Abstract
BACKGROUND AND PURPOSE: Inclusion of oligodendroglial tumors may confound the utility of MR based glioma grading. Our aim was, first, to assess retrospectively whether a histogram-analysis method of MR perfusion images may both grade gliomas and differentiate between low-grade oligodendroglial tumors with or without loss of heterozygosity (LOH) on 1p/19q and, second, to assess retrospectively whether low-grade oligodendroglial subtypes can be identified in a population of patients with high-grade and low-grade astrocytic and oligodendroglial tumors.
MATERIALS AND METHODS: Fifty-two patients (23 women, 29 men; mean age, 52 years; range, 19–78 years) with histologically confirmed gliomas were imaged by using dynamic susceptibility contrast MR imaging at 1.5T. Relative cerebral blood volume (rCBV) maps were created, and 4 neuroradiologists defined the glioma volumes independently. Averaged over the 4 observers, a histogram-analysis method was used to assess the normalized histogram peak height of the glioma rCBV distributions.
RESULTS: Of the 52 patients, 22 had oligodendroglial tumors. The histogram method was able to differentiate high-grade gliomas (HGGs) from low-grade gliomas (LGGs) (Mann-Whitney U test, P < .001) and to identify low-grade oligodendroglial subtypes (P = .009). The corresponding intraclass correlation coefficients were 0.902 and 0.801, respectively. The sensitivity and specificity in terms of differentiating low-grade oligodendroglial tumors without LOH on 1p/19q from the other tumors was 100% (6/6) and 91% (42/46), respectively.
CONCLUSION: With histology as a reference, our results suggest that histogram analysis of MR imaging–derived rCBV maps can differentiate HGGs from LGGs as well as low-grade oligodendroglial subtypes with high interobserver agreement. Also, the method was able to identify low-grade oligodendroglial tumors without LOH on 1p/19q in a population of patients with astrocytic and oligodendroglial tumors.
MR imaging is the technique of choice to characterize brain tumor malignancy before treatment, with dynamic susceptibility contrast perfusion imaging as a widely used imaging technique for tumor grading.1–8 Using the WHO histopathologic criteria,9 we refer to glioma grades I–II to as low-grade gliomas (LGGs), whereas grades III-IV are referred to as high-grade gliomas (HGGs). Differentiation of HGGs from LGGs by MR imaging is based on the higher relative cerebral blood volume (rCBV) values of HGGs compared with LGGs. Image-based glioma grading is currently performed by assessing the maximal ratio between a rCBV area within the glioma and an unaffected contralateral rCBV value (rCBVmax, “hot-spot” method). A well-known problem with this method is that most oligodendroglial tumors (oligodendrogliomas or oligoastrocytomas) exhibit higher rCBVmax values than astrocytomas irrespective of glioma grade.1,7 A reason for this might be that most oligodendroglial tumors are located in cortical areas and have direct involvement with gray matter.10 Also, it has been suggested that the degree of oligodendroglial vascularity is associated with loss of heterozygosity (LOH) on the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q), seen in 40%–90% of oligodendroglial tumors.11,12 Most (∼90%) oligodendroglial tumors with LOH on 1p/19q are known to have “classic histopathologic” features.13
An oligodendroglial tumor can be defined as having classic histopathologic features if >50% of the tumor area exhibits uniform round nuclei with perinuclear halos.12,14 Such tumors often have an attenuated network of branching capillaries resembling a “chicken wire” pattern.12 Recent studies have reported that rCBVmax measurements by using the hot-spot method might be predictive of 1p/19q status, showing that oligodendroglial tumors with LOH on 1p/19q have significantly higher rCBVmax values than oligodendroglial tumors without LOH on 1p/19q.15,16 However, on the basis of the rCBVmax values, there was no significant difference between HGGs and LGGs in either study. Because oligodendroglial tumors constitute ∼10% of all diffuse gliomas (astrocytic and oligodendroglial tumors),11,17,18 it would represent a major limitation to the hot-spot method if these tumors had to be characterized by other diagnostic techniques. Little focus has been placed on whether the distribution of rCBV values in low-grade oligodendroglial tumors is more homogeneous than that in HGGs, irrespective of genotype. If so, a more rigorous grading method based on histogram analysis19,20 might differentiate between oligodendroglial subtypes without losing the ability to differentiate between HGGs and LGGs.
Patients diagnosed with low-grade oligodendroglial tumors with LOH on 1p/19q have been shown to respond more favorably to treatment and have prolonged survival rates compared with patients without this genotype.21,22 Hence, patients diagnosed with low-grade oligodendroglial tumors without LOH on 1p/19q are expected to have a reduced response to treatment and may, therefore, require a more aggressive treatment plan. To date, few studies have identified clear correlations between oligodendroglial subtype, tumor growth, and conventional MR imaging features. One study reported, however, that oligodendroglial tumors with indistinct/irregular borders, as seen on conventional MR images, were more likely to have LOH on 1p/19q compared with tumors with sharp/smooth borders.23 Unfortunately, these findings were not related to glioma grade. Although perfusion MR imaging is considered a good technique for grading gliomas, little focus has been placed on whether perfusion MR imaging can identify low-grade oligodendroglial subtypes in a population of patients with astrocytic and oligodendroglial tumors.
