Abstract
BACKGROUND AND PURPOSE: Eccentric stenosis of the coronary artery is associated with plaque disruption and acute coronary syndrome. The purpose of the present study was to determine whether eccentric stenosis of the carotid artery contributes to cerebrovascular events.
MATERIALS AND METHODS: Of 6859 patients with vascular diseases who underwent duplex carotid ultrasonography, we studied 512 internal carotid arteries in 441 patients who had a maximum area stenosis at or more than 70%, which corresponds with approximately 50% or more by the NASCET method. The maximal (A) and minimal wall thicknesses (B) were measured on cross-sectional sonography images, and an eccentricity index was calculated using the following formula: (A − B)/A. Arteries in the lowest quartile of the eccentricity index (<0.69) were defined as having a concentric stenosis, whereas the others were defined as having eccentric stenosis. The underlying clinical characteristics and plaque morphologies, as well as the occurrence of ipsilateral ischemic stroke or transient ischemic attack in the preceding year, were compared between patients with eccentric and concentric stenosis.
RESULTS: Patient characteristics and plaque morphology were similar between the 2 groups. Cerebrovascular events occurred more frequently ipsilaterally to the artery with eccentric stenosis (13.5%) than to the artery with concentric stenosis (5.5%; P = .013); the difference was more evident when cerebrovascular events of presumed carotid arterial origin were assessed (P = .005). After adjusting for risk factors and plaque morphology, eccentric stenosis was independently related to the presence of recent cerebrovascular events (odds ratio = 2.76; 95% confidence interval = 1.19–6.40).
CONCLUSIONS: In patients with an area carotid stenosis of 70% or more, eccentric plaque was associated with a significantly increased incidence of ipsilateral cerebrovascular events compared with patients with concentric stenosis.
It is critical to identify patients with carotid stenosis that can lead to ischemic stroke. Several randomized prospective trials demonstrated that the degree of carotid stenosis is a common indicator that can be used to assess the risk of stroke.1–4 However, because most patients with carotid stenosis without surgical revascularization are free from occurrence or recurrence of ischemic cerebrovascular events after many years, the use of stenosis severity as a measure of stroke risk has a relatively poor specificity.5 Thus, additional indicators are needed to identify carotid artery lesions associated with a higher risk of stroke. The vulnerability of the carotid plaque morphology has been recognized as an important predictor for stroke.6 Hypoechoic7–10 or heterogenous10–12 plaques on sonography, as well as the presence of intraplaque hemorrhage or a thin or ruptured fibrous cap on multicontrast weighted MR imaging,13 have been reported to predict subsequent stroke. Plaque characteristics other than stenosis severity may be essential for assessing the risk of artery-to-artery embolism from a carotid plaque.
In the coronary artery, plaque distribution eccentricity is strongly associated with the acute coronary syndrome.14–19 A prospective observational study using intravascular sonography found that, in all of the acute coronary syndrome patients, the pre-existing coronary lesions showed eccentric stenosis.16 The reason for this association appears to be that the eccentric plaque is a vulnerable plaque and may indicate disruption and superimposed thrombus.17
In the carotid arteries, plaque eccentricity may also be an important marker of subsequent ischemic stroke. However, the correlation between the geometry of the carotid artery stenosis and cerebrovascular events has not been determined. The purpose of the present study was to identify differences in the clinical findings between eccentric and concentric carotid artery stenosis and to elucidate the relationship between the geometry of the stenosis and cerebrovascular events.
Methods
From January 2004 to February 2006, a total of 6859 consecutive patients were examined with duplex carotid ultrasonography in our hospital. Of them, 995 inpatients underwent ultrasonography for acute ischemic stroke, and the other patients were screened for carotid artery disease, because they had recent or chronic stroke, asymptomatic cerebrovascular disease, carotid bruit, vascular risk factors, cardiovascular disease, peripheral artery disease, or nonspecific symptoms, such as headache and dizziness. Of these, 370 patients had a plaque with maximum area stenosis at 70% or more in the unilateral internal carotid artery (ICA), and 71 patients had such plaques in both ICAs; these 512 arteries were studied. Area stenosis of the carotid artery at 70% or more on sonography roughly corresponds with stenosis of 50% or more by the North American Symptomatic Carotid Endarterectomy Trial Collaborators (NASCET) criteria.1 Arteries with severe calcification of which the degree of stenosis could not be measured due to the presence of an acoustic shadow on sonography were excluded from the study.
