Abstract
BACKGROUND AND PURPOSE: A simple classification instrument based on imaging that predicts outcomes in patients with actute ischemic stroke is lacking. We tested the hypotheses that the Boston Acute Stroke Imaging Scale (BASIS) classification instrument effectively predicts patient outcomes and is superior to the Alberta Stroke Program Early CT Score (ASPECTS) in predicting outcomes in acute ischemic stroke.
MATERIALS AND METHODS: Of 230 prospectively screened, consecutive patients with acute ischemic stroke, 87 had noncontrast CT (NCCT)/CT angiography (CTA), and 118 had MR imaging/MR angiography (MRA) at admission and were classified as having major stroke by BASIS criteria if they had a proximal cerebral artery occlusion or, if no occlusion, imaging evidence of significant parenchymal ischemia; all of the others were classified as minor strokes. Outcomes included death, length of hospitalization, and discharge disposition. BASIS was compared with ASPECTS (dichotomized > or ≤7) in 87 patients who had NCCT/CTA.
RESULTS: BASIS classification by NCCT/CTA was equivalent to MR imaging/MRA. Fifty-six of 205 patients were classified as having major strokes including all 6 of the deaths. A total of 71.4% and 15.4% of major and minor stroke survivors, respectively, were discharged to a rehabilitation facility, whereas 14.3% and 79.2% of patients with major and minor strokes were discharged to home. The mean length of hospitalization was 12.3 and 3.3 days for the major and minor stroke groups, respectively (all outcomes, P < .0001). In 87 NCCT/CTA patients, BASIS and ASPECTS agreed in 22 major and 44 minor strokes. BASIS classified 21 patients as having major strokes who were classified as having minor strokes by ASPECTS. The BASIS major/ASPECTS minor stroke group had outcomes similar to those classified as major strokes by both instruments.
CONCLUSIONS: The BASIS classification instrument is effective and appears superior to ASPECTS in predicting outcomes in acute ischemic stroke.
Classification instruments are important tools that enable progress in clinical medicine. They provide benchmarks to assess current medical practice and to evaluate new therapies. To be useful for monitoring new therapies, a classification instrument must be practical, reproducible, and able to be performed in a variety of clinical settings suitable for inclusion in multicenter trials. In ischemic stroke, clinical rating instruments are commonly used, and they have been used to demonstrate the efficacy of new treatments.1 However, as pointed out by Caplan,2 clinical classification instruments used in stroke do not provide information on the first event (arterial occlusion) in the chain of causality that produces the stroke syndrome and cerebral infarction. Thus, they are limited in their ability to assess the effects of treatments on arterial occlusions, and they provide little guidance on how therapy may be improved.
Classification instruments based on imaging for ischemic stroke have been proposed. The most widely used is the Alberta Stroke Program Early CT Score (ASPECTS) system, which is based on a systematic review of parenchymal changes observed on noncontrast CT (NCCT) scans.3 MR imaging-based instruments have also been proposed using diffusion MR imaging to detect parenchymal changes.4 Similar to clinical rating methods, a shortcoming of these systems is that the primary cause of the infarct, arterial occlusion, is not a part of the classification instrument. Advances in intracranial vascular imaging by CT and MR make possible a classification method that can focus on the immediate cause of the ischemia, arterial occlusion, which could be widely implemented. Moreover, it has been demonstrated that proximal cerebral artery occlusions detected by CT angiography (CTA) are independent predictors of poor outcomes in acute stroke patients.3
Here we propose a classification instrument that focuses primarily on the status of the major cerebral arteries as revealed by CTA or MR angiography (MRA) and incorporates the status of the parenchyma only if those arteries are patent. We group patients as having major or minor strokes based on whether a proximal cerebral artery is occluded. Only if the major arteries are patent is the status of the brain parenchyma considered for the presence of a large completed infarct, and if present the individual is classified as having a major stroke. All of those not meeting these criteria, by exclusion, are grouped in the minor stroke classification. We tested this new classification instrument in a consecutive series of patients who presented to a large medical center emergency department (ED) with acute neurologic symptoms suggesting acute stroke.
Materials and Methods
Patients
This study was approved by the hospital institutional review board. In a prospective fashion, all of the patients who presented to the hospital ED with stroke symptoms on 221 consecutive days were screened. On a daily basis, the ED patient encounter logs were reviewed. The names and identification numbers of all of the patients who presented with symptoms suggesting an acute stroke syndrome were collected. Patients who were admitted to the hospital from the ED with acute stroke symptoms were further evaluated through a review of the clinical records. Patients who had transient symptoms (a diagnosis of transient ischemic attack) were excluded. All of the patients fulfilling these criteria are included without regard to treatment, including 16 who received thrombolytic therapy.
The patient records, including CT and MR imaging data, were available on-line, and the following information was analyzed: demographic data, discharge diagnosis, date and time of registration in the ED, time of conclusion of imaging study, imaging findings at initial work-up, length of stay (LOS), whether death occurred during hospitalization, and discharge disposition. The latter were categorized as discharged to home, to a rehabilitation facility, or to a skilled nursing facility.
