Abstract
BACKGROUND AND PURPOSE: The corpus callosum is an important predilection site for traumatic axonal injury but may be unevenly affected in head trauma. We hypothesized that there were local differences in axonal injury within the corpus callosum as investigated with diffusion tensor imaging (DTI), varying among patients with differing severity of traumatic brain injury (TBI).
MATERIALS AND METHODS: Ethics committee approval and informed consent were obtained. Ten control subjects (7 men, 3 women; mean age, 37 ± 9 years) and 39 patients with TBI (27 men, 12 women; 34 ± 12 years) were investigated, of whom 24 had mild; 9, moderate; and 6, severe TBI. Regions of interest were selected in the callosal genu, body, and splenium to calculate fractional anisotropy (FA), apparent diffusion coefficient (ADC), and the number of fibers passing through. Statistical comparison was made through analysis of variance with the Scheffé post hoc analysis.
RESULTS: Compared with controls, patients with mild TBI investigated <3 months posttrauma (n = 12) had reduced FA (P < .01) and increased ADC (P < .05) in the genu, whereas patients with mild TBI investigated ≥3 months posttrauma (n = 12) showed no significant differences. Patients with moderate and severe TBI, all investigated <3 months posttrauma, had reduced FA (P < .001) and increased ADC (P < .01) in the genu compared with controls and reduced FA in the splenium (P < .001) without significant ADC change.
CONCLUSION: Mild TBI is associated with DTI abnormalities in the genu <3 months posttrauma. In more severe TBI, both the genu and splenium are affected. DTI suggests a larger contribution of vasogenic edema in the genu than in the splenium in TBI.
Traumatic axonal injury is a frequent cause of impaired clinical outcome in patients with traumatic brain injury (TBI).1 The location and severity of traumatic axonal injury is related to various factors,2,3 including the characteristics of acceleration/deceleration forces during the trauma incident and differences in attenuation and rigidity between adjacent cerebral structures. One of the predilection sites of traumatic axonal injury is the corpus callosum.2,4 This structure forms the largest commissural white matter bundle in the brain. Its genu connects orbitofrontal and frontal cortices, whereas its body and splenium connect temporal, parietal, and occipital regions.5 CT and MR imaging studies have indicated that the corpus callosum is unevenly affected in TBI in the sense that the splenium more commonly shows traumatic axonal injury than the genu and body.2–4,6,7 However, the frequency of axonal injury is underestimated with CT and various MR imaging techniques, such as fluid-attenuated inversion recovery (FLAIR), T2-weighted fast spin-echo, and T2*-weighted gradient-echo (GE) sequences.7,8 Diffusion tensor imaging (DTI) has evolved in recent years as a valuable complementary technique to investigate traumatic axonal injury.9–15 DTI allows quantification of tissue architecture through an extensive description of water diffusion.16,17 DTI parameters such as fractional anisotropy (FA) describe the level of structural integrity of the tissue that is investigated.16 Traumatic axonal injury is associated with FA reduction and changes of the apparent diffusion coefficient (ADC).9–15 Fiber tracking algorithms can be used to reconstruct white matter fibers in 3D and may demonstrate reduction of fiber attenuation in TBI.9
Several DTI studies have investigated the corpus callosum in head trauma.9,11,13–15 The extent of traumatic axonal injury in the corpus callosum and its relation to trauma severity are not clear from these studies. First, a number of studies investigated only the genu and/or splenium rather than genu, body, and splenium.11,13–15 Second, various studies included only patients with mild or severe TBI, instead of patients with a range of trauma severities.9,11,15
The purpose of the present study was to investigate DTI characteristics of the corpus callosum in patients with mild, moderate, and severe TBI. We hypothesized that there were local differences in DTI characteristics within the corpus callosum, which varied among patients with different TBI severities.
Materials and Methods
Patients and Control Subjects
The study was approved by our local ethics committee. Informed consent was obtained from each patient or from the patient's first-degree relatives. We prospectively investigated 39 patients with TBI (27 men, 12 women; mean age, 34 ± 12 years). They were selected from 46 consecutive patients who were referred to our neuroradiology department for DTI evaluation of TBI between June 2006 and June 2007 and who had no known history or MR imaging evidence of additional central nervous system disease. Patients with movement artifacts on MR images (n = 7) were excluded. Of the 39 included patients, 24 had mild; 9, moderate; and 6, severe TBI, defined as traumatic head injury with an initial Glasgow Coma Scale score ≥13 (mild), 9–12 (moderate), or ≤8 (severe). We investigated 10 age-matched control subjects (7 men, 3 women; mean age, 37 ± 9 years) for reference values. They were volunteers from our department who gave informed consent to participate in the study and who had no known history or MR imaging evidence of central nervous system disease.
