003, P = .011, respectively).
VBM Results
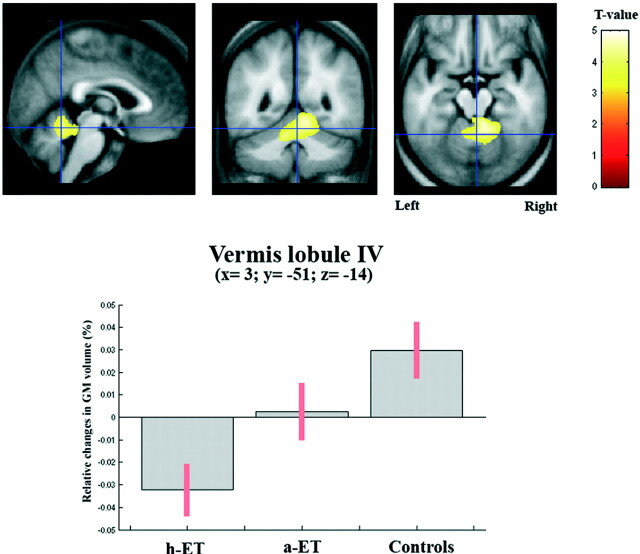
Optimized VBM analyses revealed no significant differences in GM volume when both ET groups (all ET patients) were compared with healthy control subjects. However, reductions in GM volume were detected in the right cerebellar clusters (x = 9, y = −39, z = −14), right insula (x = 33, y = −20, z = 13), and right hippocampus (x = 24, y = −34, z = 2) in patients at a lower statistical threshold (Puncorrected < .01). When the 50 patients with ET were grouped according to the presence or absence of the head tremor, additional significant changes in regional GM volume were observed (Table 2). In particular, patients with h-ET showed significant cerebellar atrophy at the level of the anterior lobe, with a marked atrophy of the vermis and partially of the paravermal regions with respect to controls (Pcorrected < .001) (Fig 2). Examination of the reverse contrast did not yield any significant results. Patients with a-ET showed only a tendency toward a CV loss, namely at the level of the right paravermal region compared with controls, though statistical significance was not reached (Puncorrected < .01). No significant difference in regional GM was observed between a-ET and h-ET groups. Because sex distribution and age varied between the 3 groups, additional ANCOVA analyses were performed to rule out the possibility that GM differences were caused by these specific demographic factors. When the analyses were performed with age and sex as covariates, no correlation was found between these 2 variables and cerebellar GM changes (Table 2). Our analyses also assessed possible links between changes in cerebellar GM volume and clinical data. In the whole ET group, the only variable that was significantly associated with cerebellar GM loss was the presence of head tremor (P = .006), whereas no association was found between cerebellar atrophy and age at onset (P = .156), duration of the disease (P = .685), or severity of the disease (Fahn-TRS-A scale score, P = .535; Bain score, P = .493).
Table 2:
Location and Talairach coordinates of significant clusters of GM volume loss in patients with h-ET or a-ET compared with controls, when total GM or TIV was included in the ANCOVA*
| Pcorrected | Cluster (κ) | Talairach Coordinates (x/y/z) | Location | |
|---|---|---|---|---|
| GM | ||||
| h-ET group | .000 | 9769 | 8/−48/−7 | R paravermal lob. IV |
| 3/−51/−14 | Vermis lob. IV | |||
| −10/−45/−15 | L paravermal lob. IV | |||
| a-ET group† | .598 | 3770 | 13/−46/−17 | R paravermal lob. IV |
| TIV | ||||
| h-ET group | .000 | 18728 | 15/−48/−10 | R paravermal lob. IV |
| 5/−46/−12 | Vermis lob. IV | |||
| −14/−47/−18 | L paravermal lob. IV | |||
| a-ET group† | .998 | 530 | 11/−51/−4 | R paravermal lob. III |
Note:—R indicates right; L, left; Lob., lobule; GM, gray matter; h-ET, arm and head tremor; a-ET, arm tremor; TIV, total intracranial volume.
GM volume loss in the h-ET and a-ET groups relative to controls, accounting for differences in total GM and TIV. A threshold of Pcorrected < .001, corrected for multiple comparisons, is used to identify the most significant peaks. Data analyses have been further corrected for age and sex distribution.
The statistical threshold is released (P < .01, uncorrected for multiple comparisons) to detect subtle morphologic changes in the a-ET group.
