Abstract
BACKGROUND AND PURPOSE: Four-section multisection CT angiography (MSCTA) accurately detects aneurysms at or more than 4 mm but is less accurate for those less than 4 mm. Our purpose was to determine the accuracy of 64-section MSCTA (64MSCTA) in aneurysm detection versus combined digital subtraction angiography (DSA) and 3D rotational angiography (3DRA).
MATERIALS AND METHODS: In a retrospective review of patients studied because of acute symptoms suspicious for arising from an intracranial aneurysm, 63 subjects were included who had undergone CT angiography (CTA). Of these, 36 underwent catheter DSA; all but 4 were also studied with 3DRA. The most common indication was subarachnoid hemorrhage (SAH; n = 43). Two neuroradiologists independently reviewed each CTA, DSA, and 3DRA.
RESULTS: A total of 41 aneurysms were found in 28 patients. The mean size was 6.09 mm on DSA/3DRA and 5.98 mm on 64MSCTA. κ was excellent (0.97) between the aneurysm size on 64MSCTA and DSA/3DRA. Ultimately, 37 aneurysms were detected by DSA/3DRA in 25 of the 36 patients who underwent conventional angiography. The reviewers noted four 1- to 1.5-mm sessile outpouchings only on 3DRA; none were considered a source of SAH. One 64MSCTA was false positive, whereas one 2-mm aneurysm was missed by CTA. The sensitivity of CTA for aneurysms less than 4 mm was 92.3%, whereas it was 100% for those 4–10 mm and more than 10 mm, excluding the indeterminate, sessile lesions.
CONCLUSIONS: In comparison with the available literature, 64MSCTA may have improved the detection of less than 4-mm aneurysms compared with 4- or 16-section CTA. However, the combination of DSA with 3DRA is currently the most sensitive technique to detect untreated aneurysms and should be considered in suspicious cases of SAH where the aneurysm is not depicted by 64MSCTA, because 64MSCTA may occasionally miss aneurysms less than 3–4 mm size.
Aneurysms are one of the most important causes of subarachnoid hemorrhage (SAH), with a fatality rate between 40% and 60%, whereas misdiagnosis is associated with further increased morbidity and mortality.1,2 Traditionally, catheter digital subtraction angiography (DSA) has been considered the “gold standard” for aneurysm detection; currently, 3D rotational DSA (3DRA; obtained via the catheter angiogram) may offer increased aneurysm detection, with improved visualization of an aneurysm's configuration and contour compared with DSA alone.3–6 However, the combination of DSA/3DRA is invasive, time consuming, and may involve neurologic complications in 1%–2%.7,8 Hence, an accurate, noninvasive test would be invaluable in the emergent screening for SAH.
In this regard, multisection CT angiography (MSCTA) has shown potential in the noninvasive detection of intracranial aneurysms. Recent literature has demonstrated a high accuracy of detecting aneurysms more than 3–4 mm in size using 4-section (4MSCTA) and 16-section (16MSCTA) in the ranges of 92%–100%, but with a much lower sensitivity in detecting smaller aneurysms (<3–4 mm), in the range of 74%–84%.9–13 With the advent of newer, faster multidetector CT scanners, there can be thinner collimation, improved z-axis resolution, and contrast bolus timing, potentially leading to improved detection of these smaller aneurysms. Because there has been little literature comparing combined DSA/3DRA with 64-section MSCTA (64MSCTA), our purpose was to evaluate the accuracy of 64MSCTA in aneurysm detection with special attention to smaller (<4 mm) aneurysms.
Methods
Internal review board approval was obtained for this study.
Patient Selection
In a retrospective review of a 1-year period (May 2006 through April 2007), patients who had clinical histories requesting urgent evaluation for intracranial aneurysm via 64MSCTA (n = 66) were identified via CT logs. Three of these 66 patients were then excluded who had undergone clipped/coiled aneurysms (due to the presence of streak artifact), significant trauma (due to the unlikelihood of an aneurysm being present), or SAH with delayed presentation to CT angiography (CTA) (>24 hours, to exclude cases of vasospasm).
Review of Cases
The 64MSCTA was typically performed within an hour of presentation or detection of SAH. The interpretation of these acute examinations was performed emergently by the combination of an on-call resident physician with the staff neuroradiologist immediately after the procedure. For the purposes of this study, 2 staff neuroradiologists with experience in catheter and CTA (A.M.M. and C.S.P.), retrospectively, and independently reviewed the examinations in the remaining 63 patients via noncontrast CT and CTA. When reviewing the CTA examinations, the reviewers were blinded to each other and to the DSA/3DRA images and results, but when evaluating the DSA/3DRA results, the neuroradiologists were not blinded to the initial noncontrast CT/CTA findings. This methodology was used to simulate the typical emergent work-up of aneurysms, where the CTA results were usually available at the time of DSA evaluation. In addition, our intent was to make sure that questionable areas on the CTA were evaluated on the DSA/3DRA to include false-positives detected by CTA. In cases of interobserver discrepancies as to whether an aneurysm was present, the reviewers settled these discrepancies via consensus. DSA and 3DRA (when present) or surgical results were accepted as the “gold standard” for the presence of aneurysm. After obtaining consensus as to the presence of aneurysm, the maximum size measurements of the aneurysms were obtained. Each CTA's quality was also graded as “good” (diagnostic quality with adequate arterial visualization on 3D-volume rendered [3D-VR], maximum intensity projection [MIP], and multiplanar reformats [MPRs]); “fair” (mildly limited 3D reconstruction visualization of arterial structure due to contrast bolus or motion, though with adequate MPRs); “poor” (severely limited [but visible] arterial visualization with inadequate MPR images); or “failed” (complete lack of visible arteries).
