Abstract
BACKGROUND AND PURPOSE: Tinnitus is a common disorder, and the etiology remains mostly unclear. The purpose of this study was to investigate the causative effect of the vascular loop and compression of the vestibulocochlear nerve at the cerebellopontine angle in patients with unexplained tinnitus.
MATERIALS AND METHODS: This study was approved by our institutional review board. Written informed consent was obtained from all participants. Fifty-eight patients with unexplained tinnitus and 44 age- and sex-matched asymptomatic controls were examined with temporal MR imaging. Besides the tinnitus and control groups, a third group was formed by asymptomatic sides of patients with unilateral tinnitus. A 3D fast imaging employing steady-state acquisition (3D-FIESTA) sequence was performed in addition to the regular pre- and postcontrast axial and coronal sequences. The anatomic type of vascular loop, the vascular contact, and the angulation of the vestibulocochlear nerve at the cerebellopontine angle (CPA) were evaluated by 2 experienced neuroradiologists. The χ2 test was used for statistical analysis.
RESULTS: No statistically significant differences were found between the patient and control groups for the anatomic type of vascular loop, the vascular contact, and the angulation of the vestibulocochlear nerve at the CPA (P > .05).
CONCLUSION: Although 3D-FIESTA MR imaging correctly shows the anatomic relationships of the vestibulocochlear nerve, its vascular compression cannot be attributed as an etiological factor for tinnitus.
Tinnitus is a sound in the ear, occurring without an external stimulus. It is a common complaint with a lifetime incidence of 7%–12% in the general population.1-3 Tinnitus may be classified as either an arterial pulse synchronous type (pulsatile) or a continuous (nonpulsatile) type. Some investigators also have subdivided the tinnitus into subjective (perceived only by the patient) and objective (perceptible to another person) types. Nonpulsatile tinnitus and subjective tinnitus are more common than pulsatile tinnitus, and nonpulsatile tinnitus is usually subjective. Medical history, neuro-otologic physical examination, and neuroradiologic imaging may identify a treatable cause. Pulsatile tinnitus is most frequently the result of a vascular abnormality or a vascular tumor. However, in some cases, the etiology of tinnitus remains uncertain, and most of these patients have no imaging abnormalities.4,5
Compression of the eighth cranial nerve (eighth CN) by vascular structures has been proposed as a cause of tinnitus.6-10 However, controversial results have been reported in the medical literature about the relationship between neurovascular compression of the eighth CN and neuro-otologic symptoms.11-14
The purpose of our study was to assess the correlation between the presence of a vascular loop and its contact with the eighth CN in the cerebellopontine angle (CPA) and ipsilateral symptoms of unexplained tinnitus by using a 3D fast imaging employing steady-state acquisition (3D-FIESTA) sequence, which is an MR imaging technique that provides good contrast between CSF, nerves, and vessels.15
Materials and Methods
Patient Group
This prospective study was approved by the institutional review board. Written informed consent was obtained from all participants. During a 6-month period, 55 patients (23 men, 32 women; age range, 18–78 years; mean age, 48.5 years) with unexplained tinnitus and 43 controls (9 men, 34 women; age range, 19–88 years; mean age, 42.2 years) without tinnitus, deafness, facial palsy, or vertigo were enrolled in the study. Patients who had undergone a cranial MR imaging for headache and who were reported to be healthy were included in the control group. Patients with tinnitus underwent a neuro-otologic evaluation to exclude an underlying pathologic process. A total of 196 ears in 98 patients were evaluated.
Thirteen of the 55 patients had bilateral tinnitus, and the remaining 42 patients had unilateral tinnitus. Therefore, the number of symptomatic sides of patients was 68. Both sides of control patients were evaluated separately, constituting a group of 86 sides. Besides the tinnitus and control groups, a third group was formed by asymptomatic sides of patients with unilateral tinnitus (Table 1). The patients with neuritis or tumors at the CPA were not included in the study. No patients had previous CPA surgery or temporal bone trauma.