In view of the above, the purpose of our study was twofold: first, to assess retrospectively whether a histogram analysis method of perfusion MR images may both grade gliomas and differentiate between low-grade oligodendroglial tumors with or without LOH on 1p/19q; and, second, to assess retrospectively whether low-grade oligodendroglial subtypes can be identified in a population of patients with high-grade and low-grade astrocytic and oligodendroglial tumors.
Materials and Methods
Patient Selection
Study approval was obtained from the Regional Medical Ethics Committee, and patients were included only if informed consent was signed. Between June 2005 and June 2007, 52 patients (23 women, 29 men; mean age, 52 years; range, 19–78 years) met the inclusion criteria of a newly diagnosed glioma after perfusion MR imaging and subsequent surgery (resection or biopsy). The histologic evaluation was based on the WHO classification.9 Grade I pilocytic astrocytomas were not included because they have been shown to confound the utility of the rCBV measurements.7,10 All histologic sections from the oligodendroglial tumors were re-examined by 2 neuropathologists to establish a consensus regarding the presence or absence of classic oligodendroglial histology (yes/no). “Classic histology” was defined as the presence of tumor cells with round nuclei and perinuclear halos in >50% of the tumor area.12,14
Observers
All measurements were performed independently by 4 neuroradiologists with 4–5 years of experience with brain tumor perfusion MR imaging. The observers were blinded to the histopathologic diagnosis and patient-related information.
MR Imaging and Postprocessing
Imaging was performed at 1.5T (Sonata, Symphony, or Avanto; Siemens, Erlangen, Germany), by using an 8-channel- (Symphony/Sonata) or a 12-channel (Avanto) head coil. The protocol included an axial T2-weighted fast spin-echo sequence (TR/TE, 4000/104 ms) and an axial T1-weighted spin-echo sequence (TR/TE, 500/7.7 ms) obtained before and after intravenous contrast-agent injection. The voxel size was 0.45 × 0.45 × 5 mm3 with 19 sections. Perfusion MR imaging was performed by using a gradient-echo echo-planar imaging sequence acquired during contrast agent administration. The imaging parameters were the following: TR/TE, 1430/46 (12 axial sections) to 1590/52 ms (14 axial sections); bandwidth, 1345 Hz/pixel; FOV, 230 × 230 mm; voxel size, 1.80 × 1.80 × 5 mm3; intersection gap, 1.5 mm. For each section, 50 images were recorded at intervals equal to the TR. After approximately 8 time points, 0.2 mmol/kg of gadobutrol (Gadovist; Bayer Schering Pharma, Berlin, Germany) was injected at a rate of 5 mL/s, immediately followed by a 20-mL bolus of saline (NatriumKlorid [9 mg/ml]; B. Braun Melsungen, Melsungen, Germany) also at 5 mL/s.
The images were postprocessed by using a dedicated software package (nordicICE; NordicImagingLab, Bergen, Norway) and Matlab R2007a (MathWorks, Natick, Mass). The rCBV maps were generated by using established tracer kinetic models applied to the first-pass data.24,25 The dynamic curves were mathematically corrected to reduce contrast-agent leakage effects.26 On a pixel-by-pixel basis, the rCBV maps were normalized by dividing every rCBV value in a specific section with an unaffected white matter rCBV value defined by a neuroradiologist.27 The final rCBV maps were automatically coregistered with the conventional MR images by using a normalized mutual-information algorithm in statistical parametric mapping (SPM5, Matlab).28
Molecular Genetics
Only patients with oligodendroglial tumors were tested for LOH status. Examination of LOH status in astrocytic tumors is not routinely performed in our hospital. Assessment of LOH status was performed after the histopathologic diagnosis was made and analyzed by polymerase chain reaction (PCR) by using at least 4 of 6 microsatellite markers on 1p35–36 (D1S2660, D1S507, D1S199, D1S2734, D1S1676, D1S247) and 19q13 (D19S918, D19S219, D19S112, D19S412, D19S596, D19S206).29 Normal deoxyribonucleic acid (DNA) was extracted from blood samples of patients with oligodendroglial tumors by using QIAamp DNA Blood Mini Kit (Qiagen, Valencia, Calif) or from paraffin-embedded normal brain tissue. Tumor DNA was extracted from paraffin blocks. The tumor cell content was checked by light microscopic investigation of hematoxylin-eosin–stained sections before DNA extraction and found to reach at least 60% in each case.
