Abstract
BACKGROUND AND PURPOSE: The efficacy of intra-arterial administration of nimodipine (IAN) in patients with severe vasospasm after aneurysmal subarachnoid hemorrhage (SAH) remains unproved. The goal of the present study was to investigate the clinical effect and cerebral perfusion after IAN in patients with severe vasospasm refractory to hemodynamic treatment.
MATERIALS AND METHODS: Twenty-six of 214 patients with aneurysmal SAH were included in the prospective study, approved by the local ethics committee. All patients met the criteria of medically refractory cerebral vasospasm. Effectiveness was monitored angiographically by digital subtraction angiography and by transcranial Doppler (TCD), perfusion CT (PCT), and neurologic examination during treatment course and follow-up.
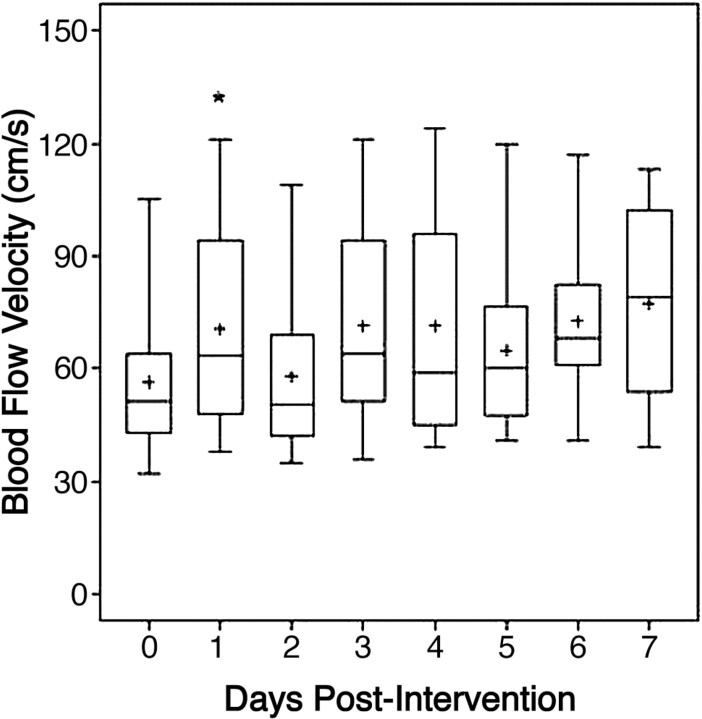
RESULTS: No angiographic effect was observed in 8 patients. The pooled PCT values revealed a reduction of time to peak (P = .03) and mean transit time (P = .17) 1 day after intervention. This effect did not persist during the following days. The pooled TCD analysis demonstrated a transient increase in flow 1 day after intervention (P = .03). No trend was evident during the next 7 days after intervention. Additional infarction was experienced by 61.1% of patients.
CONCLUSIONS: IAN in a selective patient group resulted in a positive response with reduction of angiographic vasospasm and increase in cerebral perfusion as detected by PCT after 24 hours. Therefore, IAN appears more effective than intra-arterial papaverine. Nevertheless the efficacy of IAN is temporary. Therefore, the search for more effective treatment strategies to reduce critical vasospasm and to improve cerebral perfusion must be continued.
Severe cerebral vasospasm constitutes the dominant factor of secondary morbidity and mortality after aneurysmal subarachnoid hemorrhage (SAH).1-4 The current strategies for preventing vasospasm comprise the systemic administration of the calcium channel blocker nimodipine.5-7 Moreover, the use of intracisternal thrombolysis8 and the intracisternal application of nicardipine-prolonged implants9,10 demonstrated effectiveness in preventing cerebral vasospasm. Despite these treatment methods,6,11 the rate of vasospasm-related permanent disability is estimated as totaling 10%–20%.12,13 Meta-analyses on the efficacy of hemodynamic therapy are partially contradictory but usually recommend hemodynamic optimization for symptomatic vasospasm.6,13-15
For symptomatic vasospasm refractory to hemodynamic therapy, endovascular strategies such as balloon angioplasty16 and intra-arterial spasmolysis with papaverine17 or nimodipine18 have been recommended. Balloon angioplasty was found effective, but the procedure is limited to proximal vessel segments. In addition, it demands an experienced endovascular surgeon and is associated with significant risk.19,20 Intra-arterial papaverine has been shown to reverse angiographic vasospasm and to reduce the velocities as detected by transcranial Doppler (TCD).17,21 However, clinical trials have failed to demonstrate the effectiveness of intra-arterial papaverine on outcome.22 The most likely reason for this failure is the short duration of effect that has been documented: it is limited to a mere few hours.23 Intra-arterial nimodipine (IAN) in patients with severe vasospasm has been reported by several authors.24-26 Data from a larger series by Biondi et al18 suggested that IAN is effective and safe for the treatment of vasospasm after SAH. However, no information regarding the influence of IAN on cerebral perfusion is available. Moreover, there is a lack of information about the duration of effect.
The objective of the present study was to investigate the effect and duration of the action of IAN by repeated assessment of the clinical condition with TCD, digital subtraction angiography (DSA), and perfusion measurements by means of perfusion CT (PCT).