MR Imaging Protocol
Investigations were performed on a 1.5T system (Sonata scanner, Siemens, Erlangen, Germany). Straight head positioning without tilt was aimed at in each patient and control subject. The MR imaging protocol consisted of an axial 3D T1-weighted scan (TR/TE, 11/4 ms), a FLAIR scan (TR/TE/TI, 9480/112/2390 ms), an axial T2*-weighted GE scan (TR/TE, 1330/33 ms), and an axial echo-planar imaging (EPI) DTI scan (TR/TE, 5700/110 ms; FOV, 24 × 24 cm; image matrix, 128 × 128; number of sections, 30; section thickness, 4 mm; nominal voxel size, 1.875 × 1.875 × 4 mm; number of signal intensity averages, 3) with diffusion gradients set in 25 noncollinear directions by using 2 b-values (b = 0 and 1000 s/mm2). The DTI scanning took 7 minutes 30 seconds
DTI Data Processing
DTI data were processed on a voxel-by voxel basis with dedicated software (DPTools [http://www.fmritools.org]). A correction algorithm was applied to the DTI dataset to account for distortions that were related to eddy currents induced by the large diffusion-sensitizing gradients. It relied on a 3-parameter distortion model including scale, shear, and linear translation in the phase-encoding direction.18 The 25 elements for each voxel, calculated from the images that were obtained by applying diffusion-sensitizing gradients in the 25 noncollinear directions, in addition to a non-diffusion-weighted image, were diagonalized to compute the eigenvalues (λ1, λ2, λ3) of the diffusion tensor matrix. The ADC was given by ADC = (λ1 + λ2 + λ3) / 3, and FA by
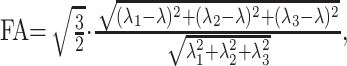 |
where
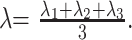 |
FA values were visualized in 2D color maps.
Fiber Tracking
Fiber tracking was performed with dedicated software (MedINRIA [http://www-sop.inria.fr/asclepios/software/MedINRIA]). White matter fiber tracts were created in 3D on the basis of similarities between neighboring voxels in shape (quantitative diffusion anisotropy measures) and orientation (principal eigenvector map) of the diffusion ellipsoid and coregistered on the FA map by using a special algorithm previously described.19,20 The principal diffusion direction method20–22 was used, in which the eigenvector corresponding to the largest eigenvalue is extracted from the diffusion tensor field generated from the DTI datasets in the region where the diffusion is linear. The FA threshold value was 0.20; and the angulation threshold, 45° to prevent fibers from sudden transition and to keep tracking based on the connectivity of the neighborhood, as described elsewhere.20,21 The 3D fiber reconstructions were color-coded so that blue represented the superior-inferior; green, the anteroposterior; and red, the left-right direction.