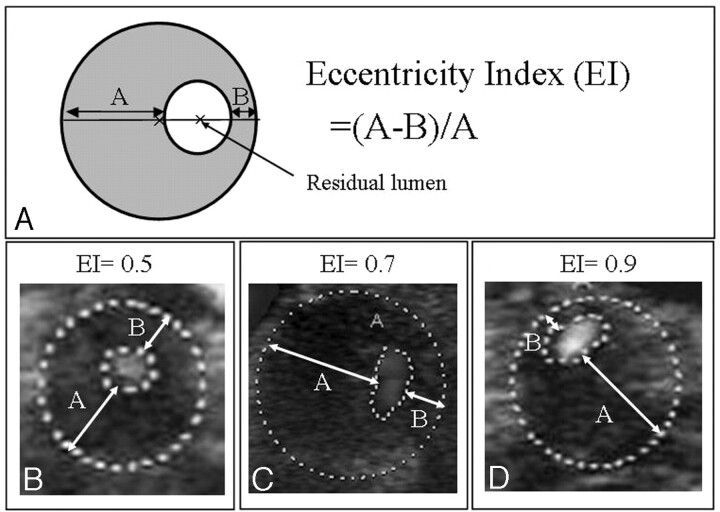
Sonography examination was performed using a duplex color-coded ultrasonographic device equipped with a linear array 7.5-MHz transducer (mainly Aplio XV; Toshiba, Tokyo, Japan). The most stenotic portion of the ICA was determined by using gray-scale and Doppler sonography, as described previously20; the cross-sectional image of the stenotic portion was stored on a computer hard disk together with the other sonography findings. Plaque morphology and the distribution of the stenotic portion were evaluated by investigators who were blinded to the patients’ clinical information. The maximal (A) and minimal (B) thicknesses of the vessel wall were measured on the cross-sectional image by using Scion Image (Scion, Frederick, Md.), and the eccentricity index of the plaque was calculated by using the following formula: (A − B)/A (Fig 1).16 The 512 ICAs studied were divided into quartiles based on the index; those in the lowest quartile were defined as having concentric stenosis, and those remaining were defined as having eccentric stenosis. The echogenicity of the carotid plaque was categorized as hypoechoic, isoechoic, or hyperechoic. A hypoechoic plaque was defined as having an echogenicity the same as that of the vessel lumen; an isoechoic plaque was defined as having an echogenicity of the soft tissues surrounding the carotid arteries; and a hyperechoic plaque was defined as having a brighter echogenicity than the surrounding soft tissues.7 A heterogeneous plaque was defined as containing a mixture of hypoechoic, isoechoic, or hyperechoic lesions.12
Fig 1.
AEccentricity index = (A − B)/A. B–D, Examples of stenotic internal carotid arteries with eccentricity index values of 0.5, 0.7, and 0.9, respectively.
The following clinical characteristics were evaluated: sex, age, hypertension (blood pressure of ≥140/90 mm Hg or use of antihypertensive medications), diabetes mellitus (fasting blood glucose ≥126 mg/dL, positive 75-g oral glucose tolerance test result, or use of insulin or oral hypoglycemic agents), hypercholesterolemia (serum total cholesterol ≥220 mg/dL or use of antihypercholesterolemic medications), ischemic heart disease, peripheral artery disease, aortic aneurysm, current smoking habit, and habitual alcohol consumption (≥2 drinks per day).
Recent cerebrovascular events, including ischemic stroke, transient ischemic attack (TIA), and transient monocular blindness (TMB) ipsilateral to the stenotic carotid artery within 1 year preceding the sonography study were reviewed from the medical records. For diagnosis of ischemic stroke, we required identification of culprit infarcts mainly on MR imaging, in addition to the episode of neurologic dysfunction. TIA was defined as a brief episode of neurologic dysfunction caused by focal brain or retinal ischemia, with clinical symptoms typically lasting less than 1 hour, and without evidence of acute infarction21; an episode caused by retinal ischemia was termed TMB here. As the cerebrovascular events of presumed carotid arterial origin, all of the cerebrovascular events other than those with the small infarct of less than 1.5 cm in diameter in the perforator arterial territory (lacune) and those with the high-risk sources of cardioembolism in Trial of Org 10172 in Acute Stroke Treatment classification22 were also assessed.