Methods
Patient Population
The prospective study was approved by the local research ethics committee as a last resort therapy. Two hundred fourteen patients with aneurysmal SAH were admitted to our department of neurosurgery between March 2006 and March 2007. All patients were managed according to a standardized treatment protocol. Angiography and surgical or endovascular obliteration of the aneurysm were performed within 24 hours after admission. The patients were subsequently cared for in the intensive care unit. No prophylactic hypertensive and hypervolemic therapy was performed. Prophylactic intravenous nimodipine at 2 mg/h was given for at least 7 days or longer in case of clinical and/or Doppler sonographic or angiographic vasospasm, followed by tapering oral administration for another week. Dexamethasone, 4 mg every 8 hours, was given for 3 days and then tapered off during another 3 days. The neurologic condition was recorded hourly for routine monitoring. TCD examinations were performed daily. According to our protocol, a routine control DSA was performed on days 5–7 after the index SAH. PCT measurements with calculation of the mean transit time (MTT), the selective time to peak of the brain parenchyma (Tmax),27,28 the regional cerebral blood volume (rCBV), and the regional cerebral blood flow (rCBF) were integrated into the investigation of cerebral vasospasm after SAH as previously suggested.29,30 According to our protocol, these perfusion investigations were performed on day 1, days 3–4, and days 9–11 after the initial SAH.28 Whenever vasospasm was suggested, additional examinations were performed.
Hypertensive and hypervolemic therapy was applied to asymptomatic and symptomatic patients with TCD flow acceleration above 120 cm/s and/or moderate perfusion reduction as demonstrated by perfusion CT. Moderate hypertensive and hypervolemic therapy was used for oligosymptomatic patients and aimed at cerebral perfusion pressure (CPP) levels between 80 and 120 mm Hg and a central venous pressure >6 mm Hg. Maximal hemodynamic therapy was used for progressively symptomatic vasospasm and aimed at CPP levels >120 mmHg and a central venous pressure in excess of 10 mm Hg.
Confirmation of Cerebral Vasospasm
The observation of cerebral vasospasm was based on clinical, sonographic, PCT, and angiographic measurements. The confirmation of cerebral vasospasm was based on the angiographically defined vascular constriction. After aneurysmal SAH, the following patient groups in addition to the routine control were referred to angiography: 1) conscious patients with new delayed ischemic neurologic deficits, 2) conscious or comatose patients with intracranial flow velocities as detected by TCD of >150 cm/s or increase of flow velocity >40 cm/s within 1 day, and 3) patients with highly pathologic perfusion parameters monitored by PCT.27 Narrowing of the arterial diameter of >30% from baseline was defined as angiographic vasospasm. Balloon angioplasty was performed whenever possible for proximal segmental vasospasm. IAN was performed for diffuse and peripheral vasospasm. In all cases, a baseline PCT was obtained before the endovascular intervention.
Application of IAN
After confirmation of hemodynamically relevant vasospasm by angiography, the standardized IAN was initiated immediately. After the selective positioning of the microcatheter within the internal carotid artery (C1/C2 segment), a bolus of 0.8 mg of nimodipine was administered slowly, followed by a second bolus application 5 minutes later. The follow-up angiography was performed 10 minutes later. In cases of minor angiographic effect after the second bolus injection, the patients were considered for continuous IAN for 2 hours (4 mg/h). In cases of no angiographic effect, IAN was considered as failed; the patients were excluded from further analysis and were treated according to our experimental algorithm with the intrathecal application of nimodipine.
Assessment of Effectiveness of IAN
The efficacy of IAN was assessed by monitoring the following: 1) the neurologic condition of the conscious patients, 2) TCD examinations, 3) PCT measurements, and 4) PCT follow-up measurements. The neurologic examination of the patients was performed according to the Glasgow Coma Scale (GCS). The flow velocities as detected by daily TCD were monitored in the segments of the internal, middle, and anterior cerebral arteries of both hemispheres. The mean value was calculated from the sum of obtained flow velocities. PCT measurements with calculation of MTT, Tmax, rCBV, and rCBF were also obtained to measure the effect of IAN. New ischemic lesions on the CT scan after initiating the IAN were considered to be related to persistent vasospasm after treatment.
Patient Outcome
The clinical outcome was assessed according to the modified Rankin Scale (mRS) at the time of discharge and again 3 months later.
Statistical Analysis
Data are presented as median (interquartile range) and relative frequencies for continuous and categoric variables, as appropriate. Statistical analyses were performed with a paired signed rank test for intraindividual comparisons. A 2-tailed value of P < .05 was considered significant. Box-plot profiles were used to present the sample dynamics by time. Spearman correlation coefficients were used to quantify the correlation between the blood flow velocity measured with TCD and the perfusion parameters (MTT, Tmax, rCBV, rCBF) measured with PCT. To examine the potential impact factors on the profile of flow velocity measured from pre- to postintervention, we used the generalized estimating equation (GEE) approach 31 because it produces a summary estimate of the group effect averaged over the follow-up time, taking into account the correlation of the repeated measurements for a patient. P values have not been adjusted for multiple testing because the present analysis is of an exploratory nature and the trial had not been powered for the present comparisons. Analyses were performed with the SAS statistical package (Version 8.0; SAS Institute, Cary, NC).
Results
Of the 214 patients with aneurysmal SAH treated between March 2006 and March 2007, 26 (12.1%) met the criteria of hemodynamically relevant cerebral vasospasm as defined previously and were selected for IAN. The mean age of patients in the study group was 48.5 years (interquartile range, 42–58 years). The male-to-female ratio was balanced. The distribution of World Federation of Neurological Surgeons (WFNS) grades, Fisher grades, localization of the aneurysm, and the occurrence of vasospasm according to the time after SAH are listed in Table 1.