Measurements
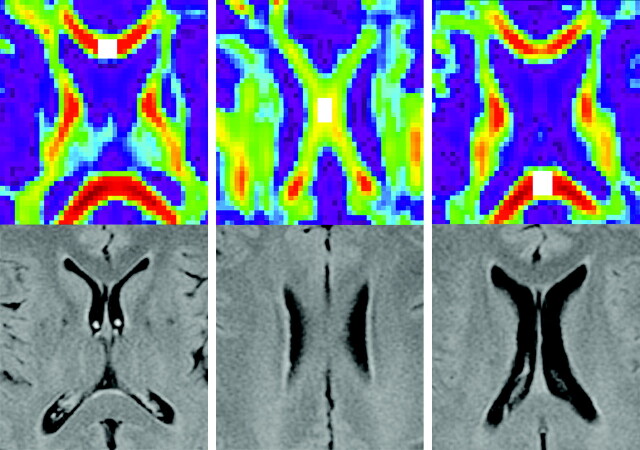
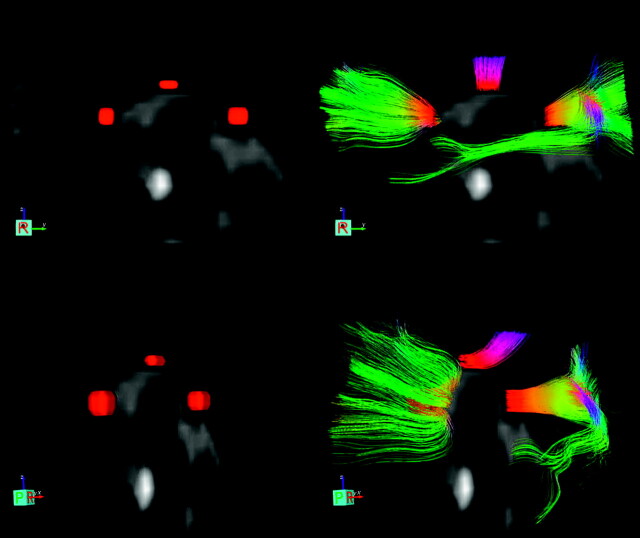
Measurements were performed by 1 investigator (D.R.R.) with the software packages described in the previous paragraphs. After realignment and spatial normalization of the DTI scans, 3 midline regions of interest were manually drawn in the corpus callosum on the FA map of each individual: a region of interest of 4 × 4 × 2 (x × y × z) voxels (7.5 × 7.5 × 8 mm = 450 mm3) in the genu, a region of interest of 3 × 5 × 1 voxels (5.625 × 9.375 × 4 mm = 211 mm3) in the body, and a region of interest of 4 × 5 × 2 voxels (7.5 × 9.375 × 8 mm = 562.5 mm3) in the splenium (Fig 1). Visual comparison was made with the corresponding low b-value diffusion, T1, and FLAIR sections to confirm region-of-interest localization in the corpus callosum without including CSF. For each region of interest, the presence of FLAIR hyperintensities was scored, as well as the presence of hypointensities on the low b-value EPI diffusion and the T2*-weighted scan. Regions of interest that contained such hypointensities, which were considered indicative of hemorrhage, were excluded. For all other regions of interest, FA and ADC were calculated. Fiber tracking software allowed reconstruction of fibers that passed through a given region of interest (Fig 2). The number of fibers passing through was calculated for each region of interest. All patient measurements were performed blinded to the clinical status of the patient.
Fig 1.
Localization of regions of interest. The upper row of images shows axial color-coded maps of FA with region-of-interest localization in the genu (left image), body (middle image), and splenium (right image) of the corpus callosum. Regions of interest are shown in white. The lower row of images shows the corresponding axial FLAIR scans.
Fig 2.
Regions of interest with reconstructed fibers in a patient with mild TBI. Regions of interest and fibers are superimposed on T2-weighted images. The upper 2 images show a lateral view with typical region-of-interest localization in the genu, body, and splenium without (left upper image) and with (right upper image) reconstructed fibers passing through. The lower images show the same regions of interest and reconstructed fibers in the anterior oblique view.
Statistical Analysis
Analysis of variance (ANOVA) with Scheffé post hoc analysis, the χ2 test, and the nonparametric Kruskal-Wallis test were used to analyze differences in baseline characteristics between patient groups. Differences in DTI and fiber tracking parameters between groups were analyzed through ANOVA with the Scheffé post hoc analysis. A P value < .05 was considered to indicate a statistically significant difference.
Results
Patient groups showed no statistically significant differences in age and sex (Table 1). The time period between the trauma incident and the MR image was longer in patients with mild TBI than in patients with moderate or severe TBI, who were all investigated <3 months posttrauma. However, the difference among groups was not statistically significant (P = .2, Kruskal-Wallis test). The frequency of FLAIR hyperintensities in the genu, body, and splenium regions of interest and the frequency of EPI/GE hypointensities in the body and splenium regions of interest were higher in patients with severe TBI than in the other 2 patient groups. However, only the difference in FLAIR hyperintensities in the splenium showed statistical significance (P < .05). In 1 patient with severe TBI, the region of interest in the callosal body contained EPI/GE hypointensities indicative of hemorrhage. The splenium region of interest contained such hypointensities in 1 patient with mild TBI and in 1 patient with severe TBI. These regions of interest were excluded from further analyses.