Table 1:
Summary of the study population
| Characteristics | Patients (n = 55) | Controls (n = 43) |
|---|---|---|
| Bilateral tinnitus | 13 | |
| Unilateral tinnitus | 42 | |
| Symptomatic sides | 68 | 0 |
| Asymptomatic sides | 42 | 86 |
Imaging and Evaluation
All MR imaging examinations were performed by using a 1.5T system (Signa Excite; GE Healthcare, Milwaukee, Wis) with an 8-channel neurovascular head coil. The imaging protocol consisted of axial T2-weighted images of the whole brain (TR/TE, 4500–5500/100 ms; NEX, 2; section thickness, 5 mm; intersection spacing, 1.5 mm; matrix size, 512 × 256) and coronal and axial T1-weighted images of the CPA before and after administration of intravenous contrast material (TR/TE, 460/11 ms; NEX, 3; section thickness, 3 mm; intersection spacing, 0.5 mm; matrix size, 288 × 256). 3D-FIESTA imaging of the CPA was performed with the parameters as follows: TR/TE, 5.9/1.6 ms; flip angle, 65°; bandwidth, 31.2; FOV, 20; matrix size, 320 × 224; section thickness, 1 mm; overlapping, 0.5 mm; 56 partitions. The control group was examined with the 3D-FIESTA sequence in addition to the routine cranial MR imaging protocol.
All images were transferred to a workstation (Advantage Windows 4.1, GE Healthcare). Optimal thresholds were used in each patient to visualize the neurovascular structures. Axial 3D-FIESTA images and sagittal reconstructions were reviewed by 2 neuroradiologists who were blinded to the clinical symptoms of the patients. The final decision was made by consensus.
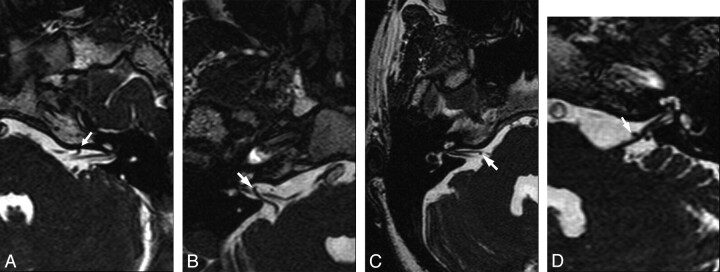
In the evaluation, the vessels were classified according to their anatomic location by using a simple method previously described by Chavda in McDermott et al11: type I, lying only in the CPA but not entering the internal auditory canal (IAC); type II, entering but not extending >50% of the length of the IAC (Fig 1A); and type III, extending >50% of the IAC (Fig 1B). In addition, we also evaluated the presence of vascular contact (Fig 1C) and the angulation of eighth CN at the contact point as a sign of vascular compression (Fig 1D).
Fig 1.
Examples of types of AICA loops and eighth CN-AICA relationships. Axial 3D-FIESTA MR images through the eighth CN show the following: AICA loop within the IAC (arrow) but not >50% of its depth (Type II) (A); vascular loop extending into >50% of the IAC (arrow) (Type III) (B); and contact of AICA (arrow) with the eighth CN, not resulting (C) and resulting (D) in angulation on the eighth CN (arrow) in the CPA.
Statistical Analysis
MR imaging findings of the 3 groups (tinnitus group, control group, and the group consisting of asymptomatic sides of patients with unilateral tinnitus) were analyzed to evaluate the correlation of ipsilateral tinnitus with the type of the vascular loop, vascular contact, and the angulation of the eighth CN. The χ2 test was applied for statistical analysis by using a commercially available software (Statistical Package for the Social Sciences, Version 11.5; SPSS, Chicago, Ill). A P value < .05 was considered to indicate a statistically significant difference.
Results
MR imaging findings (anatomic location of the vessels, the presence of vascular contact, and the angulation of eighth CN) in the 3 groups are summarized in Table 2.
Table 2:
MR imaging findings of the patient and control groups
| MR Imaging Findings | Symptomatic Sides of Patients (n = 68) (%) | Asymptomatic Sides of Patients (n = 42) (%) | Asymptomatic Sides of Controls (n = 86) (%) |
|---|---|---|---|
| Type of vascular loop in CPA | |||
| Type I | 44 (65) | 35 (83) | 62 (72) |
| Type II | 18 (26) | 3 (7) | 15 (17) |
| Type III | 6 (9) | 4 (10) | 9 (11) |
| Vascular contact with eighth CN | 36 (53) | 17 (40) | 35 (41) |
| Angulation of eighth CN | 10 (15) | 6 (14) | 11 (13) |
Note:—CPA indicates cerebellopontine angle; CN, cranial nerve.