Table 1:
Characteristics of patients with cerebral vasospasm who were treated with IAN
| Patient No. | Age (yr)/Sex | WFNS Grade | Fisher Grade | Aneurysm Location | Treatment | Vasospasm Confirmed by DSA* |
|---|---|---|---|---|---|---|
| 1 | 67/F | IV | IV | PICA left | Clipping | 15 |
| 2 | 47/M | I | IV | MCA left | Clipping | 11 |
| 3 | 47/F | III | IV | MCA left | Clipping | 8 |
| 4 | 59/M | III | IV | Pericallosal left | Clipping | 6, 9 |
| 5 | 49/F | I | III | BA | Coiling | 7 |
| 6 | 65/F | IV | IV | AcomA | Clipping | 16 |
| 7 | 48/M | III | IV | MCA right | Clipping | 8 |
| 8 | 54/M | III | IV | ICA left | Coiling | 11 |
| 9 | 36/F | III | IV | AcomA | Clipping | 9 |
| 10 | 46/F | IV | IV | BA | Coiling | 5, 6 |
| 11 | 46/F | I | II | AcomA | Coiling | 6 |
| 12 | 33/M | I | III | MCA left | Clipping | 7 |
| 13 | 37/M | I | II | AcomA | Clipping | 3 |
| 14 | 49/F | I | III | AcomA | Coiling | 6 |
| 15 | 73/F | IV | IV | PcomA left | Clipping | 4 |
| 16 | 58/M | II | III | PICA right | Coiling | 10 |
| 17 | 40/M | I | II | AcomA | Coiling | 5, 6 |
| 18 | 27/M | IV | IV | ICA left | Clipping | 5, 13 |
| 19 | 48/F | IV | IV | ICA right | Clipping | 10, 11 |
| 20 | 58/M | I | II | MCA right | Clipping | 7, 11 |
| 21 | 70/F | II | III | MCA left | Clipping | 8, 9 |
| 22 | 55/F | IV | IV | PcomA right | Coiling | 8 |
| 23 | 36/M | I | IV | ICA right | Coiling | 10 |
| 24 | 65/M | I | III | AcomA | Clipping | 5, 7, 8, 11 |
| 25 | 49/F | IV | IV | AcomA | Coiling | 0, 3, 10 |
| 26 | 42/M | III | IV | ICA left | Coiling | 7, 11, 12, 14, 18 |
Note:—ICA indicates internal carotid artery; AcomA, anterior communicating artery; MCA, middle cerebral artery; PcomA, posterior communicating artery; BA, basilar artery; PICA, posterior inferior cerebellar artery; WFNS, World Federation of Neurological Surgeons; DSA, digital subtraction angiography.
In days after the index SAH.
Individual Treatment Schedule and Postinterventional Angiographic Results
Overall the number of treatments amounted to 42 intra-arterial nimodipine applications for the 26 patients (range, 1–5 treatment sessions per patient). In 24 sessions, the IAN was bilateral, whereas a unilateral IAN was performed during 18 applications. Eight (30.7%) patients dropped out because of no measurable angiographic effect (vessel diameter and circulation time) of IAN and were, therefore, considered as treatment failures. The further analysis to determine the effect of IAN was restricted to 18 patients treated with a total of 22 sessions. The average dose per session was 1.6 mg (range, 0.8–3.2 mg). Four (22.2%) patients received a balloon angioplasty (PTA) before IAN, whereas 14 (77.8%) patients were treated with IAN initially. Due to only minor initial angiographic effect, 3 (16.6%) patients received an additional unilateral continuous infusion of 4 mg/h for 2 hours. According to the arterial diameter and the circulation time, the early positive angiographic effect was classified as “minor,” “moderate,” or “major” improvement. “Minor” changes were classified angiographically by observation of the reduced circulation time only, “moderate” changes consisted of reduced circulation time and reduction of the vasospastic segmental narrowing, and a “major” response included a complete angiographic reversal of the cerebral vasospasm. The individual characteristics are summarized in Table 2. Eleven (61.1%) of 18 patients were clinically assessable. Within this group, the neurologic condition remained unchanged during the 24 hours after IAN for 7 patients, whereas 2 patients demonstrated an improvement and another 2 patients experienced a deterioration of the neurologic condition according to the GCS.