CTA Technique
CTAs were obtained by a 64-channel multidetector CT scanner (Brilliance CT; Philips Medical Systems, Best, the Netherlands), located in the emergency department. An 18- or 20-gauge needle was placed in the antecubital vein. The CTAs were initiated via “triggering” off of the aortic arch at an HU threshold of 140 HU after the intravenous contrast bolus was initiated; this delay varied, but typically ranged from 10–25 seconds. Contrast material (Iohexol 350 [Omnipaque]; GE Healthcare Ireland, Cork, Ireland) was injected at a rate of 4 mL/s via power injection for a total volume of 80 mL in each study. The scanning parameters included 120 kV, effective milliamps of 300 per section, collimation of 0.67 mm, a reconstruction interval of 0.9 mm with a 0.45 mm overlap, and a table speed of approximately 40 mm/s. The section revolution time was 0.75 seconds. Data for the CTA were obtained in a caudal-cranial direction from the level of approximately C1–2 up through the vertex of the skull, for a scan time of 4–6 seconds.
The CTA data were sent via PACS and hospital intranet to workstations that have Vitrea 2 3D reconstruction software (Vital Images, Minnetonka, Minn) integrated into a PACS workstation; the time necessary to transfer images from the CT technologist's workstation to the combined PACS/3D workstation was typically 3–5 minutes. Once on the workstation, it took approximately 2–3 seconds to generate preset 3D-VR images; MIP and MPR display algorithms were also reviewed. The neuroradiologist could manipulate the images in a near-infinite number of projections with various amounts of time (usually 5–10 minutes) needed for review. The source, 3D-VR, MIP, and MPR images, were initially reviewed emergently by an experienced neuroradiologist and later by the 2 reviewers independently, who did not read the initial, emergent report. Figs 1–3 illustrate examples of CTA surveys of aneurysms. Outpouchings of less than 2 mm in size with a vessel arising from the apex of the cone were considered infundibula. After the 2 reviewers reached consensus as to the presence of an aneurysm in each positive MSCTA and after the 3DRA sequences (if available) were reviewed, a single staff neuroradiologist reviewer (A.M.M.) measured the aneurysm's maximum size on each positive CTA in a similar projection as that of the 3DRA to obtain a correlation of the maximum size between modalities. The largest diameter of each aneurysm was measured in millimeters to 1 decimal place and graded as large (>10 mm), medium (4–10 mm), or small (<4 mm), in accordance with previous work.10
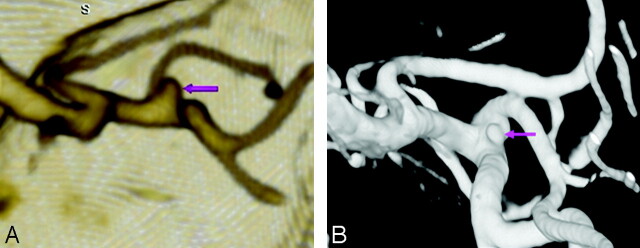
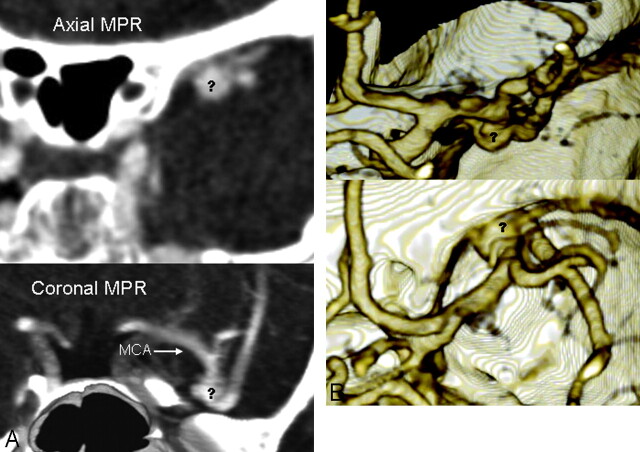
Fig 1.
An 83-year-old woman with SAH and an aneurysm less than 4 mm. Emergent CTA showed a 2-mm MCA bifurcation aneurysm. This was difficult to visualize on conventional DSA (not shown) but was confirmed on 3DRA (B) and surgically.
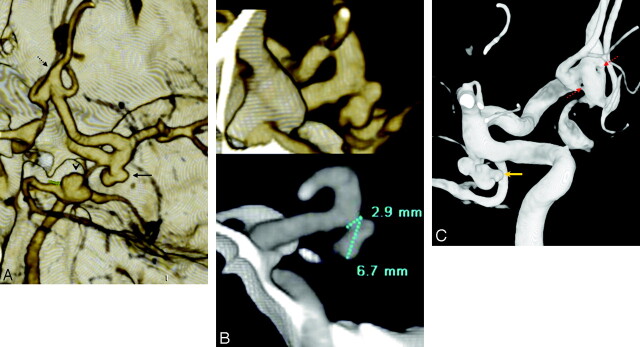
Fig 2.
A 47-year-old woman with SAH and multiple 4- to 10-mm aneurysms; aneurysm measurement technique is demonstrated. MSCTA showed 3 aneurysms: a 7 mm basilar tip aneurysm (arrowhead, A), a right posterior communicating artery (PcomA) segment aneurysm (solid arrow, A), and a fenestrated, fusiform anterior communicating artery (AcomA, dashed arrow, A). Sectioned 3D (B, top) and MPR (B, bottom) images were used to measure the PcomA aneurysm. 3DRA demonstrated the PcomA (gold arrow denotes the site of hemorrhage, C) and the double-fenestration AcomA (dashed red arrows, C).
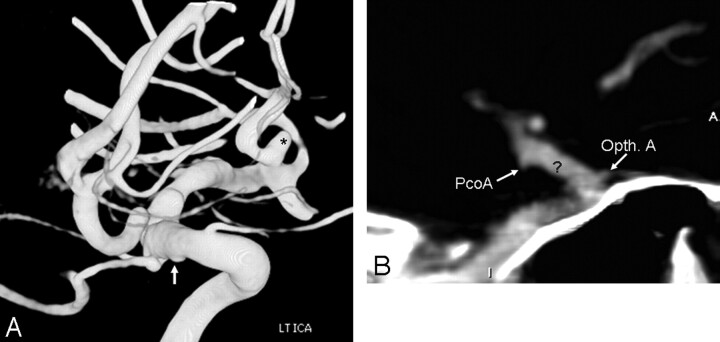
Fig 3.
