Abstract
BACKGROUND AND PURPOSE: It is known that the motor cortex shows hypointensity on T2-weighted images in older patients. The goal of this study was to assess the signal intensity of the motor cortices on the phase-weighted imaging performed with a Windows-based software program that we developed ourselves.
MATERIALS AND METHODS: All studies were performed at 3T MR imaging. First, the TE for the phase-weighted image was optimized; the best contrast between the motor and other cortices was obtained with a TE of 40 ms. The study population consisted of 45 healthy subjects (23 females, 22 males; mean age, 32.1 years). The signal intensity of the motor cortices was divided into 3 grades by 2 neuroradiologists in comparison with that of the superior frontal cortex (SFC): In grade I, the motor cortex was isointense to the SFC; in grade II, the motor cortex was slightly hypointense to the SFC; and in grade III, the motor cortex was markedly hypointense to the SFC.
RESULTS: The motor cortex was classified as either grade II or III in all subjects older than 20 years of age on the phase-weighted images. Even at 10–19 years of age, the grade II or III appearance was found in 14 (88%) of 16 motor cortices (8 subjects) on the phase-weighted images.
CONCLUSION: In adolescents, the motor cortex is hypointense to other cerebral cortices on phase-weighted MR imaging, which probably reflects differences in the concentration of nonheme iron and/or in the tissue architecture.
Haacke et al1 designed a high-spatial-resolution 3D fast low-angle shot MR imaging technique that can enhance subtle differences in subvoxel magnetic inhomogeneities.2 This technique is called susceptibility-weighted (SW) imaging. It has initially been used to enhance the visualization of the venous blood vessels and has also been reported to generate blood oxygen level–dependent venograms.2–4 Recently, SW imaging has been applied to the various diseases such as arterial venous malformations, trauma,5 tumors,6,7 and multiple sclerosis.8
Previous studies have reported that the hypointensity of the motor and visual cortices on T2-weighted images is more conspicuous in older patients, and this finding has been attributed to differences in nonheme iron concentration.9,10 The T2-weighted hypointensity of the motor cortices has also been reported in several neurodegenerative disorders, such as amyotrophic lateral sclerosis, Alzheimer disease, or multiple system atrophy. Oba et al11 have reported that the histologic analysis of patients with amyotrophic lateral sclerosis showed increased nonheme iron deposition in the regions of T2 shortening.
The phase-weighted images, based on principles similar to those of SW imaging, are obtained by multiplication of the phase and magnitude images by using a Windows-based software. Because this technique would have higher sensitivity for nonheme iron, we hypothesized that the phase-weighted image could visualize the hypointensity of the motor cortices even in younger subjects. The purpose of our study was, therefore, to assess the signal intensity of the motor cortices on the phase-weighted imaging at 3T MR imaging.
Materials and Methods
MR Imaging
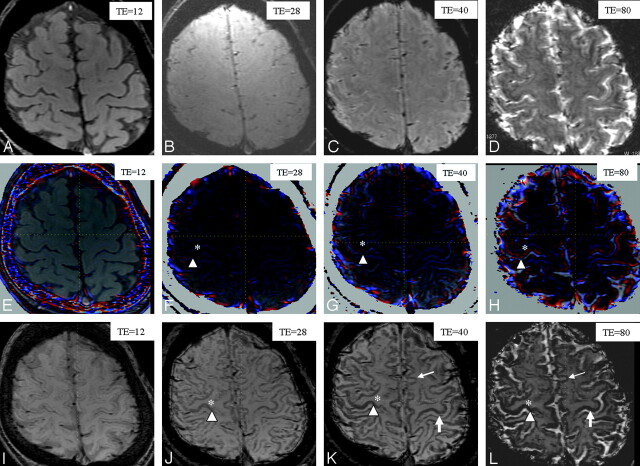
All studies were performed with a 3T MR imaging system (Signa Excite 3T; GE Healthcare, Milwaukee, Wis) by using a dedicated 8-channel phased-array coil (USA Instruments, Aurora, Ohio). Our phase-weighted imaging consisted of a 3D fast spoiled gradient-recalled sequence, which was performed with the following parameters: TR of 55 ms; TI/imaging time, 700/5 minutes; flip angle of 20°; bandwidth of 15 kHz; 22-cm FOV; 288 × 128 matrix; 2-mm-thick sections, which resulted in a voxel size of 0.8 × 0.5 × 2.0 mm3. All images were obtained in the axial plane. An application, running on Windows Office Systems (Microsoft, Bothell, Wash) with the standard specifications of an Intel-based personal computer and loaded with these images and the phase-weighted images, was created by using the magnitude and phase images, as previously explained by Reichenbach et al.2 The phase image was low-pass-filtered (by using a 32 × 128 exclusion of low-spatial-frequency information) to remove much of the low-spatial-frequency background static-field variation of the brain. A phase mask was created by setting all positive-phase values (between 0° and 180°) to unity and normalizing the negative-phase values ranging from 0° to 180° to a gray-scale of values ranging linearly from 1 to 0, respectively. This normalized phase mask was multiplied 9 times against the original magnitude image. Finally, a minimal intensity projection (phase-weighted image) over 2 sections was performed to display the processed data by using contiguous 4-mm-thick sections in the transverse plane (Fig 1I–L).