Table 2:
Individual treatment schedules and postinterventional angiographic results
| Patient No. | Distribution of Vasospasm | IAN | IAN Dosage per Session (mg) | Continuous IAN | Former PTA | Direct DSA Result |
|---|---|---|---|---|---|---|
| 1 | ACA/MCA b | ICA b | 3.2 | – | – | + |
| 2 | ACA/MCA l | ICA l | 1.6 | 4 mg/h, 2 hours | – | (+) |
| 3 | MCA l | ICA l | 0.8 | – | – | ++ |
| 4 | 1) ACA b | 1) ICA b | 1) 3.2 | 1) − | 1) − | 1) ++ |
| 2) ACA b | 2) ICA b | 2) 3.2 | 2) – | 2) – | 2) + | |
| 5 | MCA b | ICA b | 3.2 | – | – | ++ |
| 6 | ACA/MCA l | ICA l | 1.6 | – | – | ++ |
| 7 | MCA r | ICA r | 1.6 | – | Yes | ++ |
| 8 | MCA l | ICA l | 1.6 | – | – | (+) |
| 9 | MCA l | ICA l | 0.8 | – | Yes | ++ |
| 10 | 1) ICA l | 1) ICA l | 1) 3.2 | 1) − | 1) − | 1) + |
| 2) ICA l | 2) ICA l | 2) 3.2 | 2) – | 2) – | 2) + | |
| 11 | ACA l | ICA l | 1.6 | – | Yes | ++ |
| 12 | MCA l | ICA l | 1.6 | – | – | ++ |
| 13 | ACA r | ICA r | 1.6 | – | – | (+) |
| 14 | ACA b | ICA b | 3.2 | – | – | + |
| 15 | ICA l | ICA l | 1.6 | – | – | ++ |
| 16 | MCA l | ICA l | 1.6 | – | (+) | |
| PCA l | VA l | 1.6 | 4 mg/h, 2 hours | |||
| 17 | 1) ICA b | 1) ICA b | 1) 3.2 | 1) − | 1) − | 1) ++ |
| 2) ICA b | 2) ICA b | 2) 3.2 | 2) 4 mg/h, 2 hours | 2) – | 2) (+) | |
| 18 | 1) ICA left | 1) ICA left | 1) 1.6 | 1) − | 1) Yes | 1) + |
| 2) ICA right | 2) ICA right | 2) 1.6 | 2) – | 2) Yes | 2) + |
Note:—ACA, anterior cerebral artery; MCA, middle cerebral artery; ICA, internal carotid artery; PCA, posterior cerebral artery; VA, vertebral artery; l, left-sided; r, right-sided; b, bilateral; DSA, digital subtraction angiography; PTA, balloon angioplasty; (+), minor changes; +, moderate changes; ++, major changes.
Effects of IAN on Flow Velocity as Detected by TCD
The pooled TCD analysis of the study group comparing the results obtained directly before and the day after the IAN demonstrated a transient increase of flow velocity. This increase was statistically significant (P = .03, with the signed rank test for intraindividual comparison). However, no trend was evident during the 7 days following the intervention (Fig 1). Those patients who had undergone PTA first had 20.7-cm/s lower TCD values on average with time than those patients without PTA (P = .0051 with the GEE model). Patients with a total dose of <3.2 mg had 13.9-cm/s higher TCD values averaged with time than those patients receiving doses of >3.2 mg (P = .08 with the GEE model).
Fig 1.
The box-plot profiles of blood-flow velocity measured with TCD, from day 0 (preintervention) to the 7 days postintervention. The plus represents the sample mean value. The asterisk indicates P < .05 for the change from baseline.
Effects of IAN on Cerebral Perfusion as Detected by PCT
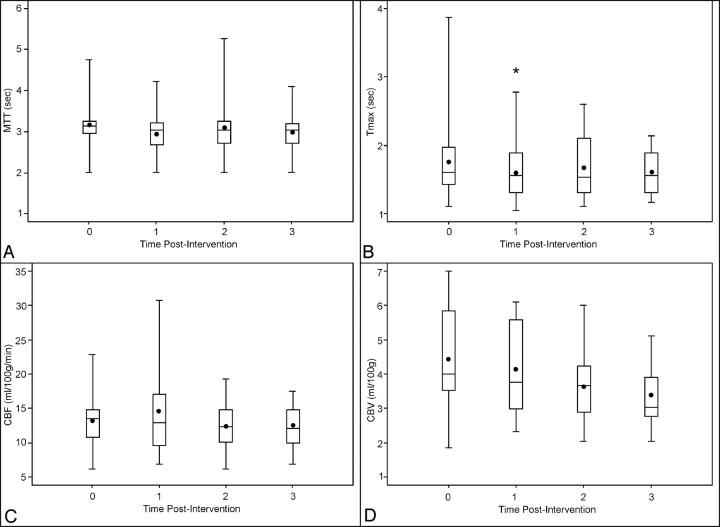
The MTT, Tmax, rCBV, and rCBF were monitored for each hemisphere in terms of hemispheric mean values.27 One day after IAN, the pooled Tmax data demonstrated a statistically significant reduction (P = .03, with the signed rank test for intraindividual comparison). Moreover, the pooled MTT 1 day after IAN indicated a trend toward reduction (P = .17, with the signed rank test for intraindividual comparison) as detected by PCT (Table 3). This effect was not measurable during further follow-up (Figs 2, 3).
Table 3:
Change of PCT parameters between immediately before and the day after the IAN intervention*
| Preintervention | 1 Day Postintervention | P† | |
|---|---|---|---|
| MTT | 3.27 (2.99–3.84) | 2.72 (2.24–3.38) | .17 |
| Tmax | 1.66 (1.43–2.29) | 1.55 (1.12–1.94) | .03 |
| CBF | 13.67 (10.86–15.45) | 13.90 (9.52–25.96) | .15 |
| CBV | 4.0 (3.51–5.87) | 3.74 (2.97–5.59) | .52 |
Note:—CBF indicates cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time; Tmax, selective time to peak of brain parenchyma.
Data are presented as medians (interquartile range).
With the signed rank test for paired samples.
Fig 2.
The box-plot profiles of perfusion parameters measured with perfusion CT: MTT (A), Tmax (B), cerebral blood flow (C), and cerebral blood volume (D), by time 0 (preintervention), 1 (first day after intervention), 2 (2–5 days postintervention), to 3 (5–9 days postintervention). The plus signs are the sample mean values. The asterisk indicates P < .05 for the change from baseline.
Fig 3.
