Abstract
SUMMARY: Carotidynia is an idiopathic syndrome characterized by pain over the carotid bifurcation without associated luminal pathologic process. The classification of carotidynia as a distinct disease rather than as a symptom has generated controversy in the literature. Recent reports, however, suggest that carotidynia is a distinct disease characterized by the presence of enhancing soft tissue in the carotid sheath. We describe findings of carotidynia on positron-emission tomography and CT that further support the classification of carotidynia as a distinct inflammatory disease.
Carotidynia, as described by Fay1 in 1927 and by the International Headache Society (IHS)2 in 1988, is an idiopathic neck pain syndrome characterized by radiating pain and tenderness over the common carotid bifurcation without apparent structural abnormality of the carotid artery. The classification of carotidynia has been controversial, with some authors suggesting that carotidynia is merely a symptom referable to multiple causes of neck pain rather than a clinically distinct disease.3,4 This controversy in the literature led to the removal of carotidynia as a clinically distinct entity from the IHS headache classification in 2004.
Recent reports in the radiology literature, however, have demonstrated the presence of amorphous enhancing soft tissue surrounding the carotid bifurcation on MR, CT, and sonography in patients with idiopathic carotidynia, suggesting the presence of an inflammatory process.5–10 In this report, we describe a patient with idiopathic carotidynia and [18F] fluorodeoxyglucose (18F-FDG) positron-emission tomography (PET)-CT findings that further support the classification of idiopathic carotidynia as a distinct inflammatory disease.
Case Report
A 68-year-old woman with a history of coronary artery disease presented with severe pain focally overlying the right carotid bifurcation. She reported no fever, headache, recent upper respiratory tract infection, visual disturbance, jaw claudication, history of migraine, or strokelike symptoms. Carotid pulses were symmetric without bruit, and blood pressure was symmetric in both arms. There was no palpable neck mass, but the patient reported severe pain radiating to the head and right shoulder with palpation (positive Fay sign).1 Laboratory work-up was significant for an elevated erythrocyte sedimentation rate (ESR) of 51 mm/h.
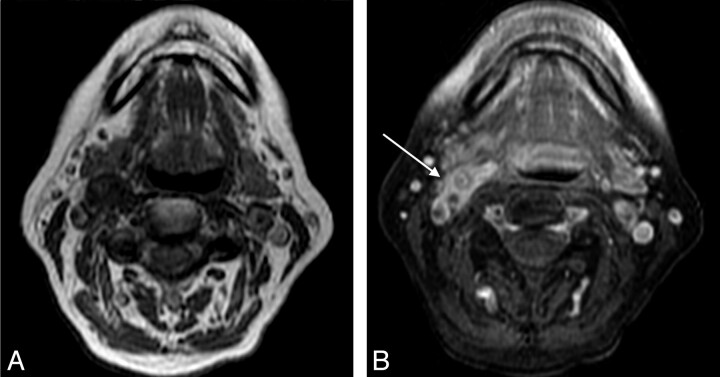
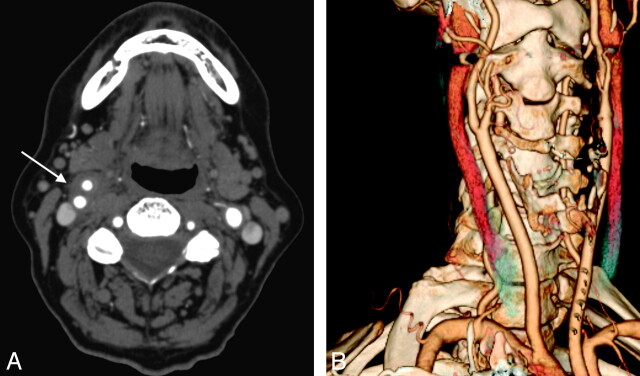
Carotid Doppler sonography evaluation (not shown) revealed echolucent wall thickening of the right carotid bulb without luminal abnormality or stenosis. MR imaging of the neck revealed a 1.6-cm length of amorphous soft tissue surrounding the right carotid bifurcation with marked enhancement and increased signal intensity on T2 fat-saturated images (Fig 1). CT angiography (CTA) of the neck showed similar amorphous soft tissue around the carotid bifurcation without luminal narrowing (Fig 2).
Fig 1.