Table 1:
Patient baseline characteristics and MR Imaging abnormalities in regions of interest in patients with TBI
| Mild TBI (n = 24) | Moderate TBI (n = 9 | Severe TBI (n = 6) | |
|---|---|---|---|
| Patient baseline characteristics | |||
| Age (yr, mean ± SD) | 33 ± 10 | 34 ± 15 | 36 ± 22 |
| M:W (No.) | 16:8 | 6:3 | 5:1 |
| Months between trauma and MRI scan (median, Q1–Q3) | 2.8 (0.4–26.2) | 0.5 (0.3–1.2) | 1.4 (0.4–2.4) |
| MRI abnormalities in ROIs | |||
| FLAIR hyperintensities (No., %) | |||
| Genu | 1 (4) | 0 | 1 (17) |
| Body | 0 | 0 | 1 (17) |
| Splenium | 1 (4) | 1 (11) | 2 (34)* |
| EPI diffusion/GE hypointensities (No., %) | |||
| Genu | 0 | 0 | 0 |
| Body | 0 | 0 | 1 (17) |
| Splenium | 1 (4) | 0 | 1 (17) |
Note:—M indicates man; W, woman; Q1, first quartile; Q3, third quartile; MRI, MR imaging; ROIs, regions of interest; TBI, traumatic brain injury; FLAIR, fluid-attenuated inversion recovery; EPI, echo-planar imaging.
P < .05.
Compared with control subjects, patients with mild TBI showed no significant difference in FA, ADC, and number of fibers for the genu, body, and splenium (Table 2).
Table 2:
DTI and fiber tracking characteristics of the corpus callosum in control subjects and patients with TBI*
| Control Subjects (n = 10) | Mild TBI (n = 24) | Moderate TBI (n = 9) | Severe TBI (n = 6) | |
|---|---|---|---|---|
| Genu | ||||
| FA | 0.74 (0.70–0.78) | 0.66 (0.63–0.69) | 0.56 (0.47–0.65)†‡ | 0.53 (0.43–0.64)†‡ |
| ADC (mm2/s) | 2.43 (2.27–2.59) | 2.63 (2.52–2.74) | 3.10 (2.76–3.45)§‡ | 3.24 (2.67–3.81)§ |
| Fibers (No.) | 495 (442–548) | 491 (451–532) | 368 (273–462)‡ | 372 (236–508) |
| Body | ||||
| FA | 0.54 (0.49–0.59) | 0.51 (0.47–0.55) | 0.46 (0.42–0.51) | 0.48 (0.41–0.55) |
| ADC (mm2/s) | 2.71 (2.25–3.18) | 2.71 (2.51–2.92) | 2.92 (2.51–3.32) | 3.00 (2.38–3.62) |
| Fibers (No.) | 254 (221–288) | 221 (199–243) | 171 (133–210)‡ | 172 (70–275) |
| Splenium | ||||
| FA | 0.82 (0.79–0.85) | 0.79 (0.77–0.81) | 0.70 (0.65–0.75)† | 0.63 (0.55–0.71)†‖ |
| ADC (mm2/s) | 2.24 (2.11–2.37) | 2.39 (2.29–2.48) | 2.41 (2.11–2.71) | 2.58 (2.35–2.81) |
| Fibers (No.) | 937 (815–1058) | 958 (882–1035) | 766 (660–873)‡ | 508 (273–744)§‖ |
Note:—DTI indicates diffusion tensor imaging; TBI, traumatic brain injury; FA, fractional anisotropy; ADC, apparent diffusion coefficient.
Values are given as mean (95% confidence intervals).
P < .001, versus control subjects.
P < .05.
P < .01.
P < .001, versus mild TBI.
Patients with moderate TBI (Table 2) had significantly lower FA and significantly higher ADC in the genu compared with control subjects (FA, P < .001; ADC, P < .01) and with patients with mild TBI (P < .05). The number of genu fibers in patients with moderate TBI was significantly lower than that in patients with mild TBI (P < .05). In the callosal body of patients with moderate TBI, FA and ADC were not significantly different from those in the other groups, whereas the number of fibers passing through was significantly lower than that in control subjects (P < .05). In the splenium of patients with moderate TBI, FA was significantly lower than that in control subjects (P < .001) and in patients with mild TBI (P < .01), whereas ADC did not differ significantly from that in other groups. The number of splenium fibers in patients with moderate TBI was significantly lower than that in patients with mild TBI (P < .05).