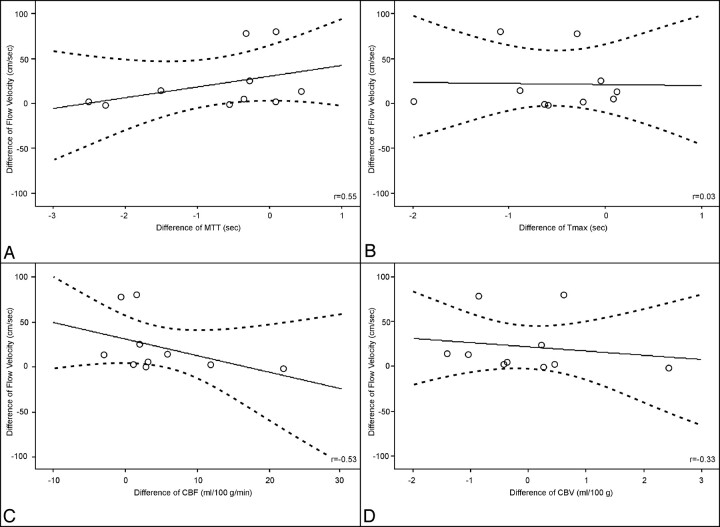
Correlation between the first day change of flow velocity measured with TCD and the first day change of perfusion parameters measured with perfusion CT: MTT (A), Tmax (B), cerebral blood flow (C), and cerebral blood volume (D). Data are presented with correlation coefficients (r) and univariate linear regression with 95% confidence intervals.
Patient Outcome
Additional ischemic lesions after IAN related to cerebral vasospasm were documented in 11 (61.1%) patients. Nine ischemic lesions were classified as minor lesions, whereas 2 were identified as major infarction on CT. Seven (38.9%) patients developed no additional cerebral minor or major infarction. The outcome as classified by mRS is summarized in Table 4. At the time of discharge, the clinical examination of 11 patients revealed a good condition (mRS 0–2), 1 patient showed a moderate status (mRS 3–4), and 6 patients were classified as having a poor outcome (mRS 5–6). mRS on 3-month follow-up is listed in Table 4.
Table 4:
Outcome of patients
| Patient No. | Posttreatment Ischemic Lesions (CT) | mRS at Discharge* | mRS at 3-Month Follow-Up* | Adverse Effects |
|---|---|---|---|---|
| 1 | Minor | 5 | 4 | None |
| 2 | Minor | 5 | 4 | Hypotension |
| 3 | Minor | 5 | 2 | Hypotension |
| 4 | Minor | 6 | – | None |
| 5 | No | 0 | 0 | None |
| 6 | No | 2 | 1 | None |
| 7 | Minor | 4 | – | Hypotension |
| 8 | No | 0 | 0 | Hypotension |
| 9 | No | 2 | 1 | None |
| 10 | Minor | 1 | 1 | None |
| 11 | Minor | 0 | 0 | None |
| 12 | No | 0 | 0 | None |
| 13 | No | 0 | 0 | Hypotension |
| 14 | No | 0 | 0 | None |
| 15 | Major | 6 | – | Hypotension |
| 16 | Minor | 1 | – | None |
| 17 | Minor | 0 | 0 | None |
| 18 | Minor | 5 | 5 | None |
Note:—mRS, indicates modified Rankin Scale.
Scores of 0–2 indicate good outcome; 3–4, moderate outcome; 5–6, poor outcome.
Adverse Effects of IAN
In the study group, 6 patients experienced an arterial blood pressure decrease of >20 mm Hg systolic after the initial bolus despite hypertensive and hypervolemic therapy. This adverse effect was temporary and could be treated easily and quickly with an increase of the vasoactive medication. One patient died 1 day after IAN due to progressive intracranial hypertension.
Illustrative Case
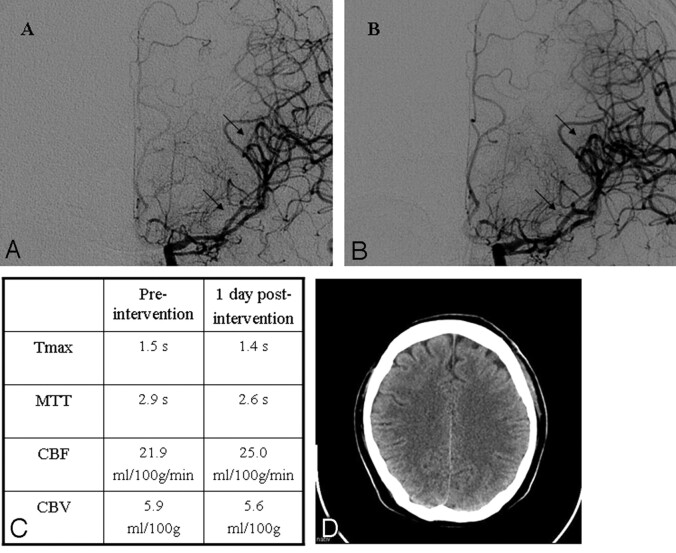
Case 12 is a 33-year-old male patient with aneurysmal SAH, WFNS grade I, and Fisher grade III. DSA revealed a middle cerebral artery aneurysm on the left side, surgically occluded. Seven days after the index bleeding, the patient's neurologic condition deteriorated, DSA demonstrated a severe vasospasm in the left M1 and M2 segments (Fig 4A), and the patient was selected for IAN. The DSA performed 10 minutes after the injection of 1.6-mg nimodipine revealed a reduction of the vasospasm (Fig 4B). PCT approximately 24 hours afterIAN demonstrated a further reduction of MTT and Tmax (Fig 4C). No ischemic lesions were documented on CT at the time of discharge (Fig 4D), and the patient did not develop neurologic impairment.
Fig 4.