Image Analysis
MR imaging findings for patients with oligodendroglial tumors were recorded by the observers. Contrast enhancement on postcontrast T1-weighted images was reported as none, moderate, or extensive. As previously described, regions of interest of the complete tumor were drawn in each section according to the combined overlay/underlay information.26,30 Areas of necrosis, cysts, or nontumor macrovessels evident on the postcontrast T1-weighted images were not included. Even though gliomas are infiltrating tumors with indistinct borders beyond the radiographic margins,11 signal-intensity hyperintensity, thought to represent tumor tissue and edema on T2-weighted images, was used to define the outmost tumor margin. Areas of contrast enhancement on postcontrast T1-weighted images were always included. Using a histogram-based grading method previously shown to grade astrocytic and oligodendroglial tumors successfully,19 we generated histograms for each subject by using the rCBV values from the complete tumor regions of interest. The range of the rCBV values along the x-axis was kept constant (values, 0–20), and the area under each histogram curve was normalized to 1. Glioma malignancy was assessed by measuring the maximal normalized peak height of the distribution. The histograms were divided into 108 bins, a number previously shown to give the best diagnostic accuracy.19 Also, to compare our results with the literature, we derived rCBVmax values for each patient by using a previously published hot-spot method.27
Statistical Analysis
Mean histogram peak heights with SEs were recorded for all HGGs, all LGGs, all high-grade oligodendroglial tumors, all low-grade oligodendroglial subtypes, and all low-grade diffuse astrocytomas. The correlation between LOH-status and classic histopathologic features in oligodendroglial tumors was assessed by using the Spearman rank correlation (Rs). Exact Mann-Whitney U tests with a significance level of P = .05 were used to assess whether the histogram method could differentiate between 1) HGGs and LGG, and 2) low-grade oligodendroglial LOH status. To compare our results with the literature values,15,16 we performed the same analysis by using rCBVmax values. The peak histogram height and rCBVmax value for each patient were average values across the 4 observers. The interobserver agreement between observers was assessed by using a single-measure intraclass correlation coefficient (ICC) parameter. The ICC values ranged from 0 to 1, with an ICC value of 1 suggesting perfect agreement. In addition, the difference between oligodendroglial tumor grades and oligodendroglial tumors with or without classic histopathology was assessed for the histogram method.
In terms of identifying patients with oligodendroglial subtypes among all 54 patients, binary logistic regression and receiver operating characteristic analysis (ROC) curves were used to assess sensitivity, specificity, and area (Az) under the ROC curve. For the logistic regression, gliomas classified as a specific oligodendroglial subtype by both observer data and histology were considered as true-positive findings. Statistical analysis was performed by using the Statistical Package for the Social Sciences 13 (SPSS, Chicago, Ill).
Results
Of the 52 patients investigated, 23 received a histologic diagnosis of a LGG (WHO grade I-II) and 29 received a diagnosis of a HGG (WHO grade III-IV). Of the LGGs, there were 8 grade II diffuse astrocytomas, 5 grade II oligodendrogliomas, and 10 grade II oligoastrocytomas. Of the HGGs, there were 2 grade III anaplastic astrocytomas, 3 grade III anaplastic oligodendrogliomas, 4 grade III anaplastic oligoastrocytomas, and 20 grade IV glioblastomas. Of the 22 patients with oligodendroglial tumors, 6 patients received the histopathologic diagnosis after a biopsy instead of a complete resection (Table).
Patient demographics, histologic features, surgical procedure, and MR imaging findings for patients diagnosed with oligodendroglial tumors
| Subject | Age (yr) | Sex | HD | WHO Grade | LOH on 1p/19q | Classic Histology | Surgical Procedure | Contrast Enhancement | Histogram Peak Heights* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | OD | II | Yes | Yes | Resection | None | 0.120 |
| 2 | 36 | F | OA | II | Yes | No | Resection | None | 0.095 |
| 3 | 28 | M | OA | II | No | No | Biopsy | None | 0.118 |
| 4 | 63 | M | AOD | III | Yes | Yes | Resection | Moderate | 0.075 |
| 5 | 54 | M | AOD | III | Yes | Yes | Resection | Moderate | 0.024 |
| 6 | 54 | M | OD | II | Yes | Yes | Biopsy | None | 0.091 |
| 7 | 44 | F | OA | II | Yes | No | Resection | None | 0.093 |
| 8 | 44 | M | OA | II | No | No | Resection | None | 0.109 |
| 9 | 40 | F | OA | II | No | No | Resection | Extensive | 0.108 |
| 10 | 37 | F | OD | II | Yes | Yes | Biopsy | None | 0.070 |
| 11 | 37 | F | OA | II | No | No | Resection | None | 0.108 |
| 12 | 30 | F | OA | II | Yes | Yes | Resection | None | 0.095 |
| 13 | 59 | M | AOD | III | No | No | Biopsy | Extensive | 0.109 |
| 14 | 31 | F | OD | II | Yes | Yes | Resection | None | 0.100 |
| 15 | 51 | M | AOA | III | No | No | Resection | None | 0.081 |
| 16 | 29 | F | AOA | III | No | No | Resection | Moderate | 0.045 |
| 17 | 71 | M | AOA | III | No | No | Resection | Moderate | 0.069 |
| 18 | 63 | M | AOA | III | No | No | Resection | Moderate | 0.070 |
| 19 | 47 | M | OA | II | Yes | No | Biopsy | Moderate | 0.093 |
| 20 | 43 | F | OA | II | No | No | Resection | None | 0.116 |
| 21 | 64 | M | OD | II | Yes | Yes | Biopsy | None | 0.105 |
| 22 | 38 | M | OA | II | No | No | Resection | None | 0.122 |
Note:—HD indicates histopathologic diagnosis; OA, oligoastrocytoma; OD, oligodendroglioma; AOD, anaplastic oligodendroglioma; AOA. anaplastic oligoastrocytoma; LOH, loss of heterozygosity.