Imaging
Brain imaging is a routine procedure in the ED, and all of the patients underwent CT scanning, MR imaging, or both. All of the imaging studies were requested on a case-by-case basis by ED physicians in charge of the individual patient's care and were not influenced by the study reported here. The ED physicians may discuss imaging issues with neurologists and neuroradiologists, but the decision to obtain imaging is theirs. Specific imaging protocols are established by the radiology department. The standard protocols for evaluating patients with possible new strokes include an angiographic study by CT or MR. Imaging capabilities in the ED include 2 CT scanners with CTA capabilities that were operational 24 h/day. All of the CT studies were performed on one of these devices. Four MR imaging scanners located within the hospital were available for scanning, and at least 1 MR imaging scanner was always operational. All of the MR imaging scans were obtained on one of these instruments. For those who went to MR imaging, all underwent a diffusion MR imaging study.
NCCT and CTA acquisitions were performed according to standard protocols on 8- or 16-section multidetector CT scanners (GE Medical Systems, Milwaukee, Wis). The following are representative sample parameters (minimal variations between scanners and sites are shown as ranges): NCCT was performed in a head holder using axial technique, 120–140 kVp, 170 mA, 2-second scan time, and 5-mm section thickness. This was followed immediately by biphasic helical scanning, obtained at the same head tilt as NCCT. CTA was performed after a 25-second delay (40 seconds for patients in atrial fibrillation) after nonionic contrast administration (100–140 mL at 3 mL/s, via an 18-gauge intravenous line, by power injector), 140 kVp, 220–250 mA, 0.8–1.0-second rotation time, 2.5-mm section thickness reconstructed at 1.25-mm intervals, 3.75 mm rotation table speed, and pitch 0.75:1.00. Images were obtained from the C6 vertebral body level through the circle of Willis, followed immediately by a second set of images from the aortic arch to the skull base.
CTA source images of the intracranial circulation were reconstructed into segmented maximum intensity projection datasets (MIPS) by the radiology department 3D laboratory on dedicated workstations with 2 or more views of all of the intracranial cerebral artery segments. Both the MIPS and the source data were available for review. Only the intracranial circulation CTA reconstructed images were considered for the classification system evaluated in this study.
MR imaging was performed on a 1.5T Signa whole body scanner (GE Medical Systems) with echo-planar capabilities. Diffusion-weighted images (DWIs) were obtained by using single-shot, echo- planar imaging with sampling of the entire diffusion tensor. Six high b-value images corresponding with diffusion measurements in different gradient directions were acquired followed by a single low b-value image. The high b-value was 1000 s/mm2, and the low b value was 0 s/mm2. Imaging parameters were a TR of 5000 ms, a TE of 90 ms, a FOV of 22 cm × 22 cm, image matrix of 128 × 128 pixels, section thickness of 5 mm with a 1-mm gap, 23 axial sections, and 5 signal intensity averages. Isotropic DWIs and apparent diffusion coefficient maps were reviewed. MRA of the head was performed using a 3D time-of-flight technique with a 20° flip angle, 3.2 ms TE, 33.3 ms TR, 1-mm section thickness covering 6 cm, and a 512 × 512 matrix encoding a 22-cm FOV. Source images were reconstructed into maximum intensity projection views of the intracranial vasculature. Similar to CTAs, the 3D laboratory created segmented MIPS with standardized views of all of the intracranial cerebral artery segments. Both the MIPS and the source data were available for review.
BASIS Classification Instrument
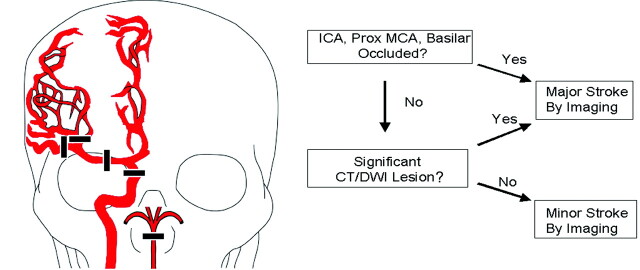
The rationale underlying the BASIS classification system is that large strokes are caused by major intracranial artery occlusions that persist for a sufficient period to cause infarction, that they can be identified by CTA or MRA, and that if spontaneous resolution of such an occlusion occurred by the time of imaging, then the presence of parenchymal abnormalities would signify an antecedent significant occlusion. All of the patients were classified using a 2-step algorithm (Fig 1), beginning with identification of an apparent proximal cerebral artery occlusion on the CTA or MRA. Proximal cerebral artery occlusions4 (Fig 1) were defined as occlusions of: distal/terminal (intracranial) internal carotid artery (ICA), proximal (M1 or M2) middle cerebral artery (MCA), and/or basilar artery. If such an occlusion was identified, the patient was classified as having a major stroke by imaging. If these arteries were not occluded, parenchymal abnormalities, if present, were identified by examination of the NCCT or diffusion MR imaging (Fig 1). A significant ischemic lesion was defined as a hypoattenuation on the NCCT that did not appear chronic or an area of reduced diffusion by MR imaging that could explain the clinical syndrome involving a substantial portion of the MCA territory defined as more than one third of the territory or 3 or more abnormal regions as defined by the ASPECTS criteria.5 Other parenchymal imaging abnormalities that could be considered as major strokes included involvement of the bilateral pons and/or bilateral thalamus. The extent of a parenchymal abnormality was determined by visual inspection. All of the other patients were classified as having a minor stroke by imaging criteria, that is, in this classification instrument, a minor stroke by imaging is by exclusion of a major stroke.