Patients with severe TBI (Table 2) had significantly lower FA and significantly higher ADC in the genu compared with control subjects (FA, P < .001; ADC, P < .01) and with patients with mild TBI (FA, P < .05; ADC, P < .01). The number of genu fibers in patients with severe TBI did not differ significantly from that in other groups. DTI and fiber tracking characteristics of the callosal body in patients with severe TBI were not significantly different from those in other groups. In the splenium of patients with severe TBI, FA was significantly lower than that in control subjects (P < .001) and in patients with mild TBI (P < .001), whereas ADC did not differ significantly from that in other groups. The number of splenium fibers in patients with severe TBI was significantly lower than that in control subjects (P < .01) and in patients with mild TBI (P < .001). There were no significant differences in DTI and fiber tracking characteristics between patients with moderate and severe TBI.
Because the time period between the trauma incident and the MR image was longer in patients with mild TBI than in the other 2 patient groups, we divided the group of patients with mild TBI into those investigated <3 months and ≥3 months posttrauma. In patients with mild TBI who were investigated <3 months posttrauma, the median time interval between the trauma incident and the MR imaging was 0.4 months (first quartile, 0.2 months; third quartile, 0.5 months) compared with 25 months in patients with mild TBI who were investigated ≥3 months posttrauma (first quartile, 8; third quartile, 42 months; P < .001; Mann-Whitney U test). Patients with Mild TBI who were investigated <3 months posttrauma had significantly lower FA in the genu compared with control subjects (P < .01; Table 3) and with patients with mild TBI investigated ≥3 months posttrauma (P < .05), whereas ADC in the genu was significantly higher than that in control subjects (P < .05). DTI and fiber tracking characteristics of the body and splenium in patients with mild TBI investigated <3 months posttrauma showed no significant differences compared with those in control subjects and patients with mild TBI investigated ≥3 months posttrauma. In the latter patient group, DTI and fiber tracking characteristics of the genu, body, and splenium did not differ significantly from those in the control subjects.
Table 3:
DTI and fiber tracking characteristics of the corpus callosum in control subjects and patients mild TBI who were investigated <3 or ≥3 months posttrauma*
| Control Subjects (n = 10) | Mild TBI <3 Months Posttrauma (n = 12) | Mild TBI ≥3 Months Posttrauma (n = 12) | |
|---|---|---|---|
| Genu | |||
| FA | 0.74 (0.70–0.78) | 0.63 (0.58–0.67)†‡ | 0.70 (0.66–0.73) |
| ADC (mm2/s) | 2.43 (2.27–2.59) | 2.73 (2.55–2.92)§ | 2.53 (2.40–2.66) |
| Fibers (No.) | 495 (442–548) | 522 (458–586) | 460 (407–514) |
| Body | |||
| FA | 0.54 (0.49–0.59) | 0.50 (0.43–0.57) | 0.51 (0.47–0.56) |
| ADC (mm2/s) | 2.71 (2.25–3.18) | 2.77 (2.46–3.08) | 2.65 (2.34–2.96) |
| Fibers (No.) | 254 (221–288) | 218 (179–257) | 225 (197–253) |
| Splenium | |||
| FA | 0.82 (0.79–0.85) | 0.78 (0.74–0.82) | 0.80 (0.78–0.82) |
| ADC (mm2/s) | 2.24 (2.11–2.37) | 2.43 (2.25–2.61) | 2.34 (2.24–2.45) |
| Fibers (No.) | 937 (815–1058) | 877 (761–994) | 1032 (937–1128) |
Note:—DTI indicates diffusion tensor imging; TBI, traumatic brain injury; FA, fractional anisotropy; ADC, apparent diffusion coefficient.
Values are given as mean (95% confidence interval).
P < .01, versus control subjects.
P < .05, versus late mild TBI.
P < .05.
Discussion
The most important findings of this study are threefold. First, patients with mild TBI investigated <3 months posttrauma showed reduced FA and increased ADC in the genu of the corpus callosum. Second, patients with mild TBI investigated ≥3 months posttrauma showed no DTI abnormalities in the corpus callosum. Third, patients with moderate and severe TBI had DTI abnormalities in the genu similar to those in patients with mild TBI investigated <3 months posttrauma and showed reduced FA in the splenium without significant ADC change.