A 78-year-old woman with SAH from an aneurysm more than 10 mm in size. MSCTA showed a 12-mm aneurysm in the periophthalmic ICA segment on 3D-VR images (data not shown), with peripheral calcifications, best seen on MPR (short white arrows, A). The aneurysm was noted to be separate from the ophthalmic artery origin on 3DRA (black arrow, B).
DSA and 3DRA Techniques
DSA was performed with femoral catheterization by the Seldinger technique with a biplane DSA unit that has rotational capabilities (Integris Allura; Philips Medical Systems). Typically, 6–9 mL of nonionic contrast (Iodixanol 320 [Visipaque]; Amersham Health AS, Oslo, Norway) was used per run, usually consisting of one anteroposterior (AP), 1 lateral, and 1–2 oblique views. The runs consisted of a 38-cm FOV (AP), 30 cm FOV (lateral and oblique), and a 1024 × 1024 matrix. The spatial resolution was 0.32 × 0.32 mm.
While the catheter was within each of the 3 major arteries (bilateral internal carotid and ≥1 vertebral artery), standard AP, lateral, and oblique DSA runs were obtained; a single rotational 3DRA acquisition was typically obtained before removing the catheter from each vessel; if the contralateral vertebral artery was not visualized on 3DRA, then a single contralateral vertebral artery DSA run was performed to clear the posterior inferior cerebellar artery. 3DRA was performed in each patient who underwent DSA unless there was clinical necessity based on patient instability and emergent need to treat, such as ventriculostomy manipulation or deteriorating mental status (n = 4). The 3DRA acquisition typically involved a 1- to 3-second delay, followed by a 4-mL/s injection for a total of 16–20 mL; the tube rotation arc was 240° (only 1 rotation used) with a rotation time of approximately 4.0–4.5 seconds. These were reconstructed in a 256 × 256 matrix.
The 3DRA runs were sent immediately on completion of the procedure to an adjacent 3D workstation (Integris 3DRA; Philips Medical Systems); the time to transfer images was typically 1–2 minutes. If a 3DRA run was not performed (n = 4), the size of each aneurysm was calculated on the DSA run after magnification correction. The source, unsubtracted images were also reviewed due to the possibility of artifacts in cases with significant metal present or patient motion. After determining consensus in each case as to the presence of an aneurysm, a single staff neuroradiologist reviewer (A.M.M.) measured each aneurysm's maximum size on 3DRA (if available) in a similar projection as that measured on the CTA to obtain a correlation of the maximum size between modalities. Again, the largest diameter of each aneurysm was measured in millimeters to 1 decimal place and graded as large (>10 mm), medium (4–10 mm), or small (<4 mm), in a fashion similar to the CTA measurements.
Statistical Analysis
For statistical analysis, 2 × 2 tables were constructed, with the sensitivity, specificity, positive/negative predictive values, and accuracy calculated both on a per-aneurysm and per-patient basis via comparison of MSCTA to 3DRA/DSA or surgical results. The detection of at least 1 aneurysm was assumed positive for each of the 63 patients when calculating the data on a per-patient basis. Pearson ρ was calculated to evaluate the correlation between the maximum aneurysm size as measured via 64MSCTA versus 3DRA; weighted κ values were calculated to evaluate the extent of the agreement between the 2 modalities regarding this size measurement. In addition, the Wilcoxon paired signed-rank test was performed to evaluate for significant differences in measurements of aneurysm sizes by using the 2 modalities. A κ value of 0.8 or above indicated excellent agreement; 0.6–0.8 indicated good agreement; 0.4–0.6 indicated moderate agreement, 0.4–0.2 indicated mild agreement, and less than 0.2 indicated no agreement. κ was also calculated to evaluate the interobserver correlation (between reviewers) for the presence or absence of aneurysm based on MSCTA.
Results
A total of 66 patients (35 women and 31 men; mean age, 54.5 years; age range, 14–93 years) clinically requiring emergent CTA for intracranial aneurysms presented during the 1-year period; 43 of these presented with SAH. Three of these patients were later excluded who had previously clipped aneurysms or SAH with delayed presentation and severe spasm. The reviewers then retrospectively reviewed each of the remaining 63 patients with CTA examinations.
Of the remaining 63 patients, a total of 41 aneurysms (in 28 patients) were suspected based on CTA. Of these 28 patients in whom an aneurysm was suspected on CTA, 25 patients underwent conventional angiography, whereas 37 aneurysms were confirmed by DSA or surgery (Table 1). Another 10 patients underwent conventional angiography after a negative CTA in the setting of SAH, all but 1 of which were negative; in 1, conventional angiography depicted an aneurysm on 3DRA that was missed on CTA by both reviewers. This false-negative CTA occurred in a patient with severe headache but without SAH, and the subsequent DSA/3DRA identified a 2-mm left internal carotid artery (ICA) periophthalmic segment aneurysm (Fig 4). However, 64MSCTA did not miss any ruptured aneurysms with SAH. One false-positive CTA did occur (without SAH), where a 6-mm MCA aneurysm was suspected and again visualized on repeat CTA but eventually excluded via DSA/3DRA; this was found to be a venous plexus in the expected location of the middle cerebral vein (Fig 5). One aneurysm was surgically confirmed without a catheter DSA. Four patients who underwent a catheter DSA did not have 3DRA due to an emergent need to perform therapy, instability of the patient, or significant patient motion not allowing 3DRA to be performed. The sensitivity, specificity, positive predictive value, negative predictive value (NPV), and accuracy of MSCTA in detecting each aneurysm size on a per-aneurysm and per-patient basis are shown in Table 2.