The histogram peak height is in units of relative frequency between 0–1.
Molecular Genetics
LOH on 1p/19q was seen in 60% (9/15) of the low-grade oligodendroglial tumors and in 29% (2/7) of the high-grade oligodendroglial tumors (Table). Of the oligodendroglial subtypes, LOH on 1p/19q was seen in 5/5 grade II oligodendrogliomas, 4/10 grade II oligoastrocytomas, 2/3 grade III anaplastic oligodendrogliomas, and 0/4 grade III anaplastic oligoastrocytomas. Classic histopathology was seen in 8 of 11 oligodendroglial tumors with LOH on 1p/19q (Table). No oligodendroglial tumors without LOH on 1p/19q had classic histopathology. The correlation between classic histopathologic features and LOH on 1p/19q was significant (Rs = 0.756, P = .01).
Glioma Grading from Perfusion MR Imaging Maps
The histogram method was able to differentiate HGGs from LGGs (P < .001), high-grade from low-grade oligodendroglial tumors (P = .004), and identify low-grade oligodendroglial LOH status (P = .009). The corresponding ICC values were 0.902, 0.918, and 0.801, respectively. Figure 1 shows conventional MR images with coregistered rCBV maps of low-grade oligoastrocytomas with and without LOH on 1p/19q. The resulting histograms from the 2 low-grade oligoastrocytomas are shown in Fig 2. A plot of the mean histogram peak heights with SEs for the different glioma types investigated is shown in Fig 3. A scatterplot of the 52 histogram peak heights is shown in Fig 4.
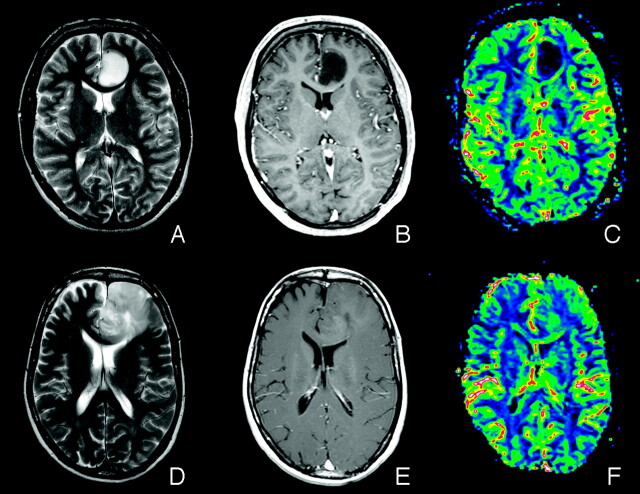
Fig 1.
A, Axial T2-weighted fast spin-echo (FSE) image (TR/TE, 4000/104 ms) of a patient (subject 3) with a low-grade oligoastrocytoma without LOH on 1p/19q. B, Axial postcontrast T1-weighted spin-echo (SE) image (TR/TE, 500/7.7 ms) of subject 3. C, Coregistered rCBV map of subject 3. D, Axial T2-weighted FSE image (TR/TE, 4000/104 ms) of a patient (subject 7) with a low-grade oligoastrocytoma with LOH on 1p/19q. E, Axial postcontrast T1-weighted SE image (TR/TE, 500/7.7 ms) of subject 7. F, Coregistered rCBV map of subject 7. Note the low rCBV values in the tumor area in image C compared with the tumor area in image F, typical of low-grade oligodendroglial tumors without LOH on 1p/19q. The corresponding normalized histogram signatures are shown in Fig 2.
Fig 2.
Resulting normalized histogram plots of the total distribution of rCBV values from the patients shown in Fig 1. The higher peak height of the low-grade oligoastrocytoma without LOH on p/19q, shown in a dotted line (subject 3, Fig 1A–C), indicates a more homogeneous rCBV distribution than the rCBV distribution of a low-grade oligoastrocytoma with LOH on 1p/19q, shown in a solid line (subject 7, Fig 1D–F).
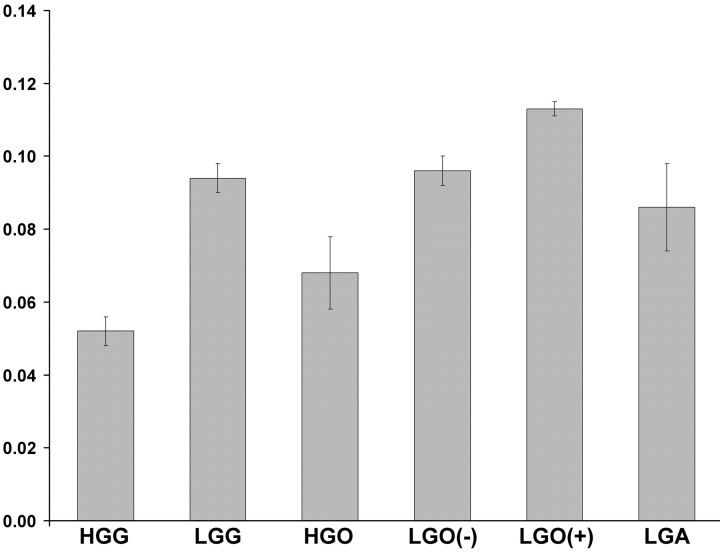
Fig 3.