Fig 1.
Comparison of various TEs in the 3 different techniques (magnitude, phase, and phase-weighted imaging) performed in a healthy volunteer (a 37-year-old man): magnitude images with TEs of 12 (A), 28 (B), 40 (C), and 80 ms (D); phase images with TEs of 12 (E), 28 (F), 40 (G), and 80 ms (H); and phase-weighted images with TEs of 12 (I), 28 (J), 40 (K), and 80 ms (L). The gray-to-white-matter contrast on magnitude images (A–D) is poor in all TEs. The gray-to-white-matter contrast on the phase (E–H) and phase-weighted images (I–L) increases by increasing the TE (asterisk versus arrowhead). The heightened contrast between motor (large arrow) and other cortices (small arrow) is most apparent on the phase-weighted image with a TE of 40 ms (K). The definition between motor (large arrow) and other cortices (small arrow) is poor on the phase-weighted image with a TE of 80 ms (L), due to the decrease in signal intensity of the other cortices.
On the phase image, the phase change was mapped into a color scale, where zero indicated no color and, as the phase change increased or decreased, the color changed to red and blue, respectively. This color change was overlaid on the magnitude image section as shown in Fig 1E–H.
Optimization of TE
Three healthy volunteers (a 27-year-old man, a 37-year-old man, and a 45-year-old man) underwent MR imaging to investigate the effects of varying the TE on the gray-to-white-matter contrasts with the 3 different techniques (magnitude, phase, and phase-weighted imaging). They gave their written informed consent before the study. For each subject, the phase-weighted image sequence was repeated with different TEs of 12, 28, 40, and 80 ms; all other imaging parameters were kept constant.
Subjects
Between April 2005 and August 2005, consecutive patients who were referred for brain MR imaging were prospectively evaluated. The main indications for phase-weighted imaging at our institution were the following: 1) detection of minor hemorrhage in relation to injury and chronic hypertension, 2) screening of vascular malformations, and 3) evaluation of brain tumors. The following criteria were used for inclusion in the study: 1) patients who underwent a brain MR examination with phase-weighted imaging on a 3T MR imaging system, 2) normal findings on a neurologic examination, 3) no history of neurologic disease or brain surgery, and 4) normal results of a brain MR imaging, which were determined in consensus by 2 neuroradiologists. Therefore, the study population consisted of 45 patients (23 females, 22 males; mean age, 32.1 years; age range, 9–59 years; age distribution, 7–9 years [n = 2], 10–19 years [n = 8], 20–29 years [n = 11], 30–39 years [n = 10], 40–49 years [n = 7], and 50–59 years [n = 7]) who fulfilled such criteria. The clinical presentations in this study included headache (n = 12), trauma (n = 10), dizziness (n = 5), screening for brain metastasis (n = 6), seizure (n = 4), stiff neck (n = 5), and healthy volunteers in a volunteer study (n = 3). The study was approved by the institutional review board, who did not require informed consent.