Table 1:
Aneurysm detection by MSCTA, confirmation by DSA/3DRA, and locations
| Aneurysm Size, mm | CTA: No. of Aneurysms* | DSA: No. of CTA True-Positives | DSA: No. of CTA False-Negatives | DSA: No. of CTA False-Positives | Aneurysm Locations (No.) of Each Size Range as on DSA |
|---|---|---|---|---|---|
| <4 | 15 | 13 | 1 | 0 | AcomA (1) |
| ACA: A1/A2 Bif (2) | |||||
| ICA: cavernous (1) | |||||
| ICA: periophthalmic (2) | |||||
| ICA: PcomA segment/origin (3) | |||||
| MCA: Bif/Trif (2) | |||||
| MCA: M2 segment (2) | |||||
| 4–10 | 20 | 18 | 0 | 1 | AcomA (4) |
| ICA: PcomA segment/origin (7) | |||||
| ICA: terminus (1) | |||||
| MCA: Bif/Trif (4) | |||||
| Basilar: tip (1) | |||||
| Basilar: midportion (1) | |||||
| >10 | 6 | 6 | 0 | 0 | AcomA (2) |
| ICA: periophthalmic (1) | |||||
| ICA: PcomA segment/origin (1) | |||||
| ICA: terminus (1) | |||||
| MCA: Bif/Trif (1) | |||||
| Total | 41 | 37 | 1 | 1 | AcomA (7) |
| ACA: A1/A2 Bif (2) | |||||
| ICA: cavernous (1) | |||||
| ICA: periophthalmic (3) | |||||
| ICA: PcomA segment/origin (11) | |||||
| ICA: terminus (2) | |||||
| MCA: Bif/Trif (7) | |||||
| MCA: M2 segment (2) | |||||
| Basilar: (2) |
Note:—PcomA indicates posterior communicating artery; AcomA, anterior communicating artery; Bif/Trif, bifurcation/trifurcation; ICA, internal carotid artery; MSCTA, multisection CT angiography; DSA, digital subtraction angiography; 3DRA, 3D rotational angiography; CTA, CT angiography; ACA, anterior cerebral artery.
Note: Number of aneurysms on CTA includes 2 patients with aneurysms who did not undergo DSA/3DRA.
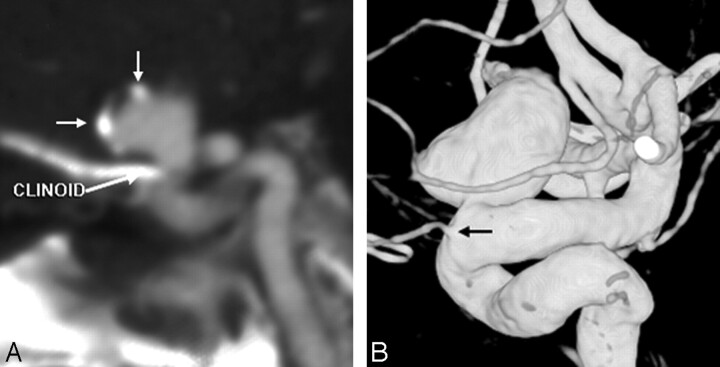
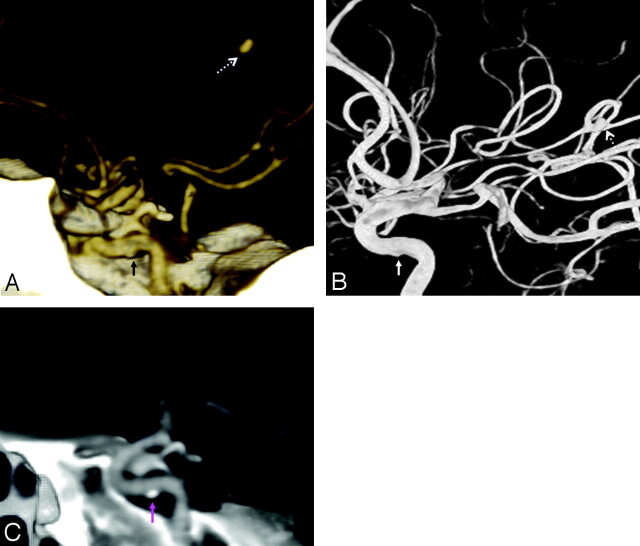
Fig 4.
The only false-negative CTA, in a 72-year-old man with severe headache, lacking hemorrhage on CT. However, the symptoms prompted an MR imaging/MR angiography (data not shown), with tiny infarcts and a questionable left supraclinoid ICA outpouching. Thereafter, the patient underwent catheter DSA to exclude vasculitis (which was negative), which showed a 2-mm periophthalmic aneurysm on 3DRA (dashed arrow, A). Closer, repeat review of the CTA showed the lesion projecting medially over the bony sella (arrow, B).
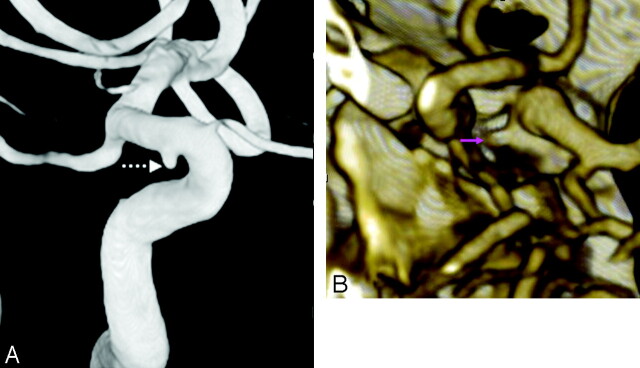
Fig 5.
The only false-positive CTA. A 38-year-old woman with confusion had a head CT negative for hemorrhage but with an isoattenuated structure in the region of the left MCA (data not shown) and corresponding flow void on T2-weighted MR imaging (data not shown), suspicious for aneurysm. Both 16-section (data not shown) and repeat 64-section CTA were performed, which showed a bizarre 6- to 7-mm outpouching (question marks, A and B) overlying the left MCA bifurcation on MPR axial (A, top) and coronal (A, bottom) and 3D MIP/VR posterior (B, top) and superior (B, bottom) views. This was considered a prominent middle cerebral venous plexus, because the catheter DSA and 3DRA (data not shown) were completely negative.