Fig 1.
Classification algorithm. Proximal cerebral artery occlusions are depicted in the drawing on the left and are defined as including the following arteries: distal (intracranial) ICA, proximal (M1 or M2) MCA, and/or basilar artery (BA). As shown in the algorithm on the right, the first step was evaluation of CTA or MRA data to identify apparent proximal cerebral artery occlusions. If no proximal cerebral artery occlusion was found, the noncontrast CT or diffusion MR imaging data were reviewed for evidence of a large acute ischemic infarct as defined in the “Materials and Methods” section. If a large CT or DWI abnormality was detected, the patient was classified as having a major stroke. All other circumstances resulted in classification as a minor stroke by imaging.
Initial classification was based on the neuroradiologic interpretation in the medical record. The original imaging data of all of the patients were then reviewed by a neuroradiologist to confirm the official interpretations and to clarify descriptions that were ambiguous with respect to the classification system used in this study. If there was conflict between the original and subsequent interpretations, a second neuroradiologist reviewed the images, and a consensus between the neuroradiologists was reached. Most patients had both CT and MR imaging within a few hours of presentation to the ED. For classification in this study, only the first imaging study that included angiography was used. A small number of patients (<10%) did not have angiography by CT or MR performed, but all had NCCT or MR imaging. They are reported for completeness.
ASPECTS Classification
The 87 patients who underwent NCCT/CTA were also classified by the dichotomized ASPECTS system.5 Each patient's NCCT was independently reviewed by 2 individual researchers trained in ASPECTS scoring, with suspicion of acute stroke the only clinical history provided. Patients with ASPECTS scores of 8, 9, or 10 were classified as having minor strokes, whereas those with scores of 7 or less were classified as having major strokes. There was agreement by both reviewers on dichotomized ASPECTS classification in 82 of the 87 patients (κ coefficient = 0.845). The 5 cases that were not in agreement were reviewed by 2 ASPECTS-trained neuroradiologists, and classification was determined by consensus.
Outcome Measures
The outcome measures included death during hospitalization, length of hospital stay (LOS), discharge to home, and discharge to a rehabilitation facility. Death and discharge to a rehabilitation facility were considered poor outcomes, whereas discharge to home was considered a good outcome. Other discharge designations, such as discharge to a skilled nursing facility, were reported in less than 5% of all patients and were not used as outcome measures.
Statistical Procedures
Fisher exact test, analysis of variance (ANOVA), and Student t test were used to test for statistical significance of the outcome measures. Differences in deaths, discharge to a rehabilitation facility, and discharge to home between strokes classified as major and minor were assessed using Fisher exact test on 3 × 2 (Tables 1 and 2) or 3 × 3 (Table 3) contingency tables to account for multiple comparisons. If significant differences were found on these, the Fisher exact test was used to isolate differences by using 2 × 2 contingency tables. Length of hospitalization was evaluated by ANOVA, and if significant differences were found, Student t test was used to isolate pair-wise differences. LOS was also assessed using the Kaplan-Meier estimator. Differences were considered significant with P < .05.
Table 1:
Outcomes of 87 patients with ischemic stroke imaged by NCCT and CTA
| Variable | Major Stroke by Imaging (n = 43) | Minor Stroke by Imaging (n = 44) | P |
|---|---|---|---|
| Deaths, n | 6 | 0 | <.0001 |
| Discharge to rehabilitation facility, n (%) | 32 (74) | 4 (9) | <.0001 |
| Discharge to home, n (%) | 5 (12) | 34 (77) | <.0001 |
| Length of stay, days (SE) | 12.1 (1.3) | 3.7 (0.4) | <.0001 |
Note:—NCCT indicates noncontrast CT; CTA, CT angiography. Significant differences in outcomes between patients with major and minor stroke were assessed using Fisher exact test (deaths, discharge to a rehabilitation facility, or discharge home) and t test (length of stay). Highly significant differences in all outcome measures were found between patients with major and minor stroke who were initially imaged with NCCT and included CTA. Not included in the table are 11 patients who had NCCT without CTA.