We found that patients with mild TBI investigated <3 months posttrauma primarily showed DTI abnormalities in the genu, without significant DTI changes in the body and splenium. Possibly, DTI abnormalities in the genu are reversible in mild TBI, because we found no abnormalities in patients investigated ≥3 months posttrauma. In more severe TBI, DTI shows that the splenium is affected in addition to the genu. In all patient groups, we found that FA reduction in the genu was accompanied by significant ADC increase, whereas FA reduction in the splenium occurred in the absence of significant ADC change. This indicates that the type of microstructural change was different in both locations. The ADC increase in the genu may imply a larger contribution of vasogenic edema23 in this location than in the splenium. Conventional imaging and pathologic-anatomic study have indicated that the posterior corpus callosum is more susceptible to fiber disruption than the anterior corpus callosum.2–4,24 In consequence, it may be hypothesized that the DTI pattern we observed in the splenium is associated with more irreversible traumatic lesions than the DTI pattern in the genu, which in turn may reflect more reversible abnormalities. This should be investigated in a longitudinal study.
The body of the corpus callosum showed no statistically significant FA and ADC change in any of our patient groups. Still, this part of the corpus callosum can show axonal injury in particular in severe head trauma.4 The relative absence of body abnormalities in our patients may indicate that head trauma was not severe enough to cause lesions in the body of the corpus callosum. We cannot fully exclude DTI abnormalities in the callosal body outside the regions of interest. However, we believe that our region-of-interest size and position included a considerable and representative part of the central body. A larger region of interest would have caused partial volume effects with inclusion of CSF and gray matter.
Patients with moderate and severe TBI had relatively few fibers passing through the regions of interest in the corpus callosum. The number of body fibers in moderate TBI and the number of splenium fibers in severe TBI showed a statistically significant reduction compared with that in controls. This apparent fiber loss is probably not explained by secondary axonal loss or degeneration. These phenomena are only supposed to manifest 3 months postinjury,25,26 which is considerably longer than the time interval after which our patients with moderate and severe TBI were investigated. Alternative explanations for the low number of fibers may be fiber disruption or edema within the region of interest or variability in the region of interest placement in the callosal body accounting for some of the fiber data variations.
The exact cause of posttraumatic DTI changes in white matter remains to be elucidated. Generally, FA reduction is attributed to a change in parenchymal structure.9,11,13–15 This may include misalignment of fibers, edema, fiber disruption, or axonal degeneration. Previous studies of the corpus callosum in TBI report varying DTI findings. In a study of 2 groups of patients with mild TBI who were investigated on average 4 days and 68 months after injury, the genu was normal, whereas the splenium showed reduced FA with increased ADC.11 In another study of mild TBI, FA was reduced in both the genu and splenium within 24 hours after injury.15 The body of the corpus callosum was not investigated in these studies of mild TBI. In severe TBI investigated on average 14 months after injury, FA reductions were found in the genu, body, and splenium.9 ADC values were not reported in that study. In 2 studies that investigated various trauma severities within 7 days after injury, reduced FA was found in the genu and splenium.13,14 Splenium ADC was reduced in 1 of these studies,13 suggesting the presence of cytotoxic edema. The callosal body was not investigated in these articles.
It is difficult to explain discrepancies of these earlier studies compared with our study because there are essential differences in methods. First, the time interval between injury and imaging varied considerably. This variance implies that the contribution of acute, subacute, and chronic posttraumatic changes inevitably differs between studies. Second, trauma severity differed among studies. Third, the DTI method in previous studies often included 6 diffusion directions,9,11,13 as opposed to the 25 directions we used. This can result in different estimations of anisotropy and diffusivity.27
A clinically relevant aspect of our study is that we found the genu to be primarily affected early on in mild TBI. This is somewhat different from the general view that primarily the posterior corpus callosum is affected in TBI.3,6,7,28 Furthermore, our results show that there are different types of microstructural injuries within the corpus callosum, as indicated by FA and ADC findings in the genu and splenium. A limitation of our study is the absence of neuropsychological correlations and long-term follow-up of our DTI findings. In addition to neuropsychological investigations and follow-up studies, histologic correlation would allow further interpretation of our DTI results. However, for obvious reasons, this may be difficult to obtain, in particular in patients with mild TBI. Furthermore, the number of patients with moderate and severe TBI was relatively small. This may limit the comparison with patients with the mild TBI in our study, who formed a larger group. Finally, our study group did not comprise patients with moderate and severe TBI who were investigated ≥3 months posttrauma. Long-term DTI changes in the corpus callosum in these patient groups remain to be investigated.
Conclusion
Our study shows that there are local differences in DTI characteristics within the corpus callosum, which are related to the clinical severity of head trauma. Mild TBI is associated with DTI abnormalities in the genu <3 months posttrauma. In more severe TBI, both the genu and splenium are affected. DTI shows different types of microstructural injuries within the corpus callosum, suggesting a larger contribution of vasogenic edema in the genu than in the splenium.