Table 2:
Accuracy of 64MSCTA on a per-aneurysm/per-patient basis, with κ and ρ per size
| Size on DSA/RA | Sensitivity of MSCTA, % | Specificity of MSCTA, % | PPV of MSCTA, % | NPV of MSCTA, % | Accuracy of MSCTA, % | κ/ρ (CTA versus DSA) Aneurysm Size |
|---|---|---|---|---|---|---|
| Overall | 97.4/96.0 | 90.0/90.0 | 97.4/96.0 | 90.0/90.0 | 95.8/94.3 | 0.965/0.967 |
| <4 mm | 92.3/90.9 | 100.0/100.0 | 100.0/100.0 | 90.9/90.9 | 95.2/95.6 | 0.711/0.753 |
| 4–10 mm | 100.0/100.0 | 90.0/90.9 | 94.7/92.3 | 100.0/100.0 | 95.8/96.4 | 0.841/0.861 |
| >10 mm | 100.0/100.0 | 100.0/100.0 | 100.0/100.0 | 100.0/100.0 | 100.0/100.0 | 0.476/0.888 |
Note:—MSCTA indicates multisection CT angiography; DSA, digital subtraction angiography; RA, rotational angiography; κ = κ reliability coefficient, ρ = ρ correlation coefficient; CTA, CT angiography. Data show a per-aneurysm/per-patient percentages.
Regarding the maximum size of each aneurysm, there was excellent correlation between the maximum size on CTA and 3DRA, as shown in Table 2 (Pearson ρ = 0.967; P < .0001), with no significant difference between the mean size on CTA (5.98 mm; SD, 3.62 mm) versus 3DRA (mean, 6.09 mm; SD, 3.75 mm) in those who underwent catheter 3DRA (Wilcoxon signed-rank test, P = .217). The κ was 0.965 overall, indicating excellent correlation between the maximum aneurysm size measured via both modalities.
In the patients with aneurysms on CTA confirmed by conventional angiography (n = 37), there were 13 aneurysms less than 4 mm in size, 18 aneurysms ranging from 4 to 10 mm, and 6 aneurysms at more than 10 mm by using the DSA/3DRA size as the standard (Table 1). On the basis of size, the sensitivity of 64MSCTA was 100% for “large” aneurysms more than 10 mm, 100% for aneurysms 4–10 mm, and 92.3% for aneurysms less than 4 mm. Of note, 4 tiny “blister-like” outpouchings less than 2 mm in size were noted in 4 patients on 3DRA that were not initially visualized by the reviewers on DSA or CTA; these were not tabulated as aneurysms (Fig 6). One was at the basilar tip, 1 at the carotid terminus, and 2 along the inferior aspect of the supraclinoid ICA in proximity to the anterior choroidal and posterior communicating artery origins. These outpouchings had a broad base, did not have a visible vessel arising from them, and had a relatively smooth appearance. Each of these patients had a larger aneurysm; 3 had SAH with an aneurysm elsewhere consistent with the site of hemorrhage. Repeat angiography in 2 of these patients (both with undersurface supraclinoid ICA lesions), both performed 2 weeks after presentation, demonstrated no increase in size. Hence, it was decided that these lesions were probably not the site of bleeding; meanwhile, the larger aneurysms had been treated. Notably, a fifth lesion seen on 3DRA on the undersurface of the supraclinoid ICA was later found to be focal atherosclerotic calcification (Fig 7).
Fig 6.
Blisterlike lesion in a 46-year-old man with SAH from a 3.5-mm MCA aneurysm, noted on CTA and 3DRA (asterisk, A). The sessile lesion was noted on the undersurface of the ICA and not noted on CTA 3D-VR (data not shown) or MPR (B) views. This was not changed on 2-week repeat catheter 3DRA.
Fig 7.
Atherosclerosis simulating a blister-like lesion. A 52-year-old woman with SAH and focal parenchymal hematoma on CT. CTA showed a 2.9-mm M3 mycotic aneurysm adjacent to the hematoma, also present on 3DRA (dashed arrows, A and B). However, a sessile outpouching was noted on the cavernous ICA undersurface (solid arrows, A and B). Further review of the CTA MPR images revealed this to be an atherosclerotic calcification (pink arrow, C).
Table 1 lists the location of aneurysms. Nine (32.1%) of the 28 patients suspected with aneurysm on CTA had more than 1; 1 patient with multiple aneurysms on CTA was never evaluated by DSA/3DRA. Of note, the only patient with a posterior inferior cerebellar artery (PICA) aneurysm (and multiple other aneurysms) was excluded due to the presence of internal carotid clips.
Regarding the quality of the CTA examinations, 58 (92.1%) of the 63 patients had “good” diagnostic quality. Two patients (3.2%) had “fair” CTA quality; in both, the use of MPR images enabled detection of the causative aneurysm, because the 3D-VR/MIP images were unreliable. Two patients (3.2%) had “poor” quality, 1 with SAH and a nondiagnostic CTA that suggested a 4-mm aneurysm (confirmed by DSA/3DRA). One patient with SAH had a completely failed CTA bolus due to injection site extravasation, and an aneurysm was later detected by DSA/3DRA.
Interobserver reliability on CTA was excellent (κ = 0.83). Two interobserver discrepancies were observed out of the 63 CTAs; both were later confirmed (by consensus) to be aneurysms on DSA/3DRA. One was a 2-mm aneurysm of the cavernous ICA; the other was 3 mm and along the periophthalmic ICA.