Table 2:
Outcomes of 118 patients with ischemic stroke imaged by MRI and MRA
| Variable | Major Stroke by Imaging (n = 13) | Minor Stroke by Imaging (n = 105) | P |
|---|---|---|---|
| Deaths, n | 2 | 0 | <.05 |
| Discharge to rehabilitation facility, n (%) | 8 (72) | 19 (18) | <.001 |
| Discharge to home, n (%) | 3 (27) | 84 (80) | <.001 |
| Length of stay, days (SE) | 12.9 (3.1) | 3.1 (0.2) | <.005 |
Note:—MRI indicates MR imaging; MRA, MR angiography. Significant differences in all outcome measures were found between patients with major and minor stroke who had MRA as the first angiographic study. Statistical tests for each outcome measure are the same as in Table 1. Not included in this table are 14 patients who had MRI including diffusion MRI but not MRA.
Table 3:
Outcomes of 87 patients with ischemic stroke imaged by NCCT and CTA and classified by BASIS and ASPECTS
| Variable | Major Stroke by BASIS and ASPECTS (n = 22) | Major by BASIS Minor by ASPECTS (n = 21) | Minor Stroke by BASIS and ASPECTS (n = 44) |
|---|---|---|---|
| Deaths, n | 5* | 1 | 0* |
| Discharge to rehabilitation facility, n (%) | 16 (94)†‡ | 16 (80)†‡ | 4 (9)† |
| Discharge to home, n (%) | 1 (6)†‡ | 4 (20)†‡ | 34 (77)† |
| Length of stay, days (SE) | 14.8 (2.4)§‖ | 9.9 (1.1)§‖ | 3.7 (0.4)§ |
Note:—NCCT indicates noncontrast CT; CTA, CT angiography; BASIS, Boston Acute Stroke Imaging Scale; ASPECTS, Alberta Stroke Program Early CT Score. There were significant differences in deaths between the BASIS and ASPECTS major stroke group and the minor stroke group classified by BASIS and ASPECTS. Highly significant differences in discharge to rehabilitation and discharge to home were found between the 2 groups that had major stroke classification and the patients classified as minor strokes by BASIS and ASPECTS.
P < .003.
All P < .0001.
No significant differences in these outcomes were found between the BASIS and ASPECTS major stroke and BASIS major/ASPECTS minor stroke groups.
P < .0001.
Significant differences were found in length of stay between the 2 groups that had major stroke classification and the patients classified as having minor strokes by BASIS and ASPECTS but not between the BASIS and ASPECTS major stroke and BASIS major/ASPECTS minor stroke groups.
Results
Diagnoses
All of the patients who presented to the hospital ED with stroke symptoms on 221 consecutive days were prospectively identified. A total of 270 patients with stroke symptoms were admitted to the hospital for further evaluation and treatment. Of these, 230 had a discharge diagnosis of ischemic stroke and are included in this study. The others were discharged with a variety of diagnoses. Other than ischemic infarction, the most common discharge diagnoses were intracerebral hemorrhage (n = 15), migraines (n = 3), and myocardial infarction (n = 2). Other discharge diagnosis were: Alzheimer disease, drug overdose with anoxia, cardiomyopathy, herpes encephalitis, multiple sclerosis, cavernous malformation, renal failure, urinary tract infection, uteropelvic obstruction, Meniere disease, vertigo of vestibular origin, vertebral artery dissection, and ruptured external iliac artery aneurysm. Seven patients had a negative work-up.
Classification by CT/CTA
Of the 230 patients discharged with a diagnosis of ischemic stroke after presenting to the hospital ED with acute neurologic symptoms, 87 underwent NCCT and CTA for evaluation of their symptoms. Apparent proximal cerebral artery occlusions were observed in 36 patients, including 34 anterior circulation occlusions and 2 basilar artery occlusions. An additional 7 patients had large MCA territory parenchymal hypodensities. The remaining 44 patients were classified as having minor stroke.
Classification by MR Imaging/MRA
A total of 118 patients underwent MR imaging, including MRA to evaluate their presenting neurologic symptoms. Of these, 13 were classified as having major strokes, including 11 with apparent anterior circulation occlusions, 1 basilar occlusion, and 1 without proximal cerebral artery occlusion but with a large MCA territory DWI abnormality. The remaining 105 patients were classified as having minor strokes.
Classification of Patients Who Did Not Undergo CT or MRA
Of the 230 patients included in this study, 25 did not undergo angiography by CT or MR, but they did undergo NCCT or MR imaging. Of these, 11 had NCCT only, and 1 of these was classified as having a major stroke, because a large thrombus was clearly identified in the left MCA. The remaining 10 did not have significant hypodensities. MR imaging without MRA was performed in 14 patients. Of these, 2 had large MCA territory DWI abnormalities.
Vascular Occlusions in Minor Strokes
Of the 205 patients who had NCCT/CTA or MR imaging/MRA, 149 were classified as having minor strokes. Evidence of arterial occlusions that did not involve the proximal cerebral arteries as defined in Fig 1 was observed in 6 patients. Occluded arteries in this group included 2 posterior cerebral, 1 anterior cerebral, 2 vertebral, and 1 distal middle cerebral artery branches.