Image Interpretation
First, for 3 different techniques (magnitude, phase, and phase-weighted imaging) performed in 3 healthy volunteers, 2 neuroradiologists analyzed the gray-to-white-matter contrast together in conference according to the following scores: 3, clear; 2, reasonably clear; 1, poor. These neuroradiologists also evaluated each image for the contrast between the signal intensity of the motor cortex and the other cortices. The motor cortex was identified on the basis of the previously defined methods.12 Then, the signal intensity of the motor cortex (90 cortices in 45 patients) obtained with the 3 techniques was interpreted by a conference of these 2 neuroradiologists in a fashion similar to that carried out in the previous studies.10 The superior frontal cortex (SFC) was used for reference, and the signal intensity of motor cortices was divided into 3 grades compared with that of SFC: In grade I, the motor cortex was isointense to the SFC; in grade II, the motor cortex was slightly hypointense to the SFC; and in grade III, the motor cortex was markedly hypointense to the SFC. Each MR image was displayed and interpreted on a high-resolution 1560 × 2048 (Coronis 3MP; Barco Medical Imaging Systems, Kortrijk, Belgium) monitor.
Results
Optimization of TE
The radiologists scored the gray-to-white-matter contrasts of all volunteers on the magnitude image as poor (score of 1) in all TEs (Fig 1A–D). On both of the phase images (Fig 1E–H) and the phase-weighted images (Fig 1I–L), the gray-to-white-matter contrasts were classified as clear (score of 3) at a TE of 80 ms, reasonably clear (score of 2) at a TE of 28 and 40 ms, and poor (score of 1) at a TE of 12 ms. Therefore, on the phase image and the phase-weighted image, the gray-to-white-matter contrasts increased by increasing the TE. The best contrast between the motor cortex and other cortices was obtained at a TE of 40 ms on the phase-weighted images (Fig 1K). The contrast between motor and other cortices was poor on the phase-weighted images at a TE of 80 ms due to the decrease in signal intensity of the other cortices (Fig 1L). As a result, a TE of 40 ms was chosen.
Signal Intensity of the Motor Cortex
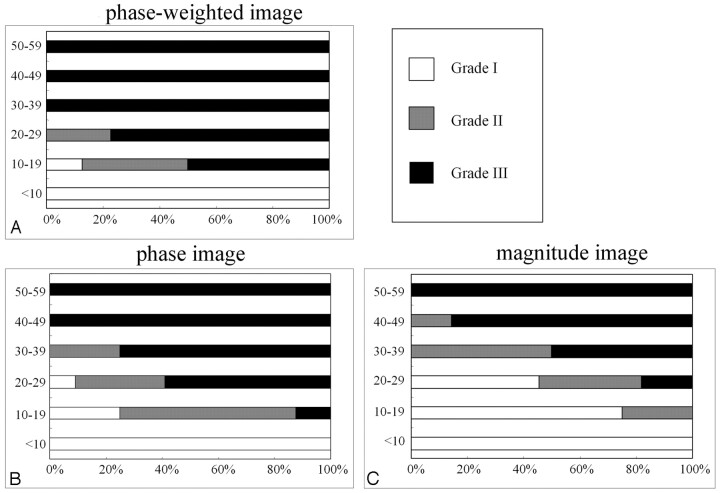
The results of the grading of 90 motor cortices (45 patients) on 3 images are summarized in Fig 2. In subjects younger than 10 years of age, no cortex was classified as grade II or III. In all subjects older than 20 years of age, the signal intensity of the motor cortex on phase-weighted images was hypointense compared with that of the SFC and was classified as grade II or III. A grade II or III appearance was found in 14 (88%) of 16 motor cortices (8 patients) in the 10- to 19-year-old group (Fig 3). In the 10- to 39-year-old group, the frequency of the grade III appearance was higher in the phase-weighted images (45 [78%] of 58 cortices) and the phase images (30 [52%] of 58) than in the magnitude images (14 [24%] of 58).
Fig 2.