Discussion
Optimally, the work-up of SAH would include the use of noninvasive testing to efficiently guide therapy of intracranial aneurysms before embolization or surgery. The need for an initial noninvasive work-up by CTA cannot be understated; the current 64-section scanners can yield an evaluation of the cervical and cranial vasculature in the same sitting and can potentially decrease the amount of time necessary to perform conventional angiography, embolization, or even surgery by depiction of the vasculature and adjacent structures. Benefits of an initial, noninvasive work-up include knowledge of the anatomy before endovascular therapy, the ability to avoid the inherent risks of invasive conventional angiography, the ability to decide that a patient should immediately undergo surgery when endovascular therapy is not feasible, and the ability to detect other, less common causes of SAH.9–17 In addition, there is significant potential for MSCTA in cases where aneurysms are of low likelihood, such as SAH in patients after significant trauma or in patients without hemorrhage but with severe headaches and a positive family history of aneurysm. Hence, with the increasing speed, coverage, and resolution of MSCTAs, it has been increasingly accepted for screening of the craniocervical arteries.9–17
Over the past 10–12 years, the sensitivity and accuracy of CTA in detecting intracranial aneurysms has progressively improved during the evolution from single-section CTA (SSCTA) to 4MSCTA to the current routine use of 16MSCTA and 64MSCTA. The sensitivities in initial studies of SSCTA varied widely, ranging from 67% to 100%, with significant difficulty noted in detecting aneurysms less than 4 mm.17–22 When 4MSCTA emerged, results among several studies were also variable but largely demonstrated that the sensitivity was more than 90% in aneurysms at or more than 4 mm, whereas the results for aneurysms less than 4 mm in size were controversial; in particular, the NPVs were reported as far under 90% on a per-aneurysm basis of evaluation, suggesting that smaller aneurysms less than 4 mm could be missed by 4MSCTA.9–11 Similar but slightly improved results have been reported with 16MSCTA, with further improved NPVs and sensitivity, though there still remains some difficulty in aneurysms less than 3–4 mm.12,13 However, these studies did not compare the 3D images from the CTA with a 3D rotational acquisition obtained by conventional angiography, which also allows free rotation and visualization in an unlimited number of planes. Hence, the reported accuracy of MSCTA potentially could have been falsely elevated if compared only with catheter DSA. In this regard, 2 early reports using 64MSCTA for detecting aneurysms described preliminarily that the sensitivity was 92%–98% and the specificity 100%, with an NPV of 82%–99% in aneurysm detection compared with DSA; however, whereas 1 study did use 3DRA as a comparison, that study did not report reliability between modalities (measuring aneurysm sizes), and neither study reported interobserver reliability.14,15 Hence, we feel that our preliminary study using DSA/3DRA more precisely reports the sensitivity of 64MSCTA, where aneurysms smaller than 4 mm can be accurately depicted, because even the single, missed 2-mm aneurysm was present in retrospect.
The one false-negative and the one false-positive case both deserve mention as scenarios that can lead to misdiagnosis. In the false-negative case, a periophthalmic segment 2-mm aneurysm projected inferomedially over the sella (Fig 4). Similarly, the 2 cases of interobserver discrepancies on CTA were both less than 4 mm size, where aneurysms were later confirmed on DSA/3DRA; 1 was of the periophthalmic ICA adjacent to bone and the other of the cavernous ICA. Previous literature has shown a propensity to miss aneurysms in both locations due to their proximity to other attenuated structures, such as bone or enhancing cavernous sinus.9–12,23,24 Interestingly, we did not miss any cases of small posterior communicating segment ICA aneurysms, as in previous studies, which may be due to our use of MPR images and our criteria that an outpouching less than 2 mm in size with a vessel arising from the apex of the cone was considered an infundibulum.12,13,25–27 This discrepancy of what should be considered an infundibulum may be a cause for differences in detection rates between studies. In our 1 false-positive case, a focal venous plexus was overlying the MCA on both 16MSCTA and 64MSCTA examinations, without aneurysm on DSA/3DRA (Fig 5). Hence, it is important to note the presence of venous contrast, because a prominent venous phase has been described as a potential source of consternation.9–14,16
3DRA via conventional angiography has become increasingly used over the past 10 years in evaluating aneurysms, because the combination of 3DRA with DSA demonstrates more aneurysms than DSA alone.3–6 However, a 3DRA examination is still dependent on conventional angiography, which is invasive with inherent risks, involves a significant amount of time and patient preparation, and is potentially long when vasculature is tortuous and difficult to catheterize. CTA can potentially avoid many of these limitations; one such example is evaluating the anterior communicating artery in SAH, where the artery can be difficult to visualize after various manipulations. Similarly, the uncommon PICA aneurysm can be excluded in SAH cases via CTA, avoiding catheterization when a small or hypoplastic vertebral artery is present. Therefore, although 3DRA may currently be slightly more sensitive for aneurysm detection than 64MSCTA, CTA is invaluable in SAH and other scenarios of acute intracranial hemorrhage.
Regarding aneurysm size, there was excellent intermodality agreement in measuring the maximum aneurysm size on CTA versus 3DRA as noted by weighted κ and ρ values (Table 2), although within each dataset of aneurysm sizes, less agreement was noted, probably related to the smaller numbers within each category. The lack of a significant difference (P = .217) between mean aneurysm sizes measured via both modalities also suggests that 64MSCTA accurately depicts the true aneurysm size. However, the only way to determine this with certainty would be to intraoperatively measure each aneurysm.
The question may arise as to whether CTA can accurately measure aneurysms less than 4 mm in size. The way to directly address that question would be to compare CTA with surgical findings for a large number of cases of small aneurysms less than 4 mm in size. To our knowledge, this direct comparison for small aneurysms has not been performed between CTA and surgery, though some previous studies may offer some insight into this subject. First, an experimental model obtained via postmortem reconstruction (by using resin) of true aneurysms demonstrated that the volumes (though the actual aneurysms sizes are not reported) measured by 3DRA are minimally more accurate than CTA, though both slightly overestimate the volume, from 7% to 11%, without a statistically significant difference between CTA and 3DRA, as noted in our study.28 In a study by Tanoue et al5 comparing 3DRA with surgery, there is a table comparing the 3DRA neck size to surgery (no table for the maximum aneurysm sizes was reported), noting that the neck size was usually underestimated by 3DRA, even for the 2- to 3-mm necks. Villablanca et al29 describe CTA's accuracy in smaller aneurysms (<3–4 mm) compared with surgery or DSA, which they considered accurate in small aneurysm characterization, but they do not provide a correlation of size measurements between modalities or with surgery. Hence, it may be that CTA and/or 3DRA may slightly overestimate or underestimate the maximum aneurysm size based on the scant data available, but overall these are thought to be generally adequate for aneurysm size and neck size measurements to clinically decide whether to perform surgery or embolization.5,9,12,17,29 The determination as to whether 64MSCTA or 3DRA can accurately measure the size of smaller (<3–4 mm) aneurysms may necessitate a study directly comparing these modalities with surgically clipped tiny aneurysms or at least an experimental model reconstructed from cadavers.