Statistical Analysis
Values are expressed as means ± SDs. The clinical variables of the concentric and eccentric plaque groups were compared by using Student t test for continuous variables and the χ2 test for categoric variables. To determine the predictors for cerebrovascular events, multivariate logistic regression analysis was performed. To ascertain the reasonableness of our dividing arteries into 2 groups by using the first quartile vale of the eccentricity index, we constructed a receiver operating characteristic (ROC) curve and obtained the eccentricity index as the cutoff point for discriminating between patients with recent cerebrovascular events and those without. Statistical test results were considered significant with a P value < .05. SPSS software (SPSS, Cary, NC) was used for the analyses.
Results
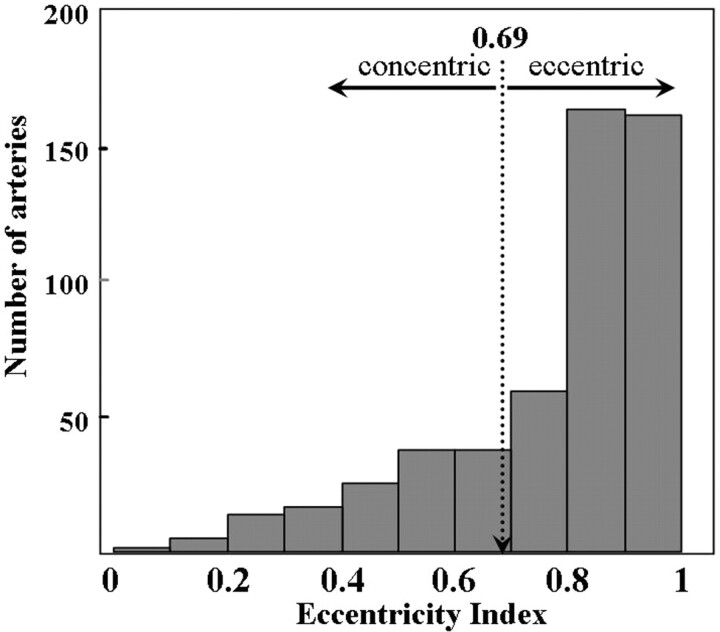
The distribution of stenosis geometry of the 512 ICAs by the eccentricity index is shown in Fig 2. The index varied from 0.00 to 0.99; the first quartile, median, and third quartile values were 0.69, 0.87, and 0.95, respectively. Thus, the index cutoff value between the lowest quartile (concentric stenosis, n = 128) and the other quartiles (eccentric stenosis, n = 384) was 0.69. There were no significant differences in patient characteristics and plaque morphology between ICAs with concentric and eccentric stenoses (Table 1).
Fig 2.
The eccentricity index histogram of 512 internal carotid arteries.
Table 1:
Patient characteristics and plaque morphology
| Variable | Concentric Stenosis (n = 128) | Eccentric Stenosis (n = 384) | P |
|---|---|---|---|
| Patient characteristics | |||
| Age, mean ± SD, y | 73.0 ± 7.5 | 72.4 ± 7.8 | .476 |
| Male, n (%) | 107 (84) | 339 (88) | .171 |
| Hypertension, n (%) | 116 (91) | 335 (87) | .306 |
| Diabetes mellitus, n (%) | 56 (44) | 159 (41) | .642 |
| Hypercholesterolemia, n (%) | 85 (66) | 232 (60) | .227 |
| Ischemic heart disease, n (%) | 54 (42) | 169 (44) | .719 |
| Peripheral artery disease, n (%) | 33 (26) | 88 (23) | .509 |
| Aortic aneurysm, n (%) | 13 (10) | 52 (14) | .319 |
| Current smoking habit, n (%) | 32 (25) | 79 (21%) | .292 |
| Alcohol consumption, n (%) | 28 (22) | 67 (17) | .264 |
| Plaque morphology | |||
| Degree of stenosis, mean ± SD, % | 83.5 ± 9.0 | 83.6 ± 8.0 | .909 |
| 70%–79%, n (%) | 48 (37) | 141 (37) | |
| 80%–89%, n (%) | 37 (29) | 131 (34) | .489 |
| 90%–99%, n (%) | 43 (34) | 112 (29) | |
| Hypoechoic, n (%) | 48 (38) | 135 (35) | .538 |
| Heterogeneous, n (%) | 91 (72) | 247 (64) | .097 |
Ipsilateral to the concentric ICA stenosis, 5 ischemic stroke events and 2 TMBs occurred in the preceding year; of these 7 events, 1 was a lacunar stroke, 1 was an event with the high-risk sources of cardioembolism, and the remaining 5 were events of presumed carotid arterial origin (Table 2). Ipsilateral to the eccentric stenosis, 36 stroke events, 9 TIAs, and 7 TMB attacks occurred in the preceding year; of these 52 events, 1 was a lacunar stroke, 2 were events with the high-risk sources of cardioembolism, and the remaining 49 were events of presumed carotid arterial origin. Overall cerebrovascular events occurred more frequently ipsilaterally to the ICA with eccentric stenosis (13.5%) than with concentric stenosis (5.5%; P = .013); the difference was more evident when cerebrovascular events of presumed carotid arterial origin were assessed (eccentric, 12.8% versus concentric, 3.9%; P = .005).