Illustrative case 12. A, Baseline angiogram on day 6 documents severe vasospasm in the left M1 and M2 segments (arrows). B, DSA obtained 10 minutes after the injection of 1.6-mg nimodipine reveals reduction of the vasospasm (arrows). C, PCT approximately 24 hours after IAN demonstrates a reduction of MTT and Tmax. D, No ischemic lesions are documented on the CT scan obtained at the time of discharge.
Discussion
The present prospective study has demonstrated the effect of IAN on severe cerebral vasospasm after aneurysmal SAH that was resistant to systemic therapy. In addition, a correlation to cerebral perfusion as detected by PCT was performed to define the duration of IAN efficacy on cerebral vasospasm.
One limitation of the study is the lack of a control group. The application of IAN was introduced exclusively as a rescue therapy. Because of imminent infarction, no control group was included in the study. All patients selected for this treatment had severe vasospasm that was refractory to all established therapies such as systemically applied nimodipine, hemodynamic treatment, or balloon angioplasty. A further limitation is presented by the small sample size, precluding subgroup analysis. In addition, due to the limited experience with IAN after SAH, dose selection had to be based on experimental data, the intravenous doses of nimodipine established in several trials, and on previous published trials of IAN.6,18,32
The endovascular methods used to treat cerebral vasospasm such as balloon angioplasty, intra-arterial papaverine (IAP), or IAN are widely accepted as rescue therapies, though their effectiveness with regard to the neurologic outcome has still not been proved.6 Balloon angioplasty has been convincingly shown to enlarge the diameter of the treated arterial segments, but the procedure is limited to proximal vessel segments. In addition, it demands an experienced endovascular surgeon and is associated with significant risks.19,20 IAP has been demonstrated to reverse angiographic vasospasm and to reduce flow velocities as detected by TCD.17,21 However, clinical trials have failed to demonstrate the effectiveness of IAP on the outcome.22 The most plausible explanation for this failure is the transient action of IAP, which is limited to a few hours.23 Moreover, IAP appears to be neurotoxic, particularly in the posterior circulation.33
The systemic administration of the calcium channel blocker nimodipine was proved to be effective on clinical outcome after SAH in a number of randomized trials.5-7 Less evidence is available with regard to the effectiveness of IAN. Firat et al32 have shown, in an experimental SAH model in rabbits, that IAN can effectively reduce basilar and vertebral artery vasospasm angiographically. Moreover, this effect was demonstrated to be superior to IAP. A limitation of this experiment was the lack of a histopathologic examination with regard to ischemic lesions.
In the context of the intra-arterial treatment of cerebral vasospasm, a number of clinical trials involving the intra-arterial application of the calcium channel blockers nimodipine or nicardipine (which belong to the group of dihydropyridines) or verapamil (as a phenylalkylamine) have been reported.18,34-36 The intra-arterial administration of verapamil was demonstrated to be safe, but beneficial effects were only documented in some patients.35 The intra-arterial administration of nicardipine was shown to be safe and effective on vasospasm34,36; nevertheless, perfusion measurements were not performed.
Against the backdrop of published experimental and clinical data and the proved effectiveness of systemic application, we chose to use the calcium channel blocker nimodipine in the current study for intra-arterial therapy. Nevertheless, a potential conflict with the receptor-binding status for the group of dihydropyridines for patients who had already been treated systemically with nimodipine is recognized.37
IAN in patients with severe vasospasm has been reported by several authors.24-26 Data from a larger series by Biondi et al18 demonstrated that IAN is effective and safe for the treatment of vasospasm after SAH. In this trial, 25 patients treated with IAN were retrospectively reviewed and analyzed. Clinical improvement was observed in 76% of the patients. Angiographic vessel dilation occurred in 43% of all the IAN sessions, and 18 of 25 patients had a favorable outcome (mRS 0–2). No complications were observed.
The present study completes the examination of the effectiveness of IAN by additionally investigating the cerebral perfusion by means of repeated PCT. Therefore, the results provide a survey of the immediate and lasting efficacy of IAN.
Due to its pharmacologic action, a direct angiographic reaction of IAN was expected. Therefore, our protocol called for exclusion of patients from further IAN and analysis who did not show any early angiographic effectiveness. IAN was considered ineffective in these patients. Eight (30.7%) of 26 patients were classified as resistant to IAN; this group was treated further with intrathecal nimodipine. In the study by Biondi et al,18 the rate of angiographic failure was 37%, and a selective analysis of this subgroup would be interesting. In the other patients, the direct short-term effect assessed by DSA was classified as minor in 5 sessions, in 7 sessions as moderate, and in 10 sessions as major with regard to vessel diameter and circulation time. However, among these 22 sessions, 5 treatments included primary balloon angioplasty and 3 treatments were followed by continuous IAN because of minor effectiveness.
Eleven (61.1%) of our 18 patients with some immediate effect of IAN were clinically assessable. In 7 patients, the neurologic condition remained unchanged, whereas 2 patients demonstrated an immediate neurologic improvement. Nevertheless, 2 patients showed a decreasing GCS. This result is in contrast to the study by Biondi et al,18 who demonstrated a clinical improvement in 76% of the patients after IAN.
The importance of the PCT parameters Tmax and MTT for analysis of cerebral vasospasm should be emphasized.30 In our study on day 1 after IAN, the pooled Tmax data demonstrated a statistically significant reduction (P = .03). The pooled MTT 1 day after IAN indicated a trend toward a reduction (P = .17). These results demonstrated the effectiveness of IAN after 24 hours in the patient population, with a positive short-term response as detected by DSA. Therefore, the effect of IAN on cerebral perfusion as measured by PCT appears to be more enduring than that with IAP.23 However, the effect of IAN as measured by PCT vanished during further follow-up.
