Abstract
SUMMARY: We report the first use of Onyx in the embolization of spinal tumors in 2 cases of aggressive vertebral hemangioma. In both cases, Onyx embolization provided effective preoperative tumor devascularization after the initial prolonged particulate embolization with Embospheres made little overall impact. Onyx enables a more rapid and visible embolization than particles and is less technically demanding than traditional liquid embolic agents, such as n-butyl cyanoacrylate.
Preoperative embolization of vascular spinal tumors minimizes perioperative hemorrhage and facilitates resection.1 Particulate agents, including Embosphere (BioSphere Medical, Rockland, Mass) and polyvinyl alcohol (PVA) are favored, because they penetrate the capillary bed beyond potential arteriolar anastomoses.2 A small number report successful use of polymerizing liquid agents, such as n-butyl cyano acrylate (n-BCA; Cordis Neurovascular, Miami Lakes, Fla), in embolizing head and neck neoplasms.3
Onyx (ev3, Irvine, Calif) is a novel, nonpolymerizing liquid agent, composed of ethylene-vinyl alcohol copolymer (EVOH) dissolved in dimethyl-sulfoxide (DMSO), which precipitates within vessels forming a spongy cast. Its unique properties enable gradual, controlled, longer, and more extensive embolizations compared with n-BCA. Already an established, Food and Drug Administration-approved treatment of central nervous system arteriovenous malformations,4 its efficacy in “off-label” vascular head and neck tumor embolization has been advocated.5
Onyx embolization of spinal tumors, 2 aggressive vertebral hemangiomas, is described for the first time, highlighting its value after a lengthy particulate embolization makes a minimal impact.
Case Reports
A 41-year-old woman with 2 months of progressive bilateral lower-extremity weakness underwent CT and MR spine imaging revealing an avidly enhancing mass centered on the L2-vertebral body with paraspinal and epidural extension (Fig 1). After emergent decompressive laminectomy with 3500 mL of blood loss and histopathologic diagnosis of hemangioma, she was referred for embolization before definitive surgery.
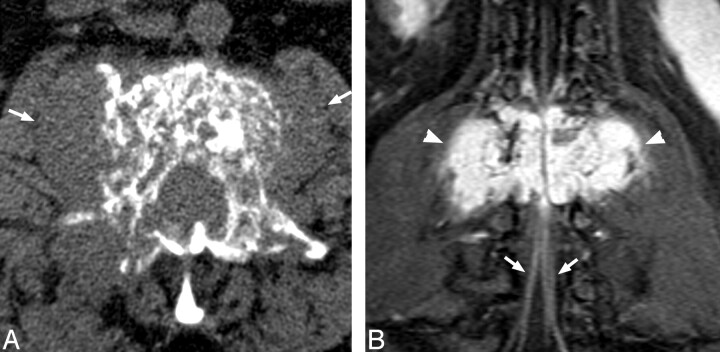
Fig 1.
A, Noncontrast CT demonstrates mixed trabecular and lytic infiltration of the L2 vertebra by a soft tissue mass, expanding the right pedicle and extending into paravertebral soft tissues (arrows). B, Postgadolinium T1 fat-sat coronal demonstrates the avidly enhancing, lobulated mass (arrowheads) with prominent flow voids. Note enhancement of the cauda equina below the level of compression (arrows).
Angiography demonstrated L2-vertebral tumor feeders from L1 and L2 lumbar segmental arteries bilaterally (Fig 2A). After infusing 5 vials of Embosphere 500- to 700-μm particles into the left L2-feeder over 1 hour and replacing 2 obstructed catheters, repeat angiography showed minimally reduced tumor supply (Fig 2B). After changing to a DMSO-compatible Rebar-18 microcatheter (ev3), injected Onyx-18 tended to reflux early, resulting in feeder occlusion without small-vessel penetration (Fig 2C).
Fig 2.
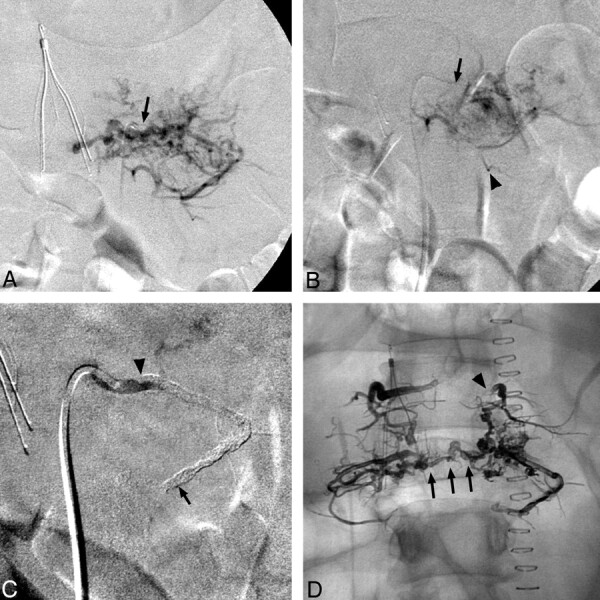
A, Selective angiography of the left L2 lumbar artery shows prominent tumor vascularity. No spinal anastomoses were identified. Note inferior vena-cava filter and diagnostic catheter in situ (arrow). B, Post-Embosphere-embolization angiography via the Prowler microcatheter (Cordis) (arrowhead) reveals persistent tumor enhancement. C, Note the guide catheter (arrow) after Onyx embolization, where the artery is occluded but the agent is largely restricted to the main artery (extending from arrowhead to arrow). D, Plain anterior/posterior radiograph shows distribution of the 4 Onyx embolizations. Note extension of Onyx from the proximal left L1 lumbar artery (arrowhead) inferior to L2 and then across the midline (arrows).