Fig 2.
Results from an optimized VBM analysis showing significant cerebellar atrophy at the level of the anterior lobe, above that occurring globally, selectively in patients with both a-ET and h-ET (Pcorrected < .001). Data analyses have been further corrected for age and sex. The maps are superimposed on the T1-weighted image averaged across all participants. The bar graphs give the mean and SD GM volume of voxels showing peak differences in the vermis. Patients with only a-ET display a tendency toward a CV loss.
When the 2 groups were analyzed separately, the age at onset was significantly correlated with cerebellar GM loss in the h-ET group (r = 0.44, P = .049), but not in the a-ET group (r = 0.1, P = .57). In none of the groups, were there significant correlations between cerebellar GM loss and disease duration (P > .2 in the h-ET group and P > .8 in the a-ET group) and the Bain and Fahn-TRS-A scale scores (P > .4 and P > .7 in the h-ET group and P > .8 and P > .5 in the a-ET group).
Region-of-Interest Results
Table 3 reports raw group values for TIV, CrV/CV, and vermal subterritories in addition to t test and ANCOVA results. As predicted by VBM analysis, significant group effects were found for the total midsagittal vermal area (t = 3.32, P = .042, df = 79) and anterior lobule area (t = 3.91, P = .025, df = 79). ANOVA resulted in probability values with marginal significance levels (P = .056 and .057) for total CV and vermal posterior inferior lobule areas. When statistical analysis was rerun including age, sex, and TIV as covariates of no interest, a significant group effect was confirmed for the total midsagittal vermal and anterior lobule area (t = 3.28, P = .044, df = 76; t = 3.46, P = .038, df = 76, respectively) as well as for the vermal posterior inferior lobule area (t = 3.21, P = .047, df = 76) and the total CV (t = 4.75, P = .012, df = 76). Means of correlation coefficients were 0.93 and 0.92 for intrarater and inter-rater reliability.
Table 3:
Region-of-interest measurements*
| Controls |
a-ET |
h-ET |
Raw Values |
ANCOVA Age-Sex-TIV |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | P | t | P | |
| TIV | 1428.5 | 130.4 | 1438.9 | 134 | 1438.8 | 105.8 | .05 | .95 | – | – |
| CV | 895.9 | 86.4 | 892.1 | 111.7 | 867.6 | 72.6 | .57 | .56 | 1.9 | .17 |
| CrV | 110.5 | 15.5 | 102.76 | 21.1 | 98.2 | 13.6 | 3.02 | .056 | 4.75 | .012 |
| Midsagittal vermal area | 898.6 | 170.6 | 849.8 | 124.6 | 790.3 | 94.5 | 3.32 | .042 | 3.28 | .044 |
| Anterior lobule area | 394.5 | 74.6 | 373.7 | 53.9 | 343.8 | 37.9 | 3.91 | .025 | 3.46 | .038 |
| Posterior sup. lobule area | 209.7 | 47.3 | 201.1 | 37.4 | 195.8 | 37.1 | .62 | .54 | .66 | .51 |
| Posterior inf. lobule area | 294.3 | 69.5 | 274.9 | 56.6 | 250.6 | 43.1 | 2.9 | .057 | 3.21 | .047 |
Note:—sup. indicates superior; inf., inferior; a-ET, arm tremor; h-ET, arm and head tremor; TIV, total intracranial volume; CV, cerebellar volume; CrV, cerebral volume; -, perform ANCOVA analysis by using 3 nuisance variables (age + sex + TIV).
Volumes are in cubic millimeters; areas are in square millimeters.
Discussion
In this study, by using complementary quantitative methodologies, we identified a unique pattern of GM atrophy in patients with a clinical subtype of ET characterized by the presence of head tremor. Indeed, patients with h-ET showed cerebellar atrophy in the anterior lobe mainly at the level of the vermis, whereas the volume of these cerebellar regions in patients without head tremor did not significantly differ from that of the controls. GM loss in the vermis was identified on both the VBM analysis and in the region-of-interest measurements. Consistent with a previous VBM report,4 no significant cerebellar atrophy was found in the whole ET group with respect to healthy subjects, suggesting that when a-ET and h-ET groups were analyzed together, the a-ET group masked the cerebellar GM loss observed in patients with h-ET.