Mean histogram peak heights with SEs of the mean for the different glioma types investigated. The values are as follows: HGG = 0.052 (0.004), LGG = 0.094 (0.004), all high-grade oligodendroglial tumors (HGO) = 0.068 (0.010), low-grade oligodendroglial tumor with LOH on 1p/19q (LGO[−]) = 0.096 (0.004), low-grade oligodendroglial tumor without LOH on 1p/19q (LGO[+]) = 0.113 (0.002), and low-grade diffuse astrocytoma (LGA) = 0.086 (0.012). The oligodendroglial tumors include both oligodendrogliomas and oligoastrocytomas.
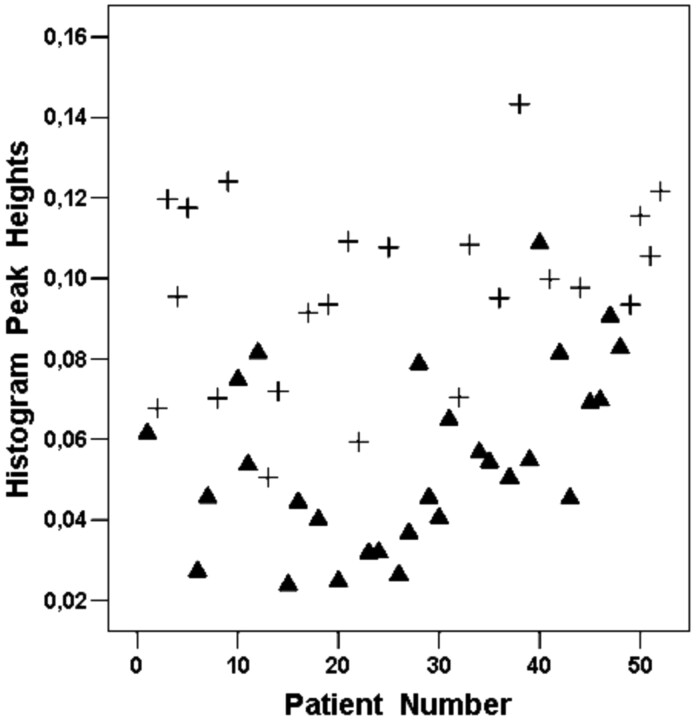
Fig 4.
A scatterplot showing histogram peak heights for the 52 patients included in our study. The histogram method is able to significantly differentiate between HGGs (▴) and LGGs (+) (P < .001), with an ICC of 0.902.
The hot-spot method was able to differentiate between HGGs and LGGs (P < .001) and high-grade and low-grade oligodendroglial tumors (P < .001) but was unable to differentiate between low-grade oligodendroglial LOH status (P = .195). The corresponding ICC values were 0.670, 0.774, and 0.283, respectively.
For the histogram method, there was no significant difference between high-grade oligodendroglial subtypes (P = .439) or between low-grade oligodendroglial tumors with and without classic histopathology (P = .194). Regardless of LOH status, there was no significant difference between low-grade oligodendroglial tumors and low-grade diffuse astrocytomas (without LOH on 1p/19q, P = .121; with LOH on 1p/19q, P = .290).