Demographics and Time to Imaging
There were no significant differences between the groups with respect to age and sex. There were 24 women and 34 men in the major stroke group and 78 women and 94 men with minor strokes (not significant by Fisher exact test). The mean age of the major and minor stroke patients were 67.0 years (SE = 1.8 years) and 69.3 years (SE = 1.0 years), respectively (not significant by Student t test). The times from registration at the ED to the end of the imaging procedures were specified for 229 of 230 patients. There were significant differences between the major and minor stroke groups with respect to time from ED registration to completion of neuroimaging examinations. The mean time from registration at the ED to CT completion was 1.2 hours (SE = 0.1 hours) for the major stroke group and 2.7 hours (SE = 0.2 hours) for the minor stroke patients; the mean times to MR imaging completion for the major and minor stroke groups were 2.5 hours (SE = 0.3 hours) and 5.6 hours (SE = 0.6 hours), respectively (P < .0001 by t test for both comparisons).
Outcomes in Patients Classified by Using CTA or MRA
In the 87 patients who were classified by NCCT and CTA, there were highly significant differences in the outcome measures between the 43 patients who were classified as having major strokes and the 44 classified as having minor strokes. Highly significant statistical differences between patients with major and minor stroke were found for all of the outcome measures, including deaths, discharge to rehabilitation facility, discharge to home, and LOS (Table 1). There were similar, highly significant differences between major and minor stroke patients who were classified with MR imaging and MRA (Table 2).
Comparisons between BASIS and ASPECTS
The 87 patients who had both NCCT and CTA were also classified using ASPECTS. Classification by ASPECTS was performed using the NCCT images only. The outcomes of patients classified by using ASPECTS are contrasted with those classified with BASIS in Table 3. There were 22 patients (25.3%) classified by both BASIS and ASPECTS as having major strokes, and 20 had proximal artery occlusions. This group had significantly worse outcomes with respect to death, discharge disposition, and LOS compared with those classified as having minor strokes by both scales (all P < .0001).
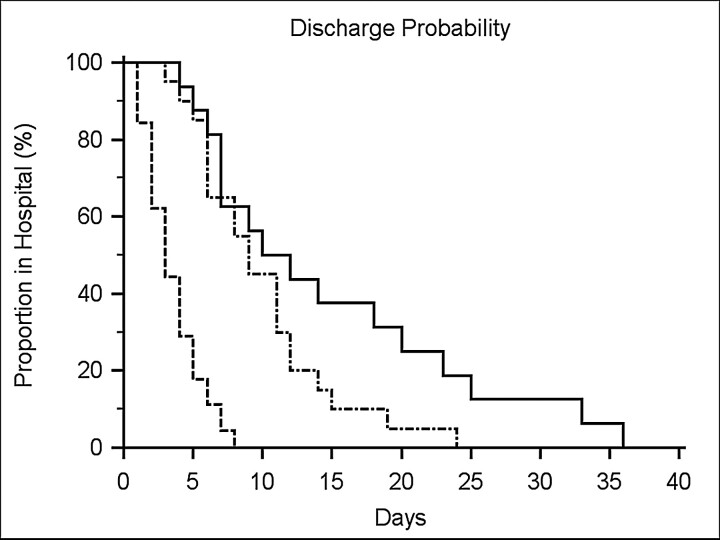
There were 21 patients who were classified as having major strokes by BASIS and contrariwise as minor strokes by ASPECTS (Table 3, second column). They had outcomes that were not significantly different from those classified as having major strokes by both BASIS and ASPECTS and significantly worse compared with BASIS and ASPECTS minor strokes with respect to discharge disposition and LOS (all P < .0001). We also evaluated LOS data using the Kaplan-Meier estimator (Fig 2). This type of analysis is commonly used to test the effect of treatment on an outcome such as survival. Here we used the Kaplan-Meier estimator to evaluate the probability of time to discharge from the hospital based on stroke classification. The probability of time to discharge was significantly different among the 3 groups (P < .0001). It was shorter for patients with minor strokes classified by both BASIS and ASPECTS compared with the other 2 groups (both P < .0001), but the probability of time to discharge was not significantly different between BASIS major/ASPECTS minor and those classified as major by both scales (P = .077).
Fig 2.
Kaplan-Meier curve of time to discharge. The Kaplan-Meier graph depicts the probability of discharge from hospital in days for patients classified as having major strokes by BASIS and ASPECT (solid line), major by BASIS but minor by ASPECT (dot and dash line), and minor by both classification instruments (dashed line). Overall, a highly significant difference (P < .0001) between the groups was found. In isolating the differences, both the BASIS major/ASPECT major and the BASIS major/ASPECT minor were significantly different from the BASIS and ASPECT minor group (P < .0001). However, there was no significant difference between the BASIS major/ASPECT major (solid line) and the BASIS major/ASPECT minor groups (P = .077).