Our findings are in agreement with the data on the somatotopic organization of the cerebellum, which showed that head and neck regions are probably located in the midline portion of the anterior lobe (mainly vermis lobule IV–V), whereas arms and legs are represented adjacent to the vermis over the intermediate cortex (paravermis) of the hemispheres.19,20 On this basis, it is possible to hypothesize that in the h-ET phenotype, the head tremor may reflect a pathologic process restricted to the medial region of the anterior vermis, whereas arm tremor could reflect a pathology located just outside the vermis, probably at the level of the paravermal regions and/or cerebellar hemispheres of the anterior lobe. However, no significant cerebellar volume loss was detected in the a-ET group; the reason for this finding is presently unknown. We cannot exclude the possibility that the lack of consistent cerebellar atrophy in the a-ET group may depend on the existence of similar phenotypes that may differ in their etiology.
The most important clinical finding in patients with ET is a kinetic and postural tremor of the arms. Head tremor, however, is common, and many patients show both arm and head tremor. A characteristic feature of ET is the somatotopic spread of tremor with time, from the arms to the head, whereas the converse is unusual, suggesting that these 2 phenotypes (arm and head tremor) may constitute a continuum of the same disease.7,21 Several clinical observations, however, argue against this hypothesis. First, only 30%–60% of the patients with arm ET develop head tremor with time, and the reason why some patients develop tremor of the head while others do not is not well understood.7,21,22 Second, isolated head tremor has been reported in 1%–10% of patients with ET.7,21,22 Third, head tremor is more frequent in female than male patients, and female sex is associated with a fourfold increased risk of head tremor.22 Fourth, the progression of a-ET is usually slower in patients with concomitant head tremor.8 Fifth, head tremor and arm tremor do not necessarily respond in the same way to treatments.23,24
Consistent with the previously mentioned findings, our study demonstrates that h-ET and a-ET, which had similar disease durations, showed different VBM patterns, because patients with h-ET revealed a marked atrophy of the vermis, whereas only a marginal cerebellar GM loss was detected in the a-ET group. Moreover, a significant correlation between age at onset of the disease and vermis atrophy was found in the h-ET group but not in the a-ET group. However, because patients with h-ET had a higher severity score than those in the a-ET group, it cannot be excluded that disease severity rather than the presence of head tremor might influence the degree of vermis atrophy, though our findings do not support this hypothesis because no correlation between the cerebellar GM volume and severity scores of ET was found.
Taken together, these observations support the hypothesis that a-ET and h-ET may represent distinct clinical forms of ET. Indeed, ET may be a family of related diseases rather than a single disease, in which clinical manifestations are dependent on the localization of pathology within the central nervous system. It is uncertain whether the observed GM volume loss of the vermis causes head ET or may result from the illness. If we assume the latter hypothesis, we would expect at least some correlation between the vermis atrophy and the duration of pathology in h-ET, but regression analysis did not show even a trend toward such a correlation, suggesting that vermis atrophy might be the cause rather than a consequence of head tremor.
Some important caveats need to be considered in discussing our findings, such as between-group differences in age, sex, the pattern of inheritance of ET, and the VBM limitations. First, because patients with a-ET were younger than patients with h-ET or the controls and sex distribution differed among the groups, we covaried for age and sex, but no difference was found in the degree of vermis atrophy between patients with ET and controls, indicating that these 2 variables did not influence cerebellar GM volume loss in h-ET. Second, all our patients had a family history of ET, and this does not allow the generalization of our results to the overall population of patients with ET. Indeed, estimates of the proportion of patients with ET with a family history range from as low as 17% to as high as 100%, and sporadic ET is a well-recognized entity. Nonetheless, including only patients with familial ET in our study may have reduced the probability of attributing spurious or coincidental pathology to ET.
Although VBM is a potentially powerful automated technique for image analysis, it is not without limitations. The interpretation of GM differences may be problematic when subtle changes are observed in small structures such as the cerebellar vermis, which also presents a complicated anatomy. For this reason, we compared the volumetric maps generated by VBM analysis with those generated by a manual volumetric region-of-interest approach. Comparing the methodologic advantages and disadvantages of VBM and manual region-of-interest analysis, Kubicki et al25 recommended the initial use of VBM in an exploratory manner and subsequent confirmatory analyses by application of manual region-of-interest tracing. Such an approach has been demonstrated to be successful in our analysis regarding the neuroanatomic correlates of ET: An initial whole-brain VBM analysis revealed a reduced volume of the cerebellar vermis, and this preliminary result was further confirmed by using manual region-of-interest tracing. Although our methodologic approach speaks to the robustness of our findings, neuropathologic confirmation of our preliminary data is warranted. A recent neuropathologic study found mild and inhomogeneous cerebellar pathologic changes in patients with sporadic ET,26 but the underlying neurodegenerative processes in head and arm ET have not yet been reported.