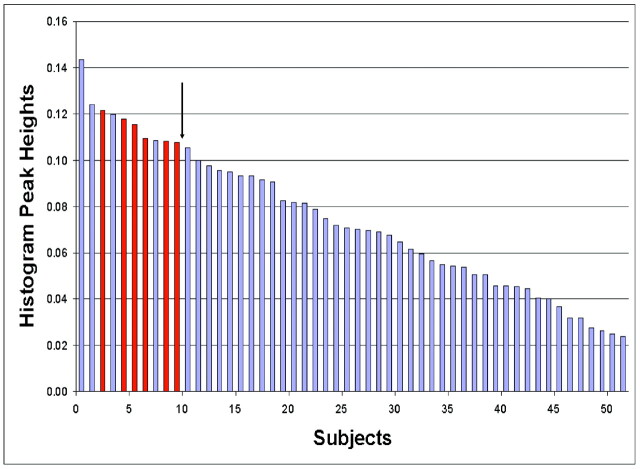
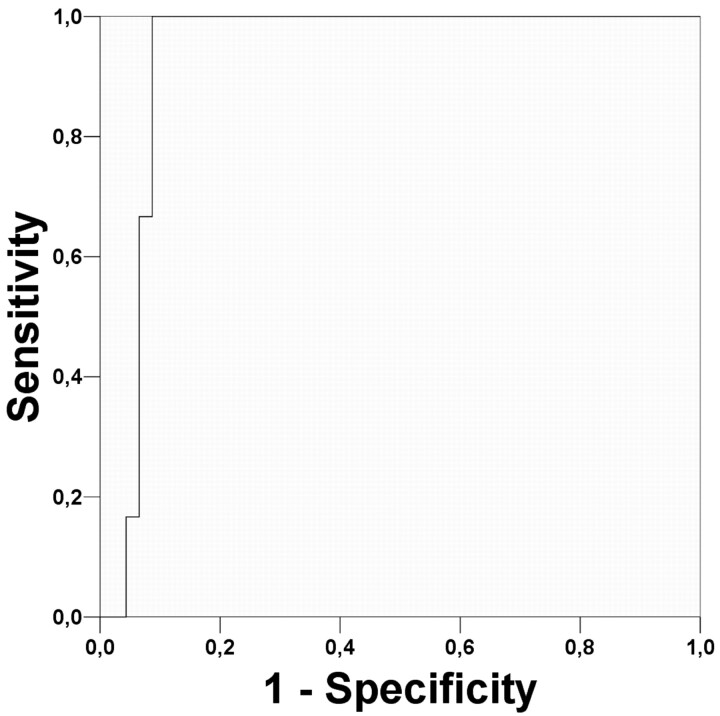
Using histology as a reference, logistic regression showed that the histogram method was unable to differentiate the 9 patients with low-grade oligodendroglial tumors with LOH on 1p/19q from the other 43 patients. However, using a cutoff value of 0.107 to distinguish the 6 patients with low-grade oligodendroglial tumors without LOH on 1p/19q from the 46 other patients (Fig 5) resulted in a sensitivity value of 100% (6/6 patients) and a specificity value of 91% (42/46 patients). The Az value (with SE) was 0.931 ± .036. The ROC curve is shown in Fig 6. The 5 other patients with cutoff values >0.107 were diagnosed as follows: 1 grade II oligodendroglioma with LOH on 1p/19q, 2 grade II diffuse astrocytomas, and 1 grade III anaplastic oligoastrocytoma without LOH on 1p/19q.
Fig 5.
Histogram peak heights for the 52 patients investigated in our study (23 LGGs and 29 HGGs). Each histogram peak height is a mean value across the 4 observers. With a cutoff value of 0.107, the sensitivity and specificity when distinguishing the 6 patients with low-grade oligodendroglial tumors without LOH on 1p/19q (red bars) from the other 46 patients (blue bars) are 100% (6/6) and 91% (42/46), respectively.
Fig 6.
ROC curve for the histogram method when distinguishing patients with low-grade oligodendroglial tumors without LOH on 1p/19q (n = 6) from the other gliomas (n = 46). The area (±SE) under the ROC curve is Az = 0.931 ± .036.
Discussion
Low-grade oligodendroglial tumors are reported having higher vascularity and higher metabolic activity than low-grade diffuse astrocytomas, showing foci of high rCBVmax values irrespective of tumor grade.3,7 Consequently, with current methods, glioma grading from MR imaging–derived rCBV maps is difficult when oligodendroglial tumors are present. A recent study suggested that the higher rCBVmax values of low-grade oligodendrogliomas might be related to 1p/19q status, showing that oligodendroglial tumors with LOH on 1p/19q had significantly higher rCBVmax values than oligodendroglial tumors without LOH on 1p/19q.15 However, the study reported no significant difference between high- and low-grade oligodendroglial tumors. A reason for this might be that 26% (22/86) of the patients in that study had recurrent tumors and received radiotherapy before the MR imaging examination. Radiation necrosis is known to reduce rCBVmax values, whereas tumor recurrence might result in higher rCBVmax values.3 Another study reported higher rCBVmax values in astrocytic and oligodendroglial tumors with LOH on 1p only, compared with tumors without LOH on 1p.16 However, on the basis of the rCBVmax values presented in that study, there was no significant difference between HGGs and LGGs.
In agreement with previously published data, a significant difference between HGGs and LGGs was observed in our study when using the hot-spot method.5,6,27 However, there was no significant difference between low-grade oligodendroglial tumors with and without LOH on 1p/19q. This result might be explained by the low interobserver agreement among the 4 observers, which may limit the utility of the hot-spot method. The incongruent results obtained with the hot-spot method suggest that the sensitivity of the method is dependent on the size and composition of the patient population. Our results were obtained in a patient population with LOH on 1p/19q in 60% of the low-grade oligodendroglial tumors.12,15 Contrary to the result of the hot-spot method obtained in our study and in the literature,15,16 the histogram method was able to differentiate between HGGs and LGGs and low-grade oligodendroglial LOH status. Because a histogram method assesses the heterogeneity of the glioma rCBV distribution,19,20 our results suggest that the rCBV distribution in low-grade oligodendrogliomas with LOH on 1p/19q is more heterogeneous than that in low-grade oligodendrogliomas without LOH on 1p/19q.