Pregadolinium T1-weighted (A) and postgadolinium fat-saturated T1-weighted (B) MR images demonstrate focal enhancing amorphous soft tissue around the right carotid bifurcation (arrow).
Fig 2.
A, Axial CTA neck demonstrates soft tissue attenuation surrounding the right carotid artery bifurcation (arrow) without luminal compromise. B, A 3D volume-rendered CTA demonstrates no evidence for stenosis or dissection at the right carotid artery bifurcation.
There was no evidence on any study for carotid dissection, atherosclerosis, aneurysm, or soft tissue mass. There was no evidence for intracranial vasculitis or arterial stenosis on any study. The temporomandibular joints and cervical vertebrae were unremarkable for age. The styloid processes were not elongated to suggest Eagle syndrome or other stylalgias.11
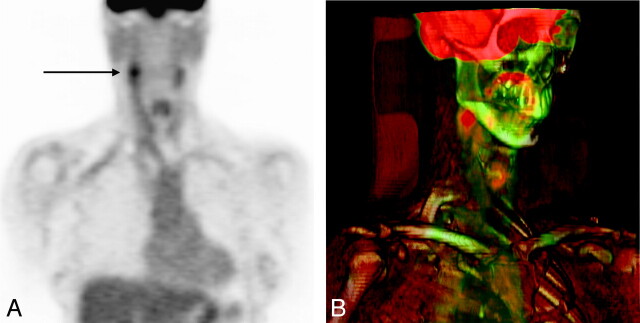
The CTA of the head revealed an incidental pituitary mass, resulting in an 18F-FDG PET-CT to exclude a systemic neoplastic or inflammatory process. PET-CT demonstrated a short segment of increased FDG activity corresponding to the region of soft tissue thickening within the right carotid sheath (Fig 3). The maximum standardized uptake value was 6.2 compared with 3.1 in the contralateral carotid sheath. There were no other areas of abnormal tracer activity in the remainder of the body. Brain MR imaging characterized the incidental pituitary mass as a Rathke cleft cyst.
Fig 3.
Coronal 18F-FDG PET (A) and corresponding 3D volume-rendered PET-CT (B) fusion images demonstrate moderately increased FDG activity confined to the right carotid bifurcation. Maximum standardized uptake value is 6.2 compared with 3.1 on the contralateral carotid artery. No other abnormal vascular FDG activity is identified to suggest a systemic vasculitis.
The patient's elevated ESR made giant cell arteritis and Takayasu arteritis differential considerations. However, the distribution of FDG activity only around the carotid bifurcation and the absence of typical symptoms suggested against these diagnoses. With a presumptive diagnosis of carotidynia, aspirin therapy was considered but was withheld because of the patient's use of clopidogrel. Without intervention, the patient's symptoms resolved in 2 weeks.
Discussion
The classification of carotidynia as a clinically distinct disease has generated much controversy in the literature. A review by Biousse and Bousser3 suggested that none of the diagnostic criteria proposed by the IHS in 1988 are consistently present in the reported cases of carotidynia and that carotidynia should be considered a “nonvalidated entity.” Hill and Hastings4 concluded that carotidynia is a “painful symptom rather than a disease” referable to migraine, viral pharyngitis, or luminal carotid disease in most cases. This controversy in the literature ultimately led to the removal of carotidynia from the second IHS headache classification in 2004.
Much of the controversy regarding carotidynia, however, has likely arisen from inconsistent use of the term to refer to both the symptom of pain over the carotid artery and to the idiopathic syndrome described by the IHS.5,9 The differential diagnosis of carotid pain includes dissection, aneurysm, atherosclerotic occlusion, and large-vessel vasculitis.4,12,13 Other causes of unilateral neck pain include migraine, trigeminal neuralgia, pharyngitis, head and neck neoplasm, temporomandibular joint disease, cervical spondylosis, and Eagle syndrome (stylalgia).3,4,11,14
Although these conditions account for many patients presenting with carotid pain, multiple reports have described a subset of patients with self-limiting carotidynia in whom none of these causes is found after careful work-up. MR, CT, and sonography imaging in these patients demonstrates amorphous enhancing soft tissue surrounding the carotid bifurcation without luminal compromise.5–10 The findings seen on MR, CT, and sonography in our patient are similar to what has been described in the literature. The PET-CT findings of idiopathic carotidynia, however, have not been described previously. We have shown that the enhancing soft tissue around the carotid bifurcation corresponds to a focus of glucose hypermetabolism surrounding the carotid bifurcation without evidence of systemic vasculitis or carotid luminal pathologic changes.