Subsequent sequential embolizations of right L1, L2, and left L1 arteries, initiated with the more concentrated Onyx-34 to form a durable plug around the catheter tip and completed with Onyx-18, resulted in deeper penetration of the tumor bed (Fig 2D). Approximately 0.5-1.0 mL was injected into each pedicle.
Postprocedure CT confirmed Onyx distribution within tumor and feeding arteries (Fig 3). Subsequent vertebrectomy and fixation resulted in nondisruptive blood loss of approximately 1500 mL.
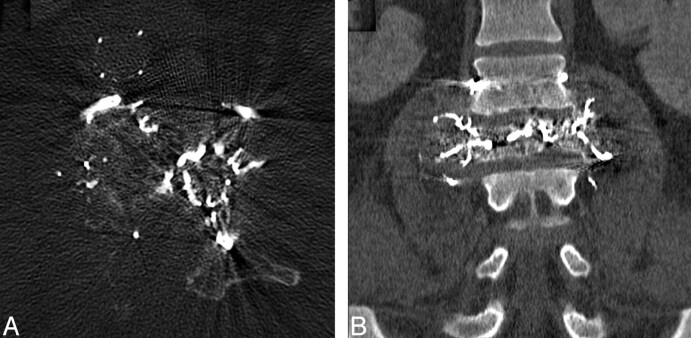
Fig 3.
Immediate postembolization axial noncontrast CT (A) and coronal reformat (B) show penetration of Onyx through tumor vessels within the vertebral body.
Case 2
A 29-year-old man underwent work-up for 6 months of progressive back pain. CT and MR imaging revealed a lytic T8-vertebral lesion with soft-tissue extension into the anterior epidural space and prominent enhancement. Tumor-embolization was requested before surgical resection and pathologic diagnosis.
On angiography, tumor supply was via a segmental branch of the right T8 intercostal artery. Artery of Adamkiewicz arose from the right T10 intercostal artery.
An Echelon-14 microcatheter (ev3) was used to coil the T8 intercostal artery distal to the feeder origin, then wedged in the more proximal tumor-feeding vessel. After vascular predilation with 300 μg of nitroglycerine, 4.5 mL of Onyx-18 was injected without reflux and penetrated the tumor extensively (Fig 4A). The microcatheter was withdrawn easily.
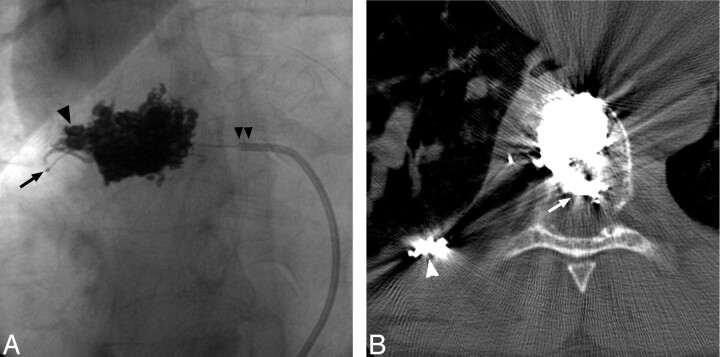
Fig 4.
Postembolization frontal radiograph of the T8 vertebra (A) shows Onyx distributed diffusely through the midlateral and right-lateral vertebral body, extending into the right pedicle (arrowhead). Note tip of Cobra catheter (Merit Medical Systems, South Jordan, Utah) (double arrowheads) and microcatheter positioned in tumor feeder (arrow). Noncontrast CT through T8 shows that the Onyx also extends into the epidural portion of the mass (arrow). Note coils positioned more distally in the right T8 intercostal artery (arrowhead).
Postprocedure CT demonstrated distribution of Onyx through most the tumor (Fig 4B), and a vertebrectomy with anterior fixation resulted in 100 mL of blood loss without blood transfusion. Histolopathology was consistent with hemangioma, and Onyx was clearly visible on gross and microscopic inspection (Fig 5).