Table 2:
Comparison of cerebrovascular events between concentric stenosis and eccentric stenosis
| Cerebrovascular Events | Concentric Stenosis (n = 128), n(%) | Eccentric Stenosis (n = 384), n(%) | P |
|---|---|---|---|
| Overall events | 7 (5.5) | 52 (13.5) | .013 |
| Ischemic stroke | 5 (3.9) | 36 (9.4) | .048 |
| Transient ischemic attack | 0 (0) | 9 (2.3) | .073 |
| Transient monocular blindness | 2 (1.6) | 7 (1.8) | .601 |
| Events of presumed carotid arterial origin | 5 (3.9) | 49 (12.8) | .005 |
After adjustment for age, sex, vascular risk factors, and plaque morphology, eccentric stenosis was independently related to overall cerebrovascular events (odds ratio = 2.76; 95% confidence interval [CI] = 1.19–6.40) and to cerebrovascular events of presumed carotid arterial origin (odds ratio = 3.69; 95% CI = 1.40–9.71; Table 3). In addition to eccentric stenosis, current smoking habit and severe stenosis at 90% or more were independently related to overall cerebrovascular events (current smoking habit, odds ratio = 2.29 and 95% CI = 1.23–4.29 and severe stenosis, odds ratio = 2.26 and 95% CI = 1.27–4.03) and to cerebrovascular events of presumed carotid arterial origin (current smoking habit, odds ratio = 2.25 and 95% CI = 1.17–4.32 and severe stenosis, odds ratio = 2.53 and 95% CI = 1.39–4.58). On the ROC curve for indicating recent cerebrovascular events, the optimal cutoff value of the eccentricity index was more than 0.71, with a sensitivity of 88% and specificity of 30%.
Table 3:
Eccentric internal carotid artery stenosis as a predictor of recent cerebrovascular events
| Model | Overall Cerebrovascular Events |
Cerebrovascular Events of Presumed Carotid Arterial Origin |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P | |
| Model 1 | 2.60 | 1.15–5.90 | 0.022 | 3.46 | 1.34–8.90 | 0.010 |
| Model 2 | 2.76 | 1.20–6.34 | 0.016 | 3.63 | 1.40–9.43 | 0.008 |
| Model 3 | 2.76 | 1.19–6.40 | 0.018 | 3.69 | 1.40–9.71 | 0.008 |
Note:—Model 1 was adjusted for age and gender. Model 2 was adjusted for age, gender, and vascular risk factors (hypertension, diabetes mellitus, hypercholesterolemia, current smoking habit, and alcohol consumption). Model 3 was adjusted for age, gender, vascular risk factors, and plaque morphologies (degree of stenosis, hypoechoic plaque, and heterogeneous plaque). CI indicates confidence interval.
Discussion
This is the first study to investigate the association between the geometry of carotid artery stenosis and ipsilateral cerebrovascular events using ultrasonography. The major new finding was that, independent of underlying risk factors and other features of plaque morphology, eccentric ICA stenosis was associated with recent cerebrovascular events within the preceding year.
The carotid bifurcation often shows uneven development of atherosclerosis between the inner and outer walls because of different flow streamline patterns and shear stress.23 The coronary artery may have a similar tendency. A previous study showed that 81% of the coronary arteries examined by intravascular sonography were eccentric, with an eccentricity index at 0.5 or more, and such eccentric plaques were strongly associated with the acute coronary syndrome.16 Using the same 0.5 index cutoff value in the present study, eccentric carotid artery stenosis was present in 88% of all of the vessels. However, this percentage may be too high to use for appropriate comparisons of clinical features between eccentric and concentric stenosis. Instead, we used the first quartile value of the eccentricity index (0.69) to divide arteries into 2 groups. This value was close to the optimal cutoff value of the eccentricity index by the ROC curve analysis (0.71). Thus, eccentricity defined as an index at 0.69 or more appears to be appropriate.