Distribution of signal intensity in the motor cortex according to the patient's age on phase-weighted (A), phase (B), and magnitude images (C). In all subjects older than 20 years, the motor cortex on phase-weighted images was classified as grade II or III. The frequency of grade III on a phase-weighted image was higher than that on the phase and the magnitude images.
Fig 3.
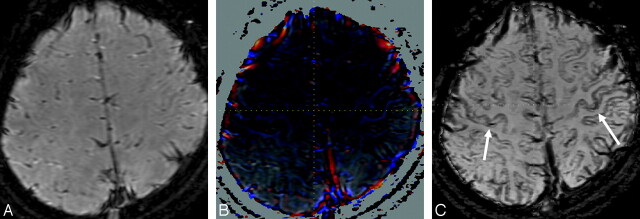
Magnitude (A), phase (B), and phase-weighted images (C) obtained in a 14-year-old girl. The signal intensity of the bilateral motor cortices (arrows) on the phase-weighted image (C) is definitely hypointense (grade III) in comparison with that in other cortices.
Discussion
At a high static field, the phase images can provide an additional source of image contrast.13,14 The sensitivity to the white and gray matter boundaries increases as a result of the increased susceptibility-induced phase shifts when using gradient-echo (GE) techniques at high field. Abduljalil et al15 have assessed phase-weighted imaging at 8T regarding the anatomic brain structures and reported that phase images can significantly improve the contrast between the gray and white matter structures over standard magnitude GE imaging techniques. The present study with the phase-weighted images at 3T also showed that the phase images enhanced the gray-to-white-matter contrast that was not apparent on the corresponding magnitude images. Therefore, the definition of the cortical gray-white-matter interface was excellent on the phase-weighted images because they are obtained by multiplication of the phase images and magnitude images.
In this study, the gray-to-white-matter contrast increased by increasing the TE on the phase image. Phase images contain direct information about the background magnetic field and chemical shift of tissues. The ability to use the phase for spectroscopic information depends on the elimination of the phase from the background field and the partial volume effects with other tissues that have a different chemical shift.16 In this theory, the long TEs typically required to obtain sufficient weighting are dependent on changes in phase that mitigate against the signal intensity loss due to partial volume effects with neighboring tissues. Moreover, the signal intensity from substances with different susceptibilities than their neighboring tissues becomes out of phase with the adjacent tissues at sufficiently long TEs.
The brain normally contains several essential metals. Iron is the most frequently encountered paramagnetic substance in the healthy brain, and it is normally present in concentrations sufficient to affect MR images.17,18 Bizzi et al18 have reported that the variations in gray matter signal intensity on T2-weighted images are due to differences in iron content. Hallgren and Sourander19 have reported that the iron staining has been observed more in the motor cortex than in the other cortices (frontal, temporal, and parietal lobes). These reports are consistent with the present findings.
The previous study regarding the quantitative age-related measurement of nonheme iron in the human brain noted that the iron staining had been observed in the motor cortex since birth and increased with age.19 Hirai et al10 evaluated the signal intensity of the motor cortices on T2-weighted images with conventional spin-echo sequences at 1.5T and reported that the hypointensity in the motor cortices in comparison with the SFC was seen in approximately 30% of subjects at 21–50 years of age. For the visual grading of the motor cortices, the grade II or III appearance was found in 35 (100%) of 35 subjects at 20–59 years of age. Moreover, the grade II or III appearance was seen in 88% at 10–19 years of age. These results may be because the phase-weighted images are extremely sensitive to susceptibility changes such as iron content compared with other sequences. Another major reason may be the concentration of perineuronal nets. Karaarslan and Arslan20 evaluated the signal intensity of the perirolandic cortex on turbo fluid-attenuated inversion recovery images in a neurologically healthy population at 1.5T and reported that decreased signal intensity of the motor cortex was observed more prominently in younger patients. The precise mechanism underlying their results remains unclear; however, the authors postulated that the concentration of perineuronal nets may affect the signal intensity of the cerebral cortex. Various factors such as the water content of brain tissue, cellular composition, and vascularization of the motor cortex may also affect the susceptibility-induced phase shifts.