By itself, glucose hypermetabolism is nonspecific and can be seen in inflammatory and neoplastic processes. The absence of a cervical mass and the self-limiting clinical course are more consistent with inflammation as an explanation for increased FDG activity. An inflammatory cause is further supported by multiple reports demonstrating resolution of imaging abnormalities with follow-up.5–8 One report confirmed histologic findings of chronic inflammation in 1 patient with idiopathic carotidynia.15
In the case of our patient, PET-CT, CTA, and MR imaging were complementary studies in excluding many causes of unilateral neck pain including carotid dissection, atherosclerotic occlusion, systemic vasculitis, head and neck mass, cervical spondylosis, and pharyngitis. Furthermore, our patient did not have a history of migraine or upper respiratory tract infection. Having excluded the major differential diagnoses of carotid pain, we conclude that the findings in our patient are best explained by a clinically distinct disease characterized by focal inflammation around the carotid bifurcation.
The term carotidynia has been used in the literature imprecisely to refer to multiple disease entities. To prevent future confusion in the literature, new terminology should be adopted to refer to the idiopathic carotid pain syndrome discussed in this report. Tardy et al9 propose the use of the term idiopathic carotiditis and make specific recommendations for its diagnosis. We concur with this more precise terminology and suggest its use in future studies of this condition.
References
- 1.Fay T. Atypical neuralgia. Arch Neurol Psychiatry 1927;18:309–15 [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society. Classification and diagnosis criteria for headache disorders, cranial neuralgias, and facial pain. Cephalalgia 1988;8 (Supp 7):1–96 [PubMed] [Google Scholar]
- 3.Biousse V, Bousser M. The myth of carotidynia. Neurology 1994;44:993–95 [DOI] [PubMed] [Google Scholar]
- 4.Hill LM, Hastings G. Carotidynia: a pain syndrome. J Fam Pract 1994;39:71–75 [PubMed] [Google Scholar]
- 5.Burton BS, Syms MJ, Petermann GW, et al. MR imaging of patients with carotidynia. AJNR Am J Neuroradiol 2000;21:766–69 [PMC free article] [PubMed] [Google Scholar]
- 6.Buetow MP, Delano MC. Carotidynia. AJR Am J Roentgenol 2001;177:947. [DOI] [PubMed] [Google Scholar]
- 7.Arning C. Ultrasonography of carotidynia. AJNR Am J Neuroradiol 2005;26:201–02 [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka N, Sagoh T, Uematsu H, et al. Imaging by multiple modalities of patients with a carotidynia syndrome. Eur Radiol 2007;17:2430–33 [DOI] [PubMed] [Google Scholar]
- 9.Tardy J, Pariente J, Nasr N, et al. Carotidynia: a new case for an old controversy. Eur J Neurol 2007;14:704–05 [DOI] [PubMed] [Google Scholar]
- 10.Kuhn J, Harzheim A, Horz R, et al. MRI and ultrasonographic imaging of a patient with carotidynia. Cephalalgia 2007;26:483–85 [DOI] [PubMed] [Google Scholar]
- 11.Beder E, Ozgursoy OB, Karatayli Ozgursoy S, et al. Three-dimensional computed tomography and surgical treatment for Eagle's syndrome. Ear Nose Throat J 2006;85:443–45 [PubMed] [Google Scholar]
- 12.Shah Q, Messé SR. Cervicocranial arterial dissection. Curr Treat Options Neurol 2007;9:55–62 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt WA, Seipelt E, Krause A, et al. Carotidynia in Takayasu arteritis. J Rheumatol 2007;34:231–32 [PubMed] [Google Scholar]
- 14.Sjaastad O, Bakketeig LS. The rare, unilateral headaches. Vȧgȧ study of headache epidemiology. J Headache Pain 2007;8:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upton PD, Smith JG, Charnock DR. Histologic confirmation of carotidynia. Otolaryngol Head Neck Surg 2003;129:443–44 [DOI] [PubMed] [Google Scholar]