The sessile, blister-like outpouchings noted only on 3DRA (Fig 6) created significant discussion at the time of treatment decision making. In all 4 of the patients there were larger aneurysms; these larger aneurysms were considered the sites of bleeding in the 3 with SAH. For several reasons, these blister-like lesions are indeterminate as to whether they should be considered as “blood-blister” aneurysms (BBAs), which lack the true outer layer of intima media that is typically seen in saccular aneurysms but retain the adventitia and a thin layer of fibrous tissue.30–32 First, the location of BBAs is usually on the superior/dorsal aspect of the supraclinoid ICA along the greater curvature; in our study, the location of both supraclinoid ICA sessile lesions was on the undersurface (lesser curvature) of the ICA.30–32 Second, BBAs usually occur apart from arterial branch sites; in 3 of the 4 tiny lesions that we noted, these occurred in expected locations of arterial branching without an arising artery visualized. Third, these lesions have shown a propensity to quickly increase in size in the setting of SAH within a matter of days or weeks, which did not occur in our 2 cases with repeat conventional angiography at 2 weeks.30–32 Fourth, BBAs have been described as usually between 2 and 8 mm; the sessile lesions in this study were all less than 2 mm.31 Finally, it is thought that atherosclerosis leads to the development of BBAs but was only mildly present in 2 of our 4 patients.30–32 Hence, although early BBA formation could not be entirely excluded regarding these sessile lesions, these did not exhibit the expected characteristics of BBAs.
We acknowledge several potential limitations of this study, including the retrospective nature and the number of patients. In addition, because most of the patients presented with SAH, expectation bias could lead to a falsely elevated sensitivity of 64MSCTA. In addition, there is no absolute evidence that an aneurysm was not present in the 64MSCTA patients deemed negative for aneurysm who did not undergo catheter DSA; however, there was 100% NPV in those who did undergo conventional angiography. Also, there is debate regarding the optimal technique for contrast bolus and area of coverage on MSCTA. For example, a “triggering” function can be used to time the optimal contrast bolus (as in our study) versus a standard delay time. In addition, although we typically scanned only the head on MSCTA, some may consider a combined head and neck MSCTA essential to evaluate the entire cervical vasculature before catheter DSA; however, this probably would cause increased interpretation time and radiation dosage and could lead to misinterpretation if bolus timing is optimized for the cervical rather than the cranial vasculature.
Other potential criticisms could also relate to the study design; some aspects of the study could be perceived as biased against MSCTA and others as biased in favor of MSCTA. In comparing the 64MSCTA results with the combination of DSA/3DRA, the reviewer was allowed to already know the results of the CTA, which could be considered as biased against MSCTA. This method of review was performed for 2 reasons. First, 64MSCTA needed to be compared against some standard, and because surgery was performed in only a minority, the combination of DSA/3DRA was used as that standard. Second, our intent was for the review of CTA and DSA/3DRA examinations to simulate the emergent work-up, where MSCTA would be the initial test. In reality, if a CTA has been performed, the angiographer does not perform the catheter angiogram unbiased and tailors the examination accordingly. Hence, it is difficult and potentially unrealistic to simulate blinding of the observer in this scenario, when the reviewer knows that, in actuality, conventional angiography is usually not performed in cases that have a high likelihood to be negative, such as in the setting of a negative CTA. Hence, the mere presence of a DSA/3DRA examination creates expectation bias. In addition, our use of the combination of DSA/3DRA as a comparison with MSCTA, rather than solely DSA (as in most previous studies) may be seen as a bias against MSCTA.9–13 However, we feel that using the DSA/3DRA as a comparison standard is more appropriate since this combination is more accurate in detecting and characterizing aneurysms than DSA alone, as suggested by previous literature.3–6
Potential biases of our study in favor of CTA could include the 4-mm cutoff size for small aneurysms and the discounting of the blister-like lesions less than 2 mm in size. The reason 4 mm (and not 3 mm) was used as a cutoff for small aneurysms was based on previous works in an attempt to use a similar system for comparison of literature, because previous works by Wintermark et al9 and Teksam et al10 have established that the sensitivity for aneurysms at or more than 4 mm was 97%–100% with 4MSCTA.11 Hence, our intent was to follow these works to focus the debate on the smaller aneurysms less than 4 mm in size, for which MSCTA has previously shown a lower sensitivity in aneurysm detection. Others have used 3 mm as a cutoff, which is also valid, and potentially future studies could use this threshold to focus on even smaller aneurysms.12,13 Regarding the potential bias in favor of CTA via our discounting of blister-like lesions less than 2 mm (noted only on 3DRA), it should be noted that, in these patients, the aneurysms that bled were from another site and that these lesions did not follow the typical configuration, appearance, and growth characteristics expected of blood-blister aneurysms, as discussed previously. To illustrate that we did not simply discard lesions just because they were tiny, we note that we did include 5 other 2-mm aneurysms, only 1 of which was confirmed as a bleeding site (via surgery), whereas the other 4 all had another larger aneurysm as the site of the hemorrhage.
Conclusion
This preliminary study suggests that 64MSCTA is excellent in the detection and delineation of intracranial aneurysms, and, in comparison with the available literature, is probably better than 4- or 16-section CTA; however, this is still not quite as sensitive as the combination of DSA/3DRA. Subsequently, if it is imperative to know whether there is an aneurysm, such as in the setting of SAH, and CTA does not reveal it, then DSA in combination with 3DRA is indicated, because 64MSCTA may still miss aneurysms less than 3–4 mm size in particular locations.
Footnotes
Data previously presented in part at: Annual Meeting of the American Society of Neuroradiology, June 11, 2007; Chicago, Ill.
Dr Charles L. Truwit currently serves on the medical advisory board of Vital Images (Minnetonka, Minn), the company that produces the 3D software used to view the CT angiographic images.