Conclusions
We used VBM to examine regional GM differences in patients with different forms of ET. Our findings demonstrate the involvement of cerebellar GM in patients with h-ET. The lack of evident cerebellar atrophy in the a-ET group suggests that ET is not a homogeneous condition and that a-ET and h-ET may represent 2 distinct clinical subtypes of the same disease. Further studies in a larger cohort of patients with a-ET are warranted to better address the role of the cerebellum in this condition.
Acknowledgments
We thank Maurizio Morelli and Sandra Paglionico for help with clinical data acquisition.
References
- 1.Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci 1993;114:45–48 [DOI] [PubMed] [Google Scholar]
- 2.Boecker H, Brooks DJ. Functional imaging of tremor. Mov Disord 1998;13:64–72 [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Shungu D, Chan S, et al. Metabolic abnormality in essential tremor: a magnetic resonance spectroscopic imaging study. Neurosci Lett 2002;333:17–20 [DOI] [PubMed] [Google Scholar]
- 4.Daniels C, Peller M, Wolff S, et al. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology 2006;67:1452–56 [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, Vonsattel JP, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol 2006;63:1189–93 [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Vonsattel JP, Honig LS, et al. Neuropathologic findings in essential tremor. Neurology 2006;66:1756–59 [DOI] [PubMed] [Google Scholar]
- 7.Louis ED. Essential tremor. Lancet Neurol 2005;4:100–10 [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Ford B, Barnes LF. Clinical subtypes of essential tremor. Arch Neurol 2000;57:1194–98 [DOI] [PubMed] [Google Scholar]
- 9.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor: Ad hoc Scientific Committee. Mov Disord 1998;13 (suppl 3):2–23 [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, eds. Parkinson's Disease and Movement Disorders. Baltimore: Lippincott William & Wilkins,1993
- 11.Bain PG, Findley LJ, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry 1993;56:868–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14 (1 Pt 1):21–36 [DOI] [PubMed] [Google Scholar]
- 13.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–21 [DOI] [PubMed] [Google Scholar]
- 14.Hagemann G, Lemieux L, Free SL, et al. Cerebellar volumes in newly diagnosed and chronic epilepsy. J Neurol 2002;249:1651–58 [DOI] [PubMed] [Google Scholar]
- 15.Lemieux L, Wieshmann UC, Moran NF, et al. The detection and significance of subtle changes in mixed-signal brain lesions by serial MRI scan matching and spatial normalization. Med Image Anal 1998;2:227–42 [DOI] [PubMed] [Google Scholar]
- 16.Mayhew TM, Olsen DR. Magnetic resonance imaging (MRI) and model-free estimates of brain volume determined using the Cavalieri principle. J Anat 1991;178:133–44 [PMC free article] [PubMed] [Google Scholar]
- 17.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2:45–53 [DOI] [PubMed] [Google Scholar]
- 18.Brett M. The MNI brain and the Talairach atlas: MRC CBU Imaging home page, 1999. http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach. Accessed July 1,2005. .
- 19.Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci 2004;5:241–49 [DOI] [PubMed] [Google Scholar]
- 20.Grodd W, Hulsmann E, Lotze M, et al. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 2001;13:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology 2004;62:932–36 [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Ford B, Frucht S. Factors associated with increased risk of head tremor in essential tremor: a community-based study in northern Manhattan. Mov Disord 2003;18:432–36 [DOI] [PubMed] [Google Scholar]
- 23.Calzetti S, Sasso E, Negretti A, et al. Effect of propranolol in head tremor: quantitative study following single-dose and sustained drug administration. Clin Neuropharmacol 1992;15:470–76 [DOI] [PubMed] [Google Scholar]
- 24.Sasso E, Perucca E, Fava R, et al. Quantitative comparison of barbiturates in essential hand and head tremor. Mov Disord 1991;6:65–68 [DOI] [PubMed] [Google Scholar]
- 25.Kubicki M, Shenton ME, Salisbury DF, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002;17:1711–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol 2008;65:101–07 [DOI] [PMC free article] [PubMed] [Google Scholar]