The association of eccentric coronary artery stenosis with acute coronary syndrome is due to the presence of disruption of the eccentric plaque or superimposed thrombus.14–19 To some extent, the same morphologic features may explain the association between eccentric carotid plaques and cerebrovascular events. In addition, hemodynamic changes induced by eccentric stenosis may be an important factor that leads to cerebrovascular events. Relatively low shear stress is reported to play a critical role in the development of atherosclerosis and vulnerable plaques.6,23,24 A computational simulation study using carotid bifurcation models demonstrated that there were differences between eccentric and concentric stenosis with respect to the size of the poststenotic recirculation zone, as well as the severity and distribution of wall shear stress.5 Using the models, the deposition of platelet and monocyte-sized particles on the vessel wall was more distinct proximal to the eccentric stenosis than proximal to the concentric stenosis.25 This suggests that eccentric stenosis is more prone to platelet activation and aggregation due to attenuated platelet deposition and plaque growth, as well as rupture due to attenuated monocyte deposition. Thus, eccentric stenosis may have a high potential for thrombus formation, which may lead to an increased risk of cerebrovascular events.
Similar plaque morphology can be easily seen by using multidetector row CT (MDCT) angiography and some types of MR techniques.13,26–30 In a recent study on MDCT, the relationship between stroke symptoms and plaque morphology was assessed.30 In the study, expansive carotid remodeling was greater in patients with cerebral ischemic symptoms than in asymptomatic patients, though there was no significant difference in the plaque eccentricity between symptomatic and asymptomatic patients. Thus, MDCT and MR techniques, as well as ultrasonography, seem to be available for evaluation of the geometry of carotid artery stenosis.
In the present study, hypoechoic plaque and heterogenous plaque were not indicative of recent cerebrovascular events, though they were often reported to be risk factors in the events.7–12 This might be because of the small patient number for appropriate statistical analysis or because the echogenicity of the carotid plaque was not evaluated objectively and quantitatively by using the gray-scale median. Other limitations of the present study include the following reasons. First, carotid arteries with advanced calcification were excluded from the study, because the severe acoustic shadow did not allow the eccentricity index to be measured on sonography. In our study population, such arteries might account for 10% of the arteries with maximum area stenosis at 70% or more. Although the contribution of carotid calcification to cerebrovascular events is uncertain,27,28,31,32 the present results may have been affected by the exclusion of these calcified arteries from the analysis. Second, although the present retrospective analysis demonstrated an association between stenosis geometry and pre-existent cerebrovascular events, a prospective trial is required to assess the contribution of eccentric stenosis to future cerebrovascular events.
In patients with a cross-sectional area carotid stenosis of 70% or more on sonography, eccentric plaque with an eccentricity index at 0.69 or more was associated with a significantly increased incidence of recent ipsilateral cerebrovascular events compared with a cohort of patients with concentric stenosis. The presence of eccentric stenosis, as well as the severity of the stenosis, may be an important indicator for use in the selection of patients for surgical revascularization of the carotid artery.
Footnotes
This study was supported in part by the Research Grant for Cardiovascular Diseases (18C-5) from the Japanese Ministry of Health, Labour, and Welfare.
Paper previously presented at: Annual International Stroke Conference, February 7–9, 2007; San Francisco, Calif.