Interestingly, of the 21 patients who were classified as having major strokes by BASIS and minor strokes by ASPECTS, 17 had proximal artery occlusions, including 2 whose basilar artery was occluded. Of these 17 with proximal occlusions, 8 patients' NCCTs were scored with perfect ASPECTS scores of 10 by both reviewers, indicating no detectable hypointensity on the NCCT that could be attributed to acute ischemia.
Outcomes in All Patients
When all of the patients, including those who had NCCT or MR imaging, but not an angiographic study, were combined, there were very significant differences in all of the outcome measures between the major and minor strokes by imaging groups. A total of 8 deaths occurred during hospitalization, and all were in the major stroke by imaging group. All involved the anterior circulation with ICA and/or MCA occlusion visualized in 5, and large MCA distribution parenchymal ischemic lesions observed in 3.
Among the survivors in the major stroke group, 72% were discharged to a rehabilitation facility, and 18% were discharged to home. Both of these outcomes were significantly worse than for the minor stroke group, of whom 17% were discharged to a rehabilitation facility and nearly 76% were discharged home (both P < .0001). The mean length of hospitalizations in days was longer for the major stroke patients (12.5 days; SE = 1.3 days) compared with those with minor stroke (3.2 days; SE = 0.2 days), which was significant (P < .0001).
Patients Who Underwent Thrombolytic Therapy
Sixteen patients were administered thrombolytic therapy (tPA). All had major strokes by imaging criteria and had outcomes comparable with the patients with major stroke who did not undergo thrombolytic therapy. Intravenous administration of tPA was given to 4 patients, and intra-arterial recanalization was attempted in 12 patients. Death during hospitalization occurred in 1 patient treated with intravenous tPA and 3 treated with endovascular therapy. Discharge to a rehabilitation facility occurred in 7 of the 12 surviving patients. Removal of these patients did not affect the outcome analyses. The odds ratio of a poor outcome for the treated group was not significantly different from patients with major stroke who were not treated.
Outliers
Potential weaknesses of the proposed classification system were probed by considering outliers with respect to LOS. For patients with minor stroke, those with LOS of 2 or more SDs greater than the mean of 3 days were identified. Two patients had an LOS of 8 days, and 1 had a 9-day LOS. One patient had a small infarct on follow-up imaging, underwent carotid endarterectomy during the hospitalization prolonging the LOS, and was discharged to home. Another had a small acute stroke on follow-up imaging but had multiple significant chronic strokes and was discharged to home with a persistent expressive aphasia. The third developed hematuria, underwent evaluation for it, and was discharged to acute rehabilitation with persistent upper extremity weakness.
Of the patients with major stroke, none were 2 SDs below the mean LOS for this group, and the 10% with the shortest LOS (3–5 days) were investigated. Five of the 6 patients were discharged to a rehabilitation facility. The one individual patient who was discharged to home presented with a left M2 occlusion, had a small stroke on follow-up imaging, and was discharged with minor weakness. Two others had mild symptoms and small infarcts on follow-up imaging, one had an MCA occlusion, whereas the other had an ICA occlusion but with distal reconstitution of the artery. Three had proximal MCA occlusions with large infarcts on follow-up imaging and were discharged to rehabilitation with significant neurologic deficits.
Discussion
A neuroimaging-based, ischemic stroke classification instrument, BASIS, was developed and tested in 205 consecutive ischemic stroke patients who had CTA or MRA as part of their initial imaging examination. BASIS classifies both anterior and posterior circulation strokes, and the primary assessment is of occlusions of proximal cerebral arteries with abnormalities of the parenchyma considered only if no major occlusions are observed. It was found to be highly effective in predicting poor outcomes and was independent of technique: classification by NCCT/CTA and MR imaging/MRA were equivalent. Importantly, the instrument was found to be significantly superior to ASPECTS, a well-validated method, in identifying patients (∼25% of patients) who have a high probability of poor outcomes despite a lack of significant parenchymal abnormalities on NCCT.
Stroke Classification Instruments and BASIS
Several clinical rating instruments have been used for the assessment of patients with acute stroke.6-8 Despite their value, they have deficiencies.9,10 The National Institutes of Health Stroke Scale (NIHSS) is a validated and the most widely used clinical rating instrument.1 Investigators improved predictive power by supplementing the NIHSS with MR imaging, sonography, and CTA data.11-14 However, clinical classification instruments are limited in their ability to assess the effects of treatments on arterial occlusions, and they provide little guidance on how therapy may be improved.2
Stroke classification instruments based on imaging alone have been proposed,5,9,13,15 including CTA source image data16 and CTA.17 Classification instruments based on diffusion and perfusion MR imaging have also been shown to have substantial predictive power.9,18-20 Recently, MR imaging and CT have been used to select patients for therapy outside the traditional 3-hour limit.21-23 BASIS incorporates many of the features of these previously described classification schemes, with the important advantage of being independent of technique. ASPECTS based on NCCT is the most widely used imaging-based instrument, has been reported to be superior to the NIHSS,5 and has been incorporated into the Interventional Management of Stroke-3 (IMS3), a large multicenter trial comparing placebo, intravenous, and combined intravenous–intra-arterial thrombolysis. Thus, ASPECTS is the standard to which any new imaging-based classification instrument must be compared.
