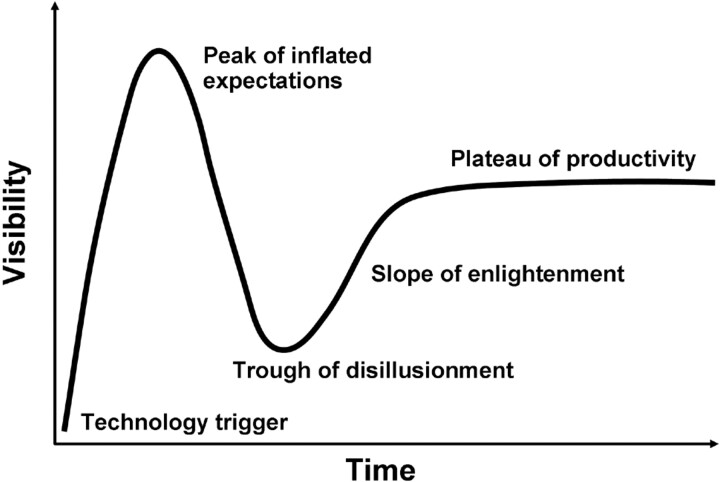
Medicine is simultaneously an art, a science, and also a business. These 3 incarnations of medicine have complex interactions. For example, the business of medicine undoubtedly distorts our scientific objectivity. A particular manifestation of such distortion that I would like to discuss is the phenomenon of hype. To “hype” is to create interest in something by flamboyant or dramatic methods. Hype has actually been studied in the field of business as it relates to the maturity, adoption, and business application of specific technologies. Such study led to the development of the Hype Cycle by Gartner.1 The Hype Cycle shows the typical time course of visibility of a new technology with time (Fig 1). Note that the Hype Cycle can have other shapes, including a shape in which the hype never recovers from the “trough of disillusionment” and declines into oblivion. With only a little retrospection, it is quite easy to come up with examples of neurointerventional devices that have followed the full course of the Hype Cycle. I need not point out specific examples by name, because they are both obvious and numerous.
Fig 1.
The Hype Cycle.
The field of neurointervention is particularly vulnerable to the hype phenomenon because of 2 particular US Food and Drug Administration (FDA) regulatory practices: the 510(k) and Humanitarian Device Exemption (HDE). These have been the primary regulatory pathways for new neurointerventional devices, and they undoubtedly amplify the Hype Cycle. With regard to the 510(k) process, some products are “spun” to the FDA as substantially equivalent to an existing approved product (eg, platinum coils), despite being specifically designed, and marketed, to be substantially different. For HDE, devices are approved by the FDA with only the barest proof of safety and efficacy to treat supposedly rare conditions but then are subtly marketed as having ever-broadening applications. Because the HDE and 510(k) approval processes each require very little data regarding safety and efficacy, there is little information available to physicians to guide therapy. This data vacuum creates an environment that promotes a whirlwind of hype. In the absence of the large amount of data necessary for rigorous proof of the safety or efficacy of a device, attempts are made to exaggerate the scientific merit of a small amount of inconclusive data through hype. This pushes the hype toward the “peak of inflated expectations.” As physicians gain some real-life experience with the device, the hype starts to burn out and we head toward the “trough of disillusionment.” Then, and only then, is the goal of a prospective randomized clinical trial finally pursued.
If we physicians would just demand a prospective randomized clinical trial in the first place, we would save a lot of time and money from being wasted on the Hype Cycle and get to the “plateau of productivity” much more quickly. Of course, randomized prospective clinical trials do not completely flatten the Hype Cycle. Drug-eluting coronary stents have been tested with randomized prospective clinical trials and, nevertheless, are now a classic example of the Hype Cycle of a medical device. However, devices supported by data from randomized prospective clinical trials are undeniably less prone to extremes of hype than those that are not.
Skepticism is essential to sort through hype. Carl Sagan said, “Skeptical scrutiny is the means, in both science and religion, by which deep thoughts can be winnowed from deep nonsense.” There is currently plenty of both deep thought and deep nonsense in the field of neurointervention. Hopefully, the future of the field will be driven by science, and the nature of science is that nonsense is ultimately unsustainable.
Reference
- 1.Understanding Hype Cycles. Available at: http://www.gartner.com/pages/story.php.id.8795.s.8.jsp. Accessed April 24,2008