Our findings showed that anterior inferior cerebellar artery (AICA) loops in CPA (type I) were found to be present in 65% of patients with tinnitus (44/68), in 72% of controls (62/86), and in 83% of patients (35/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus (Table 2). AICA loops lying in the IAC but not extending >50% of its depth (type II) were detected in 26% of patients with tinnitus (18/68), in 17% of controls (15/86), and in 7% of patients (3/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus (Table 2). AICA loops extending >50% of the length of IAC (type III) were found to be present in 9% of patients with tinnitus (6/68), in 11% of controls (9/86), and in 10% of patients (4/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus (Table 2). However, these results did not show a statistically significant difference for the presence of all types of vascular loops (P > .05).
Vascular contact with the eighth CN was depicted in 53% of patients with tinnitus (36/68), in 41% of controls (35/86), and in 40% of patients (17/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus. The angulation of the eighth CN was found to be present in 15% of patients with tinnitus (10/68), in 13% of controls (11/86), and in 14% of patients (6/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus. Again, there was no statistically significant difference between the groups for vascular contact and the angulation of the eighth CN (P > .05).
Discussion
Vascular compression syndromes, proposed by Janetta in 1975,16 describe a clinical entity characterized by compression of 1 of the cranial nerves by a vessel. Janetta was the first to perform microvascular decompression for intractable vertigo.17 Since then, many surgeons have performed operations for vascular compressions for a variety of clinical conditions like hemifacial spasm, trigeminal neuralgia, and geniculate neuralgia. On the other hand, controversy continues concerning the pathophysiology of vascular compression syndromes. One hypothesis is that continuous or pulsatile compression is thought to cause focal demyelination, reorganization, and axonal hyperactivity at the junction between the central glial and peripheral nonglial junction.18-20 The other hypothesis is that impaired blood flow by a neurovascular compression may result in reduced vascular perfusion.21 These hypotheses, however, do not explain all symptoms seen in vascular compression syndromes.
Although the concept of vascular compression has been widely accepted for hemifacial spasm and trigeminal neuralgia, their relationship with audiovestibular symptoms like vertigo and tinnitus is not clear. The vestibulocochlear nerve is composed of 3 parts: the cochlear nerve and the superior and inferior vestibular nerves. The 2 vestibular nerves join to become 1 nerve before their exit from the internal acoustic meatus. The vestibular and cochlear nerves fuse to form the 8th CN closer to brain stem.22 The introduction of MR imaging has permitted better visualization of the CPA and IAC and is the method of choice for evaluating the eighth CN in patients with audiovestibular symptoms.23,24 Advances in MR imaging techniques and application of special sequences have enabled increased spatial resolution and more detailed analysis of the CPA. The 3D-FIESTA sequence is a high-resolution heavily T2-weighted method and is capable of perfect delineation of vascular structures and nerves from CSF. The anatomic course and complex vascular relationships of cisternal and canalicular parts of the eighth CN have been visualized successfully by using the 3D-FIESTA sequence.6,9,11,14
The neurovascular compression of the eighth CN can produce vertigo, tinnitus, or hearing disturbances or various combinations of them, unlike vascular compression of the trigeminal or facial nerve. This complicated symptomatology makes vascular compression syndromes of the eighth CN difficult to understand.8 Another conflict about the vascular compressions of the eighth CN is primarily because of the cadaveric and radiologic studies that provided considerable discrepancies regarding the occurrence and the effects of vascular loops in the CPA.11 According to the cadaveric studies, the AICA loops are found within the ICA in 12.3% of human temporal bones.12,25 However, Fkuda et al26 claimed that the findings in the cadaver after formaldehyde fixation might be different from those in the viable state. In MR imaging studies, the incidence of AICA loops in contact with the eighth CN was nearly the same in symptomatic and asymptomatic patients (25% and 21.4%, respectively).13,23 Therefore, the presence of vascular loops within the IAC may not be a definite indication of vascular compression syndromes of the eighth CN.