Mapping of the precentral gyrus (motor cortex) provides the neurosurgeon with vital information regarding the motor functional organization of cortical tissue, particularly in the planning of a surgical approach route for the resection of mass lesions or epileptogenic tissues.21,22 Moreover, recently, fiber tractography by means of diffusion tensor imaging, which noninvasively allows the visualization of the functional connectivity of the neuronal pathways, has been applied clinically to assess brain tumors.23,24 In many previous reports, a process known as the fiber tracking technique has been performed with 2 manually segmented regions of interest on the basis of the known anatomic distributions of tracts. Two regions of interest for tracking the corticospinal tract have been generally placed in the cerebral peduncle and motor cortex,25–27; therefore, it may be important to determine the precise anatomic location of the motor cortex.
Our study has several limitations. We evaluated only subjects with normal results of a brain MR imaging. In brain tumors located in the perirolandic area, it is often difficult to identify the motor cortex on MR images because sulcal landmarks are obscured in the presence of mass effect and edema. Thus, further investigation may be needed to determine whether the phase-weighted image can demonstrate a contrast between the motor cortex and other cerebral cortices in patients with brain tumor. For visual grading of the motor cortices, we performed the comparison between the motor cortex and SFC only on the basis of the previously defined method. Recently, significant variation in T2 values among the cortical gray matter of the human brain such as the primary auditory cortex and the insula cortex has been documented.28 Our study would benefit from expanding the comparison beyond the SFC to include other cortices. In this study, the motor cortex was identified by 2 neuroradiologists in consensus. A confident identification of the motor cortex was possible in almost all subjects; however, a given diagnosis of motor cortex could be incorrect in limited subjects. Moreover, the partial volume effects can affect the visualization of cortical signal intensity in an image section adjacent to the cortical area, and those lead to an incorrect assumption of cortical signal intensity. In our healthy volunteer study, we arbitrarily selected 4 kinds of TE (12, 28, 40, and 80 ms) to investigate the effects of varying the TE on the phase-weighted image. Thus, the TE of 40 ms might not necessarily be optimal for the evaluation of the gray-to-white-matter contrasts.
Conclusion
In conclusion, the phase-weighted images with the optimized TE demonstrated a contrast between the motor cortex and other cerebral cortices even in adolescents. As a result, phase-weighted imaging might, therefore, provide additional valuable information when creating the cortical mapping in surgical planning for patients with hemispheric tumors or epilepsy, especially for children or young adults.
References
- 1.Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 2005;23:1–25 [DOI] [PubMed] [Google Scholar]
- 2.Reichenbach JR, Venkatesan R, Schillinger DJ, et al. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 1997;204:272–77 [DOI] [PubMed] [Google Scholar]
- 3.Cho ZH, Ro YM, Lim TH. NMR venography using the susceptibility effect produced by deoxyhemoglobin. Magn Reson Med 1992;28:25–38 [DOI] [PubMed] [Google Scholar]
- 4.Lee BC, Vo KD, Kido DK, et al. MR high-resolution blood oxygenation level-dependent venography of occult (low-flow) vascular lesions. AJNR Am J Neuroradiol 1999;20:1239–42 [PMC free article] [PubMed] [Google Scholar]
- 5.Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 2003;227:332–39 [DOI] [PubMed] [Google Scholar]
- 6.Sehgal V, Delproposto Z, Haddar D, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 2006;24:41–51 [DOI] [PubMed] [Google Scholar]
- 7.Barth M, Nobauer-Huhmann IM, Reichenbach JR, et al. High-resolution three-dimensional contrast-enhanced blood oxygenation level-dependent magnetic resonance venography of brain tumors at 3 Tesla: first clinical experience and comparison with 1.5 Tesla. Invest Radiol 2003;38:409–14 [DOI] [PubMed] [Google Scholar]