The pooled TCD analysis of the study group demonstrated a transient increase in flow velocity (P = .03) when comparing the results obtained before and the day after the IAN. However, no trend was suggested during the next 7 days after intervention. The interpretation of the TCD analysis and the results of PCT leave some room for speculation. A similar trend with regard to TCD was observed in other recent studies.23,38 The low sensitivity of TCD with regard to the effect of IAN can be explained by the fact that simultaneous dilation of proximal and peripheral arterial segments neutralizes the net effect on flow velocities as assessed by TCD.39
Additional ischemic lesions despite IAN were documented in 11 (61.1%) of our patients. Nine ischemic lesions were classified as minor lesions, whereas 2 were considered as major infarction on CT. In comparison with studies using IAP,23 this radiologic outcome after IAN with regard to infarction appears to be more favorable.
At the time of discharge, the clinical condition of 11 patients was classified as good (mRS 0–2), 1 patient had a moderate status (mRS 3–4), and 6 patients had a poor outcome (mRS 5–6). In comparison with studies using IAP,23 the clinical outcome also appears to be more favorable.
In the present trial, 2 patients were given a total dose of 0.8 mg, 7 patients received 1.6 mg, and 9 patients received ≥3.2 mg. Because of the limited number of patients in the respective subgroups, no clear trends could be identified. A dichotomization according to dosage was then conducted. With time, patients with a total dose of <3.2 mg seemed to have higher average TCD values than those patients dosed with >3.2 mg, though this was without statistical significance (P = .08 with GEE model).
Hypotension is well known as the most dominant side effect of systemically applied nimodipine.11 In our study population, 6 patients experienced an arterial blood pressure decrease of >20 mm Hg systolic after the initial bolus, despite the already-instituted hypertensive and hypervolemic therapy. In all patients, this effect was temporary and could easily be treated within 1 minute by adjusting the vasoactive medication. Nevertheless, this demonstrates that patients always need to be monitored during and after the IAN procedure.
The application of IAN was introduced exclusively as a rescue therapy. Because of imminent infarction, no control group was included in the study. All included patients had vasospasm refractory to all established therapies such as systemically applied nimodipine and hemodynamic therapy.
Conclusion
In conclusion, IAN has a direct positive response in most patients with a refractory vasospasm after standard therapy, reducing angiographic vasospasm and increasing cerebral perfusion as detected by PCT after 24 hours. However, the efficacy of IAN was shown to be temporary, and IAN can save only patients at the peak of vasospasm, who have a tendency to improve during the following days. Therefore, the search for alternative treatment strategies to reduce cerebral vasospasm and to improve patients’ outcomes, such as continuous intrathecal drug application, must be continued.
Acknowledgments
We thank the nursing staff of the Neurosurgical Intensive Care Unit NI04 for their continuing dedication and enthusiasm with regard to this study.
References
- 1.Findlay JM, Deagle GM. Causes of morbidity and mortality following intracranial aneurysm rupture. Can J Neurol Sci 1998;25:209–15 [DOI] [PubMed] [Google Scholar]
- 2.Kassell NF, Torner JC, Haley EC Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1. Overall management results. J Neurosurg 1990;73:18–36 [DOI] [PubMed] [Google Scholar]
- 3.Kassell NF, Torner JC, Jane JA, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2. Surgical results. J Neurosurg 1990;73:37–47 [DOI] [PubMed] [Google Scholar]
- 4.Rabinstein AA, Pichelmann MA, Friedman JA, et al. Symptomatic vasospasm and outcomes following aneurysmal subarachnoid hemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg 2003;98:319–25 [DOI] [PubMed] [Google Scholar]
- 5.Barker FG 2nd, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg 1996;84:405–14 [DOI] [PubMed] [Google Scholar]
- 6.Weyer GW, Nolan CP, Macdonald RL. Evidence-based cerebral vasospasm management. Neurosurg Focus 2006;21:E8. [DOI] [PubMed] [Google Scholar]
- 7.Rinkel GJ, Feigin VL, Algra A, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2005;25:CD000277. [DOI] [PubMed] [Google Scholar]
- 8.Amin-Hanjani S, Ogilvy CS, Barker FG 2nd. Does intracisternal thrombolysis prevent vasospasm after aneurysmal subarachnoid hemorrhage? A meta-analysis. Neurosurgery 2004;54:326–34, discussion 334–35 [DOI] [PubMed] [Google Scholar]
- 9.Barth M, Capelle HH, Weidauer S, et al. Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurysmal subarachnoid hemorrhage: a prospective, randomized, double-blind phase IIa study. Stroke 2007;38:330–36. Epub 2006 Dec 21 [DOI] [PubMed] [Google Scholar]
- 10.Kasuya H, Onda H, Sasahara A, et al. Application of nicardipine prolonged-release implants: analysis of 97 consecutive patients with acute subarachnoid hemorrhage. Neurosurgery 2005;56:895–902 [PubMed] [Google Scholar]
- 11.Treggiari-Venzi MM, Suter PM, Romand JA. Review of medical prevention of vasospasm after aneurysmal subarachnoid hemorrhage: a problem of neurointensive care. Neurosurgery 2001;48:249–61, discussion 261–62 [DOI] [PubMed] [Google Scholar]