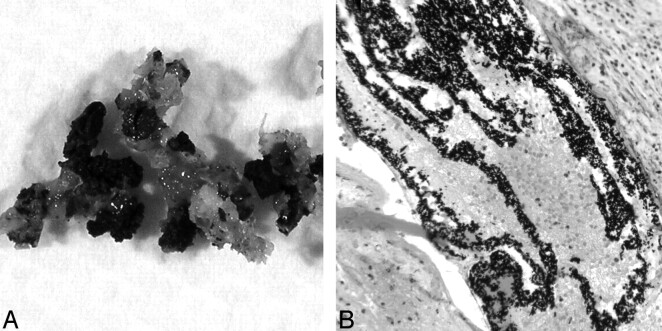
Fig 5.
Gross (A) and microscopic (B) images of postresection specimen show Onyx filling the vascular channels of the hemangioma.
Discussion
Vertebral hemangiomas have a prevalence of approximately 10%6 and a predilection for young, female patients and the thoracic spine.7 Aggressive lesions causing significant bony expansion or extraosseous extension are rare, may cause subacute neurologic impairment, and present a therapeutic challenge due to their vascularity and extent. Acosta et al8 described 16 cases, recommending combined preoperative embolization and surgical excision.
Berkefeld et al1 confirmed the efficacy of PVA embolization of hypervascular spinal tumors, finding a median perioperative blood loss of 1850 mL versus 4350 mL in controls. Hemostasis was not significantly improved if the feeder was simply coiled. However, safe particulate embolization requires large volumes and slow injection rates, resulting in lengthy injection and fluoroscopic times. Despite suspension in iodinated contrast, passage of radiolucent particles through small but dangerous collateral vessels may go unnoticed. Sedimentation and aggregation can obstruct catheters, necessitating larger caliber microcatheters. In addition, vessels embolized with particles, particularly PVA, may undergo recanalization.
Kim et al3 described n-BCA embolization of vascular head, neck, and spine tumors. They recorded only 1 temporary embolic complication in a series of 35 patients. Although tumor penetration and infarction were documented, its efficacy in limiting blood loss was not control matched. n-BCA injections were fast, permanent, and well visualized but required technical expertise and risked catheter adhesion.
Reports of Onyx tumor embolization are limited. Gobin et al5 used EVOH to embolize 14 head and neck tumors with only 2 temporary adverse events. Advantages over n-BCA included more controlled gradual injections, and they had no cases of catheter entrapment. In our first case, we allowed substantial Onyx reflux without difficulty.
Conclusion
Onyx can be used to safely embolize highly vascular tumors and has the advantages of good visibility, good control, and shorter injection times compared with particulate agents, such as Embospheres or PVA. Our cases add to the few descriptions of Onyx use in tumor embolization and are the first documented uses of Onyx in the treatment of spinal tumors. As the neuroendovascular community becomes more familiar with Onyx through its use in cerebral arteriovenous malformations, its application to other areas such as this should become more widespread.
References
- 1.Berkefeld J, Scale D, Kirchner J, et al. Hypervascular spinal tumors: influence of the embolization technique on perioperative hemorrhage. AJNR Am J Neuroradiol 1999;20:757–63 [PMC free article] [PubMed] [Google Scholar]
- 2.Manke C, Bretschneider T, Lenhart M, et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. AJNR Am J Neuroradiol 2001;22:997–1003 [PMC free article] [PubMed] [Google Scholar]
- 3.Kim LJ, Albuquerque FC, Aziz-Sultan A, et al. Low morbidity associated with use of n-butyl cyanoacrylate liquid adhesive for preoperative transarterial embolization of central nervous system tumors. Neurosurgery 2006; 2006;59:98–104 [DOI] [PubMed] [Google Scholar]
- 4.Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. AJNR Am J Neuroradiol 2007;28:371–77 [PMC free article] [PubMed] [Google Scholar]
- 5.Gobin YP, Murayama Y, Milanese K, et al. Head and neck hypervascular lesions: embolization with ethylene vinyl alcohol copolymer—laboratory evaluation in swine and clinical evaluation in humans. Radiology 2001;221:309–17 [DOI] [PubMed] [Google Scholar]
- 6.Dagi TF, Schmidek HH. Vascular tumors of the spine. In: Sundaresan N, Schmidek HH, Schiller AL, et al, eds. Tumors of the Spine: Diagnosis and Clinical Management. Philadelphia: WB Saunders;1990. :181–91
- 7.Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg 1993;78:36–45 [DOI] [PubMed] [Google Scholar]
- 8.Acosta FL, Dowd CF, Chin C, et al. Current treatment strategies and outcomes in the management of symptomatic vertebral hemangiomas. Neurosurgery 2006;58:287–95 [DOI] [PubMed] [Google Scholar]