Comparison of BASIS with ASPECTS
The comparison of BASIS with dichotomized ASPECTS in patients who had NCCT/CTA is summarized in Table 3. There was agreement between the 2 instruments in 22 major and all 44 of the minor stroke patients. Most interesting were the 21 patients who were classified as having major strokes by BASIS, minor strokes by ASPECTS, and were found to have poor outcomes. Seventeen of the patients had a proximal artery occlusion with normal or near normal NCCT. A reasonable hypothesis suggested by these observations is that BASIS is identifying a class of patient with proximal occlusions but with sufficient collateral flow to maintain parenchymal integrity at the time of imaging. It is possible that this class of patients has a higher probability of benefiting from effective recanalization therapy.
Source of BASIS Efficacy
The results reported here confirm and build upon work reported by Smith et al,3 who convincingly demonstrated the value of identifying a proximal cerebral artery occlusion by CTA in acute stroke patients. Using multivariate logistic regression analysis, they reported 2 variables that predicted poor neurologic outcome: baseline NIHSS score and the presence of intracranial large-vessel occlusion, defined similarly as proximal occlusions for BASIS. They concluded that performing routine intracranial vascular imaging on acute stroke patients may allow for more accurate determination of prognosis and may also guide therapy.3
The BASIS classification instrument is most likely effective because it is heavily influenced by proximal cerebral artery occlusion, the first event in the chain of causality that leads to major neurologic symptoms and large infarcts. A poor outcome in ischemic stroke follows an occlusion of a major artery that supplies eloquent parenchyma, if the occlusion persists long enough for the tissue to undergo infarction. Most stroke patients arrive at the hospital and are imaged hours after onset. If a proximal cerebral artery remains occluded there must be reasons (size or composition of the embolus, inadequate collateral flow, or insufficient “washout”)24 to account for its durability, and those factors are likely to ensure that a major infarction will ensue, especially if treatment is not instituted promptly. If a proximal cerebral artery occlusion spontaneously recanalizes, tissue outcome still depends on occlusion duration and collateral blood flow. If infarction has already occurred, it will be detectable with diffusion MR imaging and sometimes also by NCCT. If rapid recanalization occurs or if a peripheral artery is occluded, a small or no infarct is the result, and a good outcome is probable.
Applicability of BASIS
CTA and MRA are now widely available and are capable of accurate identification of proximal cerebral artery occlusion.25-27 The technique independence of the proposed instrument is particularly important. There are many patients who cannot have MR imaging because it is not available or because of exclusion criteria. As for CT, the administration of iodinated contrast media necessary for CTA may be contraindicated in some patients. The inclusiveness of the BASIS instrument is illustrated by the fact that 205 of 230 ischemic stroke patients admitted over the 33-week period studied had a CTA or MRA performed and suggests that it could be easily incorporated into clinical stroke trials. An additional important advantage of BASIS over systems such as ASPECTS is that it is applicable to both anterior and posterior circulation strokes.
Patients with minor strokes were more likely to have MR imaging/MRA and were imaged later compared with major stroke patients. This reflects stroke management in the ED. Patients with significant neurologic deficits are rapidly triaged to CT to exclude intracranial hemorrhage. The protocol is to perform CTA immediately after the NCCT. Those with minor or more subtle symptoms are managed with less urgency, and they are more likely to have minor strokes.
Future Directions
The positive results reported here suggest further investigations. Foremost is validation of BASIS in a prospective, blinded fashion. Its relationship to clinical rating scales and comparisons with other imaging-based classification instruments are desirable. The proposed system is amenable to modification for further stratification of stroke patients. For example, patients may be stratified according to presence of an occlusion and the presence or absence of a significant parenchymal image abnormality. Also, patients might be grouped by artery, such as basilar artery or ICA plus MCA occlusions. Such stratification may help define optimal approaches to specific occlusions. One interesting question is the prevalence of a salvageable, ischemic penumbra in patients with proximal cerebral artery occlusions. This would require, of course, the inclusion perfusion data that may be provided by CT or MR imaging.
A new acute ischemic stroke classification instrument, BASIS, was used to categorize a consecutive series of patients into 2 groups, major stroke or minor stroke, using criteria that are focused on proximal cerebral artery occlusions as detected by CT or MR angiography. BASIS is highly effective in identifying patients who are likely to have poor outcomes defined as death, discharge to rehabilitation, and long hospital stays. BASIS classification with NCCT/CTA is equivalent to MR imaging/MRA. In a comparison in patients who underwent NCCT/CTA, BASIS was able to identify a group of patients destined to have poor outcomes that was not identified by ASPECTS. This classification instrument may be useful in the evaluation of the therapeutic management of patients with acute ischemic stroke. If verified, it may useful for suggesting prognosis and helping to guide therapy in individual patients.