References
- 1.Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke 1994;25:2315–28 [DOI] [PubMed] [Google Scholar]
- 2.Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004;291:866–69 [DOI] [PubMed] [Google Scholar]
- 3.Sugahara T, Korogi Y, Nakashima K, et al. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002;23:1545–52 [PMC free article] [PubMed] [Google Scholar]
- 4.Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002;23:1199–1205 [PMC free article] [PubMed] [Google Scholar]
- 5.Tanoue S, Kiyosue H, Kenai H, et al. Three-dimensional reconstructed images after rotational angiography in the evaluation of intracranial aneurysms: surgical correlation. Neurosurgery 2000;47:866–71 [DOI] [PubMed] [Google Scholar]
- 6.Tu RK, Cohen WA, Maravilla KR, et al. Digital subtraction rotational angiography for aneurysms of the intracranial anterior circulation: injection method and optimization. AJNR Am J Neuroradiol 1996;17:1127–36 [PMC free article] [PubMed] [Google Scholar]
- 7.Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003;227:522–28 [DOI] [PubMed] [Google Scholar]
- 8.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317–20 [DOI] [PubMed] [Google Scholar]
- 9.Wintermark M, Uske A, Chalaron M, et al. Multislice computerized tomography angiography in the evaluation of intracranial aneurysms: a comparison with intraarterial digital subtraction angiography. J Neurosurg 2003;98:828–36 [DOI] [PubMed] [Google Scholar]
- 10.Teksam M, McKinney A, Casey S, et al. Multi-section CT angiography for detection of cerebral aneurysms. AJNR Am J Neuroradiol 2004;25:1485–92 [PMC free article] [PubMed] [Google Scholar]
- 11.Dammert S, Krings T, Moller-Hartmann W, et al. Detection of intracranial aneurysms with multislice CT: comparison with conventional angiography. Neuroradiology 2004;46:427–34 [DOI] [PubMed] [Google Scholar]
- 12.Tipper G, U-King-Im JM, Price SJ, et al. Detection and evaluation of intracranial aneurysms with 16-row multislice CT angiography. Clin Radiol 2005;60:565–72 [DOI] [PubMed] [Google Scholar]
- 13.Yoon DY, Lim KJ, Choi CS, et al. Detection and characterization of intracranial aneurysms with 16-channel multidetector row CT angiography: a prospective comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2007;28:60–67 [PMC free article] [PubMed] [Google Scholar]
- 14.Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients’ treatment. Neuroradiology 2006;48:787–94 [DOI] [PubMed] [Google Scholar]
- 15.Pozzi-Mucelli F, Bruni S, Doddi M, et al. Detection of intracranial aneurysms with 64 channel multidetector row computed tomography: Comparison with digital subtraction angiography. Eur J Radiol 2007;64:15–26 [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman MV, Mayo-Smith WW, Tung GA, et al. Detection of intracranial aneurysms: multi-detector row CT angiography compared with DSA. Radiology 2004;230:510–18 [DOI] [PubMed] [Google Scholar]
- 17.Villablanca JP, Martin N, Jahan R, et al. Volume-rendered helical computerized tomography angiography in the detection and characterization of intracranial aneurysms. J Neurosurg 2000;93:254–64 [DOI] [PubMed] [Google Scholar]
- 18.Lenhart M, Bretschneider T, Gmeinwieser J, et al. Cerebral CT angiography in the diagnosis of acute subarachnoid hemorrhage. Acta Radiol 1997;38:791–796 [DOI] [PubMed] [Google Scholar]
- 19.Liang EY, Chan M, Hsiang JH, et al. Detection and assessment of intracranial aneurysms: value of CT angiography with shaded surface display. AJR Am J Roengenol 1995;165:1497–1502 [DOI] [PubMed] [Google Scholar]
- 20.Preda L, Gaetani P, Rodriguez y Baena R, et al. Spiral CT angiography and surgical correlations in the evaluation of intracranial aneurysms. Eur Radiol 1998;8:739–45 [DOI] [PubMed] [Google Scholar]
- 21.Vieco PT, Shuman WP, Alsofrom GF, et al. Detection of circle of willis aneurysms in patients with acute subarachnoid hemorrhage: a comparison of CT angiography and digital subtraction angiography. AJR Am J Roentgenol 1995;165:425–30 [DOI] [PubMed] [Google Scholar]
- 22.Korogi Y, Takahashi M, Katada K, et al. Intracranial aneurysms: detection with three-dimensional CT angiography with volume rendering—comparison with conventional angiographic and surgical findings. Radiology 1999;211:497–506 [DOI] [PubMed] [Google Scholar]
- 23.Imakita S, Onishi Y, Hashimoto T, et al. Subtraction CT angiography with controlled-orbit helical scanning for detection of intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:291–95 [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto S, Kiura Y, Shibukawa M, et al. Subtracted 3D CT angiography for evaluation of internal carotid artery aneurysms: comparison with conventional digital subtraction angiography. AJNR Am J Neuroradiol 2006;27:1332–37 [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson GB, Steinke DE, Petruk KC, et al. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery 1999;45:1315–20 [DOI] [PubMed] [Google Scholar]
- 26.Velthuis BK, Van Leeuwen MS, Witkamp TD, et al. Computerized tomography angiography in patients with subarachnoid hemorrhage: from aneurysm detection to treatment without conventional angiography. J Neurosurg 1999;91:761–67 [DOI] [PubMed] [Google Scholar]
- 27.Karamessini MT, Kagadis GC, Petsas T, et al. CT angiography with three-dimensional techniques for the early diagnosis of intracranial aneurysms. Comparison with intra-arterial DSA and the surgical findings. Eur J Radiol 2004;49:212–23 [DOI] [PubMed] [Google Scholar]
- 28.Piotin M, Gailloud P, Bidaut L, et al. CT angiography, MR angiography and rotational digital subtraction angiography for volumetric assessment of intracranial aneurysms. An experimental study. Neuroradiology 2003;45:404–09 [DOI] [PubMed] [Google Scholar]
- 29.Villablanca JP, Jahan R, Hooshi P, et al. Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. AJNR Am J Neuroradiol 2002;23:1187–98 [PMC free article] [PubMed] [Google Scholar]
- 30.Abe M, Tabuchi K, Yokoyama H, et al. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg 1998;89:419–24 [DOI] [PubMed] [Google Scholar]
- 31.Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery 2000;47:578–83 [DOI] [PubMed] [Google Scholar]
- 32.Sim SY, Shin YS, Cho KG, et al. Blood blister-like aneurysms at nonbranching sites of the internal carotid artery. J Neurosurg 2006;105:400–05 [DOI] [PubMed] [Google Scholar]