References
- 1.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 2.European Carotid Surgery Trialists Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–87 [PubMed] [Google Scholar]
- 3.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421–28 [PubMed] [Google Scholar]
- 4.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet 2004;363:1491–502 [DOI] [PubMed] [Google Scholar]
- 5.Tambasco M, Steinman DA. Path-dependent hemodynamics of the stenosed carotid bifurcation. Ann Biomed Eng 2003;31:1054–65 [DOI] [PubMed] [Google Scholar]
- 6.Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque. Current imaging methods and new perspectives. Stroke 2005;36:2764–72 [DOI] [PubMed] [Google Scholar]
- 7.Polak JF, Shemanski L, O'Leary DH, et al. Hypoechoic plaque at US of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology 1998;208:649–54 [DOI] [PubMed] [Google Scholar]
- 8.Mathiesen EB, Bonaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the Tromso Study. Circulation 2001;103:2171–75 [DOI] [PubMed] [Google Scholar]
- 9.Bock RW, Gray-Weale AC, Mock PA, et al. The natural history of asymptomatic carotid artery disease. J Vasc Surg 1993;17:160–71 [DOI] [PubMed] [Google Scholar]
- 10.Langsfeld M, Gray-Weale AC, Lusby RJ. The role of plaque morphology and diameter reduction in the development of new symptoms in asymptomatic carotid arteries. J Vasc Surg 1989;9:548–57 [DOI] [PubMed] [Google Scholar]
- 11.Sterpetti AV, Schultz RD, Feldhaus RJ, et al. Ultrasonographic features of carotid plaque and the risk of subsequent neurologic deficits. Surgery 1988;104:652–60 [PubMed] [Google Scholar]
- 12.AbuRahma AF, Wulu JT, Crotty B. Carotid plaque ultrasonic heterogeneity and severity of stenosis. Stroke 2002;33:1772–75 [DOI] [PubMed] [Google Scholar]
- 13.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke 2006;37:818–23 [DOI] [PubMed] [Google Scholar]
- 14.Ambrose JA, Winters SL, Stern A, et al. Angiographic morphology and the pathogenesis of unstable angina pectoris. J Am Coll Cardiol 1985;5:609–16 [DOI] [PubMed] [Google Scholar]
- 15.Ambrose JA, Winters SL, Arora RR, et al. Coronary angiographic morphology in myocardial infarction: A link between the pathogenesis of unstable angina and myocardial infarction. J Am Coll Cardiol 1985;6:1233–38 [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi M, Terashima M, Awano K, et al. Morphology of vulnerable coronary plaque: Insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 2000;35:106–11 [DOI] [PubMed] [Google Scholar]
- 17.Waxman S, Mittleman MA, Zarich SW, et al. Plaque disruption and thrombus in Ambrose's angiographic coronary lesion types. Am J Cardiol 2003;92:16–20 [DOI] [PubMed] [Google Scholar]
- 18.Tousoulis D, Davies G, Crake T, et al. Angiographic characteristics of infarct-related and non-infarct-related stenoses in patients in whom stable angina progressed to acute myocardial infarction. Am Heart J 1998;136:382–88 [DOI] [PubMed] [Google Scholar]
- 19.Ojio S, Takatsu H, Tanaka T, et al. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans. Coronary angiographic findings within 1 week before the onset of infarction. Circulation 2000;102:2063–69 [DOI] [PubMed] [Google Scholar]
- 20.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340–46 [DOI] [PubMed] [Google Scholar]
- 21.Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med 2002;347:1713–16 [DOI] [PubMed] [Google Scholar]
- 22.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 23.Zarins CK, Giddens DP, Bharadvaj BK, et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 1983;53:502–14 [DOI] [PubMed] [Google Scholar]
- 24.Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006;113:2744–53 [DOI] [PubMed] [Google Scholar]
- 25.Steinman DA, Poepping TL, Tambasco M, et al. Flow patterns at the stenosed carotid bifurcations: Effect of concentric versus eccentric stenosis. Ann Biomed Eng 2000;28:415–23 [DOI] [PubMed] [Google Scholar]
- 26.Yuan C, Kerwin WS, Yarnykh VL, et al. MRI of atherosclerosis in clinical trials. NMR Biomed 2006;19:636–54 [DOI] [PubMed] [Google Scholar]
- 27.Nandalur KR, Baskurt E, Hagspiel KD, et al. Carotid artery calcification on CT may independently predict stroke risk. AJR Am J Roentgenol 2006;186:547–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uwatoko T, Toyoda K, Inoue T, et al. Carotid artery calcification on multislice detector-row computed tomography. Cerebrovasc Dis 2007;24:20–26 [DOI] [PubMed] [Google Scholar]
- 29.Nandalur KR, Hardie AD, Raghavan P, et al. Composition of the stable carotid plaque: insights from a multidetector computed tomography study of plaque volume. Stroke 2007;38:935–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie AD, Kramer CM, Raghavan P, et al. The impact of expansive arterial remodeling on clinical presentation in carotid artery disease: a multidetector CT angiography study. AJNR Am J Neuroradiol 2007;28:1067–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culebras A, Otero C, Toledo JR, et al. Computed tomographic study of cervical carotid calcification. Stroke 1989;20:1472–76 [DOI] [PubMed] [Google Scholar]
- 32.Shaalan WE, Cheng H, Gewertz B, et al. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J Vasc Surg 2004;40:262–69 [DOI] [PubMed] [Google Scholar]