There was a significant correlation between classic oligodendroglial histology and the presence of LOH on 1p/19q. Although classic histopathology was only observed in oligodendroglial tumors with LOH on 1p/19q, a significant difference between the histogram peak heights of the low-grade oligodendrogliomas with and without classic histopathology was not observed. Because the decision as to whether an oligodendroglial tumor had classic histology was reached by 2 neuropathologists in consensus, it is unlikely that a subjective misclassification bias is the reason for this result. Here, “classic histology” was defined as the presence of tumor cells with round nuclei and perinuclear halo in >50% of the tumor area.12,14 However, lack of statistical power might explain this discrepancy because the result was based on 15 low-grade oligodendroglial tumors only. Although the correlation between LOH status and the presence of attenuated branching capillaries resembling a chicken wire pattern is reported in the literature,12 it was not investigated in our study. Thus, a quantitative measure of the capillary attenuation in each tumor is warranted.
Previous studies using the hot-spot method showed significantly higher rCBVmax values in low-grade oligodendrogliomas than in low-grade diffuse astrocytomas.7,10 Consequently, differentiation between HGGs and LGGs is difficult when oligodendroglial tumors are included.3,7 In our study, no significant difference between low-grade oligodendroglial tumors and low-grade diffuse astrocytomas was observed when using the histogram method. Hence, when grading a glioma as either HGG or LGG, the absence of a difference between grade II glioma subtypes is attractive, because a glioma grading method aiming at optimal differentiation between HGG and LGG should have minimal variations within each grade. Further, no significant difference between high-grade oligodendroglial subtypes was observed. This might be explained by the low number of high-grade oligodendroglial tumors with LOH on 1p/19q (n = 2) in our study or by the inherent heterogeneous vascularity of HGGs.19
The histogram method was unable to distinguish all 9 patients with oligodendroglial tumors with LOH on 1p/19q from the other 43 patients. This finding is a result of the large variation in rCBV values in this patient group relative to the observed overall difference in rCBV values between glioma grades. However, the diagnostic accuracy in terms of identifying low-grade oligodendroglial tumors without LOH on 1p/19q was high. Although high diagnostic accuracy is a critical criterion for any diagnostic test,31 it is difficult to establish the degree of diagnostic accuracy required by a method to have a therapeutic impact. Our results suggest that tumors that exhibit the highest histogram peak heights in a population of patients with astrocytic and oligodendroglial tumors have a high probability of being low-grade oligodendroglial tumors without LOH on 1p/19q.
Although a cutoff value was proposed in our study, the use of specific cutoff threshold values for identification of glioma grades and low-grade oligodendroglial subtypes is difficult. Confounding factors such as choice of contrast agent, dosage, imaging sequence and parameters, vendors, and postprocessing routines introduce variations that inherently reduce the transferability of these thresholds. However, if a PCR test for LOH status is difficult to obtain, MR perfusion imaging can aid in the diagnosis of LOH status after a histologic diagnosis of a low-grade oligodendroglial tumor has been made. Qualitatively, in combination with conventional MR imaging features,15,23 low-grade oligodendroglial tumors without LOH on 1p/19q can be identified as homogeneous tumors with low rCBV values compared with unaffected white matter. Still, as confirmed by other studies,15 low-grade oligodendroglial tumors without LOH on 1p/19q can also exhibit higher rCBV values equivalent to other LGGs.
In the current study, we assessed only the peak height of the normalized histogram distribution. As long as the histograms are normalized, the relative peak height is a direct measure of the heterogeneity of the rCBV distribution.19 Although alternative measures of histogram characteristics have recently been reported,20 the reported diagnostic accuracies with these methods were inferior to the peak-height approach used in our study. A comparison of all the different histogram metrics is still warranted and should be investigated in future studies.
Six patients received their histopathologic diagnosis after biopsy, which might be prone to the sampling error of not including the most representative part of the tumor. However, at our institution, the biopsy site is based on information from both contrast enhancement and foci of high rCBV values. It has been reported that this approach may offer a more accurate way of choosing a biopsy site.3 Also, only oligodendroglial tumors were tested for LOH status. LOH on 1p/19q has also been demonstrated in astrocytic tumors16 and thus constitutes a potential source of error. This may explain why some low-grade diffuse astrocytomas showed higher rCBVmax values than low-grade oligodendroglial tumors. However, the incidence of LOH on 1p/19q in astrocytomas is reported to be rare compared with oligodendroglial tumors.17,18 Also, a high incidence of LOH on 1p/19q in astrocytomas with corresponding high rCBVmax values would be inconsistent with the findings of previous studies.7,10 Further, a recent study reported lower rCBVmax values in patients with LOH on 1p only, compared with LOH on 1p/19q combined.16 In our study, only patients with combined loss were investigated. The correlation between results from MR perfusion imaging, measurements on capillary attenuation, and LOH status in gliomas is currently being further investigated by our group.
Conclusion
Our results suggest that histogram analysis of MR imaging–derived rCBV maps can differentiate HGGs from LGGs as well as low-grade oligodendroglial subtypes with high interobserver agreement. Also, the method was able to identify low-grade oligodendroglial tumors without LOH on 1p/19q in a population of patients with astrocytic and oligodendroglial tumors.