- 8.Tan IL, van Schijndel RA, Pouwels PJ, et al. MR venography of multiple sclerosis. AJNR Am J Neuroradiol 2000;21:1039–42 [PMC free article] [PubMed] [Google Scholar]
- 9.Korogi Y, Hirai T, Komohara Y, et al. T2 shortening in the visual cortex: effect of aging and cerebrovascular disease. AJNR Am J Neuroradiol 1997;18:711–14 [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai T, Korogi Y, Sakamoto Y, et al. T2 shortening in the motor cortex: effect of aging and cerebrovascular diseases. Radiology 1996;199:799–803 [DOI] [PubMed] [Google Scholar]
- 11.Oba H, Araki T, Ohtomo K, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 1993;189:843–46 [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki S, Nakagawa H, Fukusumi A, et al. Identification of pre- and postcentral gyri on CT and MR images on the basis of the medullary pattern of cerebral white matter. Radiology 1991;179:207–13 [DOI] [PubMed] [Google Scholar]
- 13.Reichenbach JR, Barth M, Haacke EM, et al. High-resolution MR venography at 3.0 Tesla. J Comput Assist Tomogr 2000;24:949–57 [DOI] [PubMed] [Google Scholar]
- 14.Ogg RJ, Langston JW, Haacke EM, et al. The correlation between phase shifts in gradient echo MR images and regional brain iron concentration. Magn Reson Imaging 1999;17:1141–48 [DOI] [PubMed] [Google Scholar]
- 15.Abduljalil AM, Schmalbrock P, Novak V, et al. Enhanced gray and white matter contrast of phase susceptibility-weighted images in ultra-high-field magnetic resonance imaging. J Magn Reson Imaging 2003;18:284–90 [DOI] [PubMed] [Google Scholar]
- 16.Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI). Magn Reson Med 2004;52:612–18 [DOI] [PubMed] [Google Scholar]
- 17.Schenck JF, Zimmerman EA. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed 2004;17:433–45 [DOI] [PubMed] [Google Scholar]
- 18.Bizzi A, Brooks RA, Brunetti A, et al. Role of iron and ferritin in MR imaging of the brain: a study in primates, at different field strengths. Radiology 1990;177:59–65 [DOI] [PubMed] [Google Scholar]
- 19.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3:41–51 [DOI] [PubMed] [Google Scholar]
- 20.Karaarslan E, Arslan A. Perirolandic cortex of the normal brain: low signal intensity on turbo FLAIR MR images. Radiology 2003;227:538–41. Epub 2003 Mar 27 [DOI] [PubMed] [Google Scholar]
- 21.Wood CC, Spencer DD, Allison T, et al. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg 1988;68:99–111 [DOI] [PubMed] [Google Scholar]
- 22.Sobel DF, Gallen CC, Schwartz BJ, et al. Locating the central sulcus: comparison of MR anatomic and magnetoencephalographic functional methods. AJNR Am J Neuroradiol 1993;14:915–25 [PMC free article] [PubMed] [Google Scholar]
- 23.Clark CA, Barrick TR, Murphy MM, et al. White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? Neuroimage 2003;20:1601–08 [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Kizu O, Mori S, et al. Brain fiber tracking with clinically feasible diffusion-tensor MR imaging: initial experience. Radiology 2003;227:295–301 [DOI] [PubMed] [Google Scholar]
- 25.Okada T, Miki Y, Fushimi Y, et al. Diffusion-tensor fiber tractography: intraindividual comparison of 3.0-T and 1.5-T MR imaging. Radiology 2006;238:668–78 [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Miki Y, Kikuta K, et al. Diffusion tensor fiber tractography for arteriovenous malformations: quantitative analyses to evaluate the corticospinal tract and optic radiation. AJNR Am J Neuroradiol 2007;28:1107–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K, Nagakane Y, Yoshikawa K, et al. Somatotopic organization of thalamocortical projection fibers as assessed with MR tractography. Radiology 2007;242:840–45 [DOI] [PubMed] [Google Scholar]
- 28.Georgiades CS, Itoh R, Golay X, et al. MR imaging of the human brain at 1.5 T: regional variations in transverse relaxation rates in the cerebral cortex. AJNR Am J Neuroradiol 2001;22:1732–37 [PMC free article] [PubMed] [Google Scholar]