Footnotes
This work was supported by the Institut pour la Recherche sur la Moelle épinière et l'Encéphale, Paris, France. D.R. Rutgers was supported by a grant from the Catharijne Stichting Utrecht and Sara Lee International.
References
- 1.McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am 1992;15:395–413 [PubMed] [Google Scholar]
- 2.Adams JH, Graham DI, Murray LS, et al. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 1982;12:557–63 [DOI] [PubMed] [Google Scholar]
- 3.Parizel PM, Özsarlak Ö, Van Goethem JW, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 1998;8:960–65 [DOI] [PubMed] [Google Scholar]
- 4.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 1988;150:663–72 [DOI] [PubMed] [Google Scholar]
- 5.Abe O, Masutani Y, Aoki S, et al. Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr 2004;28:533–39 [DOI] [PubMed] [Google Scholar]
- 6.Gentry LR. Imaging of closed head injury. Radiology 1994;191:1–17 [DOI] [PubMed] [Google Scholar]
- 7.Parizel PM, Van Goethem JW, Özsarlak Ö, et al. New developments in the neuroradiological diagnosis of craniocerebral trauma. Eur Radiol 2005;15:569–81 [DOI] [PubMed] [Google Scholar]
- 8.Schaefer PW, Huisman TA, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial Glasgow coma scale score and score on modified Rankin scale at discharge. Radiology 2004;233:58–66. Epub 2004 Aug 10 [DOI] [PubMed] [Google Scholar]
- 9.Nakayama N, Okumura A, Shinoda J, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 2006;77:850–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmond CH, Menon DK, Chatfield DA, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage 2006;29:117–24 [DOI] [PubMed] [Google Scholar]
- 11.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg 2005;103:298–303 [DOI] [PubMed] [Google Scholar]
- 12.Ducreux D, Huynh I, Fillard P, et al. Brain MR diffusion tensor imaging and fibre tracking to differentiate between two diffuse axonal injuries. Neuroradiology 2005;47:604–08. Epub 2005 Jun 23 [DOI] [PubMed] [Google Scholar]
- 13.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 2004;25:370–76 [PMC free article] [PubMed] [Google Scholar]
- 14.Ptak T, Sheridan RL, Rhea JT, et al. Cerebral fractional anisotropy score in trauma patients: a new indicator of white matter injury after trauma. AJR Am J Roentgenol 2003;181:1401–07 [DOI] [PubMed] [Google Scholar]
- 15.Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002;23:794–802 [PMC free article] [PubMed] [Google Scholar]
- 16.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995;8:333–44 [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002;15:435–55 [DOI] [PubMed] [Google Scholar]
- 18.Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med 1996;36:960–64 [DOI] [PubMed] [Google Scholar]
- 19.Fillard P, Gerig G. Analysis tool for diffusion tensor MRI. In: Ellis RE, Peters TM, eds. Medical Image Computing and Computer-Assisted Intervention-MICCAI 2003. Berlin: Springer;2003;2879:967–68 [Google Scholar]
- 20.Westin CF, Maier SE, Mamata H, et al. Processing and visualization for diffusion tensor MRI. Med Image Anal 2002;6:93–108 [DOI] [PubMed] [Google Scholar]
- 21.Xu D, Mori S, Solaiyappan M, et al. A framework for callosal fiber distribution analysis. Neuroimage 2002;17:1131–43 [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–69 [DOI] [PubMed] [Google Scholar]
- 23.Hergan K, Schaefer PW, Sorensen AG, et al. Diffusion-weighted MRI in diffuse axonal injury of the brain. Eur Radiol 2002;12:2536–41 [DOI] [PubMed] [Google Scholar]
- 24.Leclercq PD, McKenzie JE, Graham DI, et al. Axonal injury is accentuated in the caudal corpus callosum of head-injured patients. J Neurotrauma 2001;18:1–9 [DOI] [PubMed] [Google Scholar]
- 25.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma 1997;14:419–40 [DOI] [PubMed] [Google Scholar]
- 26.Tomaiuolo F, Carlesimo GA, Di PM, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry 2004;75:1314–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med 2004;51:807–15 [DOI] [PubMed] [Google Scholar]
- 28.Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol 1988;9:1129–38 [PMC free article] [PubMed] [Google Scholar]