Acknowledgments
We thank Ingun Benestad, Molecular Pathological Laboratory, Department of Pathology, Rikshospitalet University Hospital, Oslo, Norway, for the molecular genetic analyses of 1p and 19q losses.
Footnotes
The Norwegian Research Council provided grant support to K.E.E.
Paper previously presented in part at: Annual Meeting of the International Society for Magnetic Resonance in Medicine, May 19–25, 2007; Berlin, Germany.
References
- 1.Lev MH, Rosen BR. Clinical applications of intracranial perfusion MR imaging. Neuroimaging Clin N Am 1999;9:309–31 [PubMed] [Google Scholar]
- 2.Law M, Oh S, Babb JS, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology 2006;238:658–67 [DOI] [PubMed] [Google Scholar]
- 3.Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 2004;9:528–37 [DOI] [PubMed] [Google Scholar]
- 4.Edelman RR, Mattle HP, Atkinson DJ, et al. Cerebral blood flow: assessment with dynamic contrast-enhanced T2*-weighted MR imaging at 1.5 T. Radiology 1990;176:211–20 [DOI] [PubMed] [Google Scholar]
- 5.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994;191:41–51 [DOI] [PubMed] [Google Scholar]
- 6.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999;211:791–98 [DOI] [PubMed] [Google Scholar]
- 7.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 2004;25:214–21 [PMC free article] [PubMed] [Google Scholar]
- 8.Papadimitrou JM, Woods AE. Structural and functional characteristics of the microcirculation in neoplasms. J Pathol 1975;116:65–72 [DOI] [PubMed] [Google Scholar]
- 9.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: International Agency for Research on Cancer;2007
- 10.Cha S, Tihan T, Crawford F, et al. Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2005;26:266–73 [PMC free article] [PubMed] [Google Scholar]
- 11.Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist 2006;11:681–93 [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Nakamura M, Kros JM, et al. Phenotype versus genotype correlation in oligodendrogliomas and low-grade diffuse astrocytomas. Acta Neuropathol 2002;103:267–75. Epub 2001 Nov 22 [DOI] [PubMed] [Google Scholar]
- 13.Godfraind C, Rousseau E, Ruchoux MM, et al. Tumour necrosis and microvascular proliferation are associated with 9p deletion and CDKN2A alterations in 1p/19q-deleted oligodendrogliomas. Neuropathol Appl Neurobiol 2003;29:462–71 [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Kim H, Kim TS. Clinical, histological, and immunohistochemical features predicting 1p/19q loss of heterozygosity in oligodendroglial tumors. Acta Neuropathol 2005;110:27–38. Epub 2005 May 26. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson MD, Smith TS, Joyce KA. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 2006;48:703–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law M, Brodsky JE, Babb J, et al. High cerebral blood volume in human gliomas predicts deletion of chromosome 1p: preliminary results of molecular studies in gliomas with elevated perfusion. J Magn Reson Imaging 2007;25:1113–19 [DOI] [PubMed] [Google Scholar]
- 17.Barbashina V, Salazar P, Holland EC, et al. Allelic losses at 1p36 and 19q13 in gliomas: correlation with histologic classification, definition of a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1 as a candidate tumor suppressor gene. Clin Cancer Res 2005;11:1119–28 [PubMed] [Google Scholar]
- 18.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 2005;64:479–89 [DOI] [PubMed] [Google Scholar]
- 19.Emblem K, Nedregaard B, Nome T, et al. Glioma grading using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology 2008;247 ;808–17 [DOI] [PubMed] [Google Scholar]
- 20.Law M, Young R, Babb J, et al. Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol 2007;28:761–66 [PMC free article] [PubMed] [Google Scholar]
- 21.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 1998;90:1473–79 [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 2000;18:636–45 [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson MD, du Plessis DG, Smith TS, et al. Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain 2006;129:1884–91 [DOI] [PubMed] [Google Scholar]
- 24.Rosen BR, Belliveau JW, Vevea JM, et al. Perfusion imaging with NMR contrast agents. Magn Reson Med 1990;14:249–65 [DOI] [PubMed] [Google Scholar]
- 25.Ostergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I. Mathematical approach and statistical analysis. Magn Reson Med 1996;36:715–25 [DOI] [PubMed] [Google Scholar]
- 26.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 2006;27:859–67 [PMC free article] [PubMed] [Google Scholar]
- 27.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 2002;224:797–803 [DOI] [PubMed] [Google Scholar]
- 28.Maes F, Collignon A, Vandermeulen D, et al. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997;16:187–98 [DOI] [PubMed] [Google Scholar]
- 29.Scheie D, Andresen PA, Cvancarova M, et al. Fluorescence in situ hybridization (FISH) on touch preparations: a reliable method for detecting loss of heterozygosity at 1p and 19q in oligodendroglial tumors. Am J Surg Pathol 2006;30:828–37 [DOI] [PubMed] [Google Scholar]
- 30.Schmainda KM, Rand SD, Joseph AM, et al. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol 2004;25:1524–32 [PMC free article] [PubMed] [Google Scholar]
- 31.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991;11:88–94 [DOI] [PubMed] [Google Scholar]