Footnotes
This work was supported in part by the Stroke Pathways Project, M. Steinberg, Harvard Graduate School of Design, and National Institutes of Health grant NS50041 (to R.G.G.).
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, June 9–14, 2007; Chicago, Ill.
References
- 1.Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–31 [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR. Treatment of acute stroke: still struggling. JAMA 2004;292:1883–85 [DOI] [PubMed] [Google Scholar]
- 3.Smith WS, Tsao JW, Billings ME, et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care 2006;4:14–17 [DOI] [PubMed] [Google Scholar]
- 4.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005;36:2121–25 [DOI] [PubMed] [Google Scholar]
- 5.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 [DOI] [PubMed] [Google Scholar]
- 6.Scandinavian Stroke Study Group. Multicenter trial of hemodilution in ischemic stroke–background and study protocol. Stroke 1985;16:885–90 [DOI] [PubMed] [Google Scholar]
- 7.Cote R, Battista RN, Wolfson C, et al. The Canadian Neurological Scale: validation and reliability assessment. Neurology 1989;39:638–43 [DOI] [PubMed] [Google Scholar]
- 8.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70 [DOI] [PubMed] [Google Scholar]
- 9.Linfante I, Llinas RH, Schlaug G, et al. Diffusion-weighted imaging and National Institutes of Health Stroke Scale in the acute phase of posterior-circulation stroke. Arch Neurol 2001;58:621–28 [DOI] [PubMed] [Google Scholar]
- 10.Orgogozo JM. Advantages and disadvantages of neurological scales. Cerebrovasc Dis 1998;8(suppl 2):2–7 [DOI] [PubMed] [Google Scholar]
- 11.Baird AE, Dambrosia J, Janket S, et al. A three-item scale for the early prediction of stroke recovery. Lancet 2001;357:2095–99 [DOI] [PubMed] [Google Scholar]
- 12.Molina CA, Alexandrov AV, Demchuk AM, et al. Improving the predictive accuracy of recanalization on stroke outcome in patients treated with tissue plasminogen activator. Stroke 2004;35:151–56 [DOI] [PubMed] [Google Scholar]
- 13.Nabavi DG, Kloska SP, Nam EM, et al. MOSAIC: Multimodal Stroke Assessment Using Computed Tomography: novel diagnostic approach for the prediction of infarction size and clinical outcome. Stroke 2002;33:2819–26 [DOI] [PubMed] [Google Scholar]
- 14.Verro P, Tanenbaum LN, Borden N, et al. Clinical application of CT angiography in acute ischemic stroke. Clin Neurol Neurosurg 2007;109:138–45 [DOI] [PubMed] [Google Scholar]
- 15.Kilpatrick MM, Yonas H, Goldstein S, et al. CT-based assessment of acute stroke: CT, CT angiography, and xenon-enhanced CT cerebral blood flow. Stroke 2001;32:2543–49 [DOI] [PubMed] [Google Scholar]
- 16.Coutts SB, Lev MH, Eliasziw M, et al. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke 2004;35:2472–76 [DOI] [PubMed] [Google Scholar]
- 17.Sims JR, Rordorf G, Smith EE, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment. AJNR Am J Neuroradiol 2005;26:246–51 [PMC free article] [PubMed] [Google Scholar]
- 18.Beaulieu C, de Crespigny A, Tong DC, et al. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol 1999;46:568–78 [DOI] [PubMed] [Google Scholar]
- 19.Lovblad KO, Baird AE, Schlaug G, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol 1997;42:164–70 [DOI] [PubMed] [Google Scholar]
- 20.Tong DC, Yenari MA, Albers GW, et al. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology 1998;50:864–70 [DOI] [PubMed] [Google Scholar]
- 21.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73 [DOI] [PubMed] [Google Scholar]
- 22.Hill MD, Demchuk AM, Tomsick TA, et al. Using the baseline CT scan to select acute stroke patients for IV-IA therapy. AJNR Am J Neuroradiol 2006;27:1612–16 [PMC free article] [PubMed] [Google Scholar]
- 23.Kohrmann M, Juttler E, Fiebach JB, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol 2006;5:661–67 [DOI] [PubMed] [Google Scholar]
- 24.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–82 [DOI] [PubMed] [Google Scholar]
- 25.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr 2001;25:520–28 [DOI] [PubMed] [Google Scholar]
- 26.Sheikh S, Gonzalez RG, Lev MH. Stroke CT angiography. In: Gonzalez RG, Hirsch J, Koroshetz W, et al, eds. Acute Ischemic Stroke: Imaging and Intervention. Berlin: Springer-Verlag;2006. :57–83
- 27.Vu D, Gonzalez RG, Schaefer P. Conventional MRI and MR angiography of stroke. In: Gonzalez RG, Hirsch J, Koroshetz W, et al, eds. Acute Ischemic Stroke: Imaging and Intervention. Berlin: Springer-Verlag;2006. :115–135