Tinnitus as a part of neuro-otologic symptoms is a common disorder. Our findings showed that AICA loops in the CPA (type I) were found to be present in 65% of patients with tinnitus (44/68), in 72% of controls (62/86), and in 83% of patients (35/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus (Table 2). Similarly, AICA loops extending >50% of the length of the IAC (type III) were found to be present in 9% of patients with tinnitus (6/68), in 11% of controls (9/86), and in 10% of patients (4/42) in the group formed by asymptomatic sides of patients with unilateral tinnitus (Table 2). There was no statistically significant difference between the groups for the presence of all types of vascular loops (P > .05). We also evaluated the presence of vascular contact and the angulation of the eighth CN (Table 2), for which we did not find any statistically significant differences between the groups. Therefore, radiologic demonstration of a vascular compression may be a normal anatomic variation and should not be used as a diagnostic option in a decision regarding decompressive surgery.12,13
Our results are comparable with some previous reports, though their study groups included not only tinnitus but also other audiovestibular symptoms. McDermott et al11 reported that tinnitus was not associated with the presence of vascular loops, though they showed a clear relationship between AICA loops and unilateral hearing loss. Sirikci et al12 stated that there was no relationship between nonspecific cochleovestibular symptoms and the type of vascular compression.
However, some studies do not support our results.6-9,16 In their 2 articles involving a retrospective analysis of patients who had undergone surgical decompression, Ryu et al7,8 proposed the concept of neurovascular compression syndrome of the eighth CN and recommended early decompression before irreversible impairment of the nerve function occurred. Similarly, Nowé et al6 stated that nonpulsatile tinnitus may result from a microvascular compression at the cisternal segment of the eighth CN and showed a correlation between the clinical presentation of nonpulsatile tinnitus (high and low pitch) and perceptive hearing loss. Nowé et al6 and De Ridder et al9 found a strong correlation between the presence of vascular loops in the IAC seen on MR imaging and pulsatile tinnitus. They theorized that pulsatile tinnitus is caused by direct transmission of pulsations to the cochlea via a resonance effect in the petrous bone. Nowé et al stated that the resolution of symptoms after microvascular decompression surgery should be accepted as a confirmation of their theory. The wide discrepancies among the studies may be explained by interobserver differences that can alter the results of estimation or evaluation of the various types of audiovestibular diseases in different studies.
Our study showed that symptomatic patients had as much neurovascular contact or even angulation of the eighth CN in CPA as the asymptomatic patients had. Similarly, no statistically significant association was found between tinnitus and the anatomic configuration of the vascular loop in the CPA. We assume that the grouping of tinnitus would not change our findings.
Conclusion
Findings of this study showed that the presence of a vascular loop either in contact with the eighth CN and causing angulation of the cisternal component of the nerve or its penetration into the IAC does not correlate with reports of unexplained tinnitus by the patient. Therefore, we think that the diagnosis of the eighth CN vascular compression syndrome should not be based solely on imaging findings, and this recommendation may prevent considering unnecessary surgery.
Footnotes
Paper previously presented as a poster at: Annual Meeting of the American Society of Neuroradiology, May 6–12, 2006; San Diego, Calif.
References
- 1.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med 2002;347:904–10 [DOI] [PubMed] [Google Scholar]
- 2.Nondahl DM, Cruickshanks KJ, Wiley TL, et al. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol 2002;13:323–31 [PubMed] [Google Scholar]
- 3.Marsot-Dupuch K. Pulsatile and nonpulsatile tinnitus: a systemic approach. Semin Ultrasound CT MR 2002;22:250–70 [DOI] [PubMed] [Google Scholar]