- 12.Broderick JP, Brott TG, Duldner JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994;25:1342–47 [DOI] [PubMed] [Google Scholar]
- 13.Rinkel GJ, Feigin VL, Algra A, et al. Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2004. :CD000483. [DOI] [PMC free article] [PubMed]
- 14.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med 2006;354:387–96 [DOI] [PubMed] [Google Scholar]
- 15.Treggiari MM, Walder B, Suter PM, et al. Systematic review of the prevention of delayed ischemic neurological deficits with hypertension, hypervolemia, and hemodilution therapy following subarachnoid hemorrhage. J Neurosurg 2003;98:978–84 [DOI] [PubMed] [Google Scholar]
- 16.Dion JE, Duckwiler GR, Vinuela F, et al. Pre-operative micro-angioplasty of refractory vasospasm secondary to subarachnoid hemorrhage. Neuroradiology 1990;32:232–36 [DOI] [PubMed] [Google Scholar]
- 17.Kassell NF, Helm G, Simmons N, et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg 1992;77:848–52 [DOI] [PubMed] [Google Scholar]
- 18.Biondi A, Ricciardi GK, Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol 2004;25:1067–76 [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenwasser RH, Armonda RA, Thomas JE, et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery 1999;44:975–79, discussion 979–80 [DOI] [PubMed] [Google Scholar]
- 20.Polin RS, Coenen VA, Hansen CA, et al. Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 2000;92:284–90 [DOI] [PubMed] [Google Scholar]
- 21.Elliott JP, Newell DW, Lam DJ, et al. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 1998;88:277–84 [DOI] [PubMed] [Google Scholar]
- 22.Polin RS, Hansen CA, German P, et al. Intra-arterially administered papaverine for the treatment of symptomatic cerebral vasospasm. Neurosurgery 1998;42:1256–64; discussion 1264–67 [DOI] [PubMed] [Google Scholar]
- 23.Vajkoczy P, Horn P, Bauhuf C, et al. Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke 2001;32:498–505 [DOI] [PubMed] [Google Scholar]
- 24.Boker DK, Solymosi L, Wassmann H. Immediate postangiographic intraarterial treatment of cerebral vasospasm after subarachnoid hemorrhage with nimodipine: report on 3 cases. Neurochirurgia (Stuttg) 1985;28 (suppl 1):118–20 [DOI] [PubMed] [Google Scholar]
- 25.Bracard S, Arrue P, Barral FG, et al. Management of vasospasm from subarachnoid hemorrhage: attitude of French centers—French Society of Neuroradiology [in French]. J Neuroradiol 1999;26 (1 suppl):S44–47 [PubMed] [Google Scholar]
- 26.Grotenhuis JA, Bettag W, Fiebach BJ, et al. Intracarotid slow bolus injection of nimodipine during angiography for treatment of cerebral vasospasm after SAH: a preliminary report. J Neurosurg 1984;61:231–40 [DOI] [PubMed] [Google Scholar]
- 27.Turowski B, Haenggi D, Wittsack HJ, et al. Computerized analysis of brain perfusion parameter images [lsqb]in German]. Rofo 2007;179:525–29 [DOI] [PubMed] [Google Scholar]
- 28.Turowski B, Haenggi D, Wittsack J, et al. Cerebral perfusion computerized tomography in vasospasm after subarachnoid hemorrhage: diagnostic value of MTT [in German [. Rofo 2007;179:847–54 [DOI] [PubMed] [Google Scholar]
- 29.Nabavi DG, LeBlanc LM, Baxter B, et al. Monitoring cerebral perfusion after subarachnoid hemorrhage using CT. Neuroradiology 2001;43:7–16 [DOI] [PubMed] [Google Scholar]
- 30.Wintermark M, Ko NU, Smith WS, et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006;27:26–34 [PMC free article] [PubMed] [Google Scholar]
- 31.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–60 [PubMed] [Google Scholar]
- 32.Firat MM, Gelebek V, Orer HS, et al. Selective intraarterial nimodipine treatment in an experimental subarachnoid hemorrhage model. AJNR Am J Neuroradiol 2005;26:1357–62 [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi AI, Suarez JI, Bhardwaj A, et al. Early predictors of outcome in patients receiving hypervolemic and hypertensive therapy for symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med 2000;28:824–29 [DOI] [PubMed] [Google Scholar]
- 34.Badjatia N, Topcuoglu MA, Pryor JC, et al. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. AJNR Am J Neuroradiol 2004;25:819–26 [PMC free article] [PubMed] [Google Scholar]
- 35.Feng L, Fitzsimmons BF, Young WL, et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol 2002;23:1284–90 [PMC free article] [PubMed] [Google Scholar]
- 36.Tejada JG, Taylor RA, Ugurel MS, et al. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. AJNR Am J Neuroradiol 2007;28:844–48 [PMC free article] [PubMed] [Google Scholar]
- 37.Dacquet C, Mironneau C, Mironneau J. Effects of calcium entry blockers on calcium-dependent contractions of rat portal vein. Br J Pharmacol 1987;92:203–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuknecht B, Fandino J, Yuksel C, et al. Endovascular treatment of cerebral vasospasm: assessment of treatment effect by cerebral angiography and transcranial colour Doppler sonography. Neuroradiology 1999;41:453–62 [DOI] [PubMed] [Google Scholar]
- 39.Brauer P, Kochs E, Werner C, et al. Correlation of transcranial Doppler sonography mean flow velocity with cerebral blood flow in patients with intracranial pathology. J Neurosurg Anesthesiol 1998;10:80–85 [DOI] [PubMed] [Google Scholar]