- 4.Weissman JL, Hirsch BE. Imaging of tinnitus: a review. Radiology 2000;216:342–34 [DOI] [PubMed] [Google Scholar]
- 5.Branstetter BF, Weissman JL. The radiologic evaluation of tinnitus. Eur Radiol 2006;16:2792–802 [DOI] [PubMed] [Google Scholar]
- 6.Nowé V, De Ridder D, Van de Heyning PH, et al. Does the location of a vascular loop in the cerebellopontine angle explain pulsatile and non-pulsatile tinnitus? Eur Radiol 2004;14:2282–89. Epub 2004 Oct 21 [DOI] [PubMed] [Google Scholar]
- 7.Ryu H, Yamamoto S, Sugiyama K, et al. Neurovascular compression syndrome of the eight cranial nerve: what are the most reliable diagnostic signs? Acta Neurochir (Wien) 1998;140:1279–86 [DOI] [PubMed] [Google Scholar]
- 8.Ryu H, Yamamoto S, Sugiyama K, et al. Can the site of compression explain the symptoms? Acta Neurochir (Wien) 1999;141:495–301 [DOI] [PubMed] [Google Scholar]
- 9.De Ridder D, De Ridder L, Nowé V, et al. Pulsatile tinnitus and the intrameatal vascular loop: why do we not hear our carotids? Neurosurgery 2005;57:1213–17 [DOI] [PubMed] [Google Scholar]
- 10.Brookes GB. Vascular decompression surgery for severe tinnitus. Am J Otol 1996;17:569–76 [PubMed] [Google Scholar]
- 11.McDermott AL, Dutt SN, Irving RM, et al. Anterior inferior cerebellar artery syndrome: fact or fiction. Clin Otolaryngol 2003;28:75–80 [DOI] [PubMed] [Google Scholar]
- 12.Sirikci A, Beyazıt Y, Ozer E, et al. Magnetic resonance imaging based classification of anatomic relationship between the cochleovestibular nerve and anterior inferior cerebellar artery in patients with nonspecific neuro-otologic symptoms. Surg Radiol Anat 2005;27:531–35. Epub 2005 Nov 19 [DOI] [PubMed] [Google Scholar]
- 13.Makins AE, Nikolopoulos TP, Ludman C, et al. Is there a correlation between vascular loops and unilateral auditory symptoms? Laryngoscope 1998;108:1739–42 [DOI] [PubMed] [Google Scholar]
- 14.Levy RA, Arts AH. Predicting neuroradiologic outcome in patients referred for audiovestibular dysfunction. AJNR Am J Neuroradiol 1996;17:1717–24 [PMC free article] [PubMed] [Google Scholar]
- 15.Casselman JW, Kuhweide R, Deimling M, et al. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol 1993;14:47–57 [PMC free article] [PubMed] [Google Scholar]
- 16.Janetta PJ. Neurovascular cross-compression in patients with hyperactive dysfunction of the eighth cranial nerve. Surg Forum 1975;26:467–69 [PubMed] [Google Scholar]
- 17.Janetta PJ, Moller MB, Mollar AR. Disabling positional vertigo. N Eng J Med 1984;310:1700–05 [DOI] [PubMed] [Google Scholar]
- 18.Girard N, Poncet M, Caces F, et al. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology 1997;39:46–51 [DOI] [PubMed] [Google Scholar]
- 19.McCabe BF, Gantz BJ. Vascular loops as a cause of incapacitating dizziness. Am J Otol 1989;10:117–20 [PubMed] [Google Scholar]
- 20.Nielsen VK. Pathophysiology of hemifacial spasm. I. Ephaptic transmission and ectopic excitation. Neurology 1984;34:418–26 [DOI] [PubMed] [Google Scholar]
- 21.Applebaum EL, Valvassori G. Internal auditory canal vascular loops. audiometric and vestibular system findings. Am J Otol 1985;Suppl:110–13 [PubMed]
- 22.De Ridder D, Ryu H, Moller AR, et al. Functional anatomy of the human cochlear nerve and its role in the microvascular decompressions for tinnitus. Neurosurgery 2004;54:381–90 [DOI] [PubMed] [Google Scholar]
- 23.Schick B, Brors D, Koch O, et al. Magnetic resonance imaging in patients with sudden hearing loss, tinnitus and vertigo. Oto Neurotol 2001;22:808–12 [DOI] [PubMed] [Google Scholar]
- 24.Park SU, Kim HJ, Cho YK, et al. The usefulness of MR imaging of the temporal bone in the evaluation of patients with facial and audiovestibular dysfunction. Korean J Radiol 2002;3:13–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reisser C, Schuknecht HF. The anterior inferior cerebellar artery in the internal auditory canal. Laryngoscope 1991;101:761–66 [DOI] [PubMed] [Google Scholar]
- 26.Fkuda H, Ishikawa, Okumura R. Demonstration of neurovascular compression in trigeminal neuralgia and hemifacial spasm with magnetic resonance imaging: comparison with surgical findings in 60 consecutive cases. Surg Neurol 2003;59:93–100 [DOI] [PubMed] [Google Scholar]