Abstract
BACKGROUND AND PURPOSE: A cavernous angioma is a developmental vascular malformation with a high risk of hemorrhage. The purpose of this work was to retrospectively determine whether an MR sign of T1 hyperintense perilesional signal intensity is useful for the differentiation of cavernous angioma from other hemorrhagic cerebral masses.
MATERIALS AND METHODS: The institutional review board approved this study. We retrospectively evaluated the MR images of 72 patients with acute or subacute cerebral hemorrhagic lesions with perilesional edema (29 cavernous angiomas, 13 glioblastomas, 1 oligodendroglioma, 16 metastatic tumors, and 13 intracerebral hemorrhages) for the presence of T1 hyperintense perilesional signal intensity. In addition, T1 signal intensities of a perilesional edema were quantitatively analyzed. In cavernous angiomas, volumes of hemorrhagic lesions and perilesional edemas, lesion locations, presence of contrast enhancement, and time intervals between symptom onset and MR imaging were also assessed. Data were analyzed using unpaired t test or Fisher exact test.
RESULTS: T1 hyperintense perilesional signal intensity sign was found in 18 (62.1%) of 29 cavernous angiomas, in 1 (6.3%) of 16 metastases, and in 0 primary brain tumors or intracerebral hemorrhages. Sensitivity, specificity, and positive predictive value of this sign for cavernous angioma were 62%, 98%, and 95%, respectively. The perilesional T1 hyperintensity was significantly higher in cavernous angiomas (P = .045) than in normal white matter. Perilesional edema volumes were larger in cavernous angiomas with the MR sign than in cavernous angiomas without the sign (P = .009).
CONCLUSION: When the MR sign of T1 hyperintense perilesional signal intensity is present, there is a high probability of cavernous angioma being present in the brain, and this MR sign may be helpful for differentiating cavernous angioma from hemorrhagic tumors and intracerebral hemorrhages.
A cavernous angioma, also known as a cavernous malformation or cavernoma, is a developmental vascular malformation that is typically a discrete multilobulated, berrylike lesion containing hemorrhage in various stages of evolution. Hemorrhage is a common complication of a cavernous angioma and is the cause of the first presentation in 30% of cases.1,2 The reported annual risk of hemorrhage in a cavernous angioma varies widely (1%–6.8%).3–5
MR imaging is the most important diagnostic technique for the detection of cavernous angiomas and frequently produces highly characteristic images. Typically, cavernous angiomas show a mixed signal intensity core, a reticulated “popcorn ball” appearance, and a “T2 blooming sign,” which is due to a low signal intensity hemosiderin rim that completely surrounds the lesion.6,7 Susceptibility-weighted imaging, such as a T2* gradient-echo image, is more useful for the detection of the hemosiderin deposit and the diagnosis of a cavernous angioma. The typical MR signs of “popcorn ball” appearance and “T2 blooming sign” on T2-weighted images have been reported to be found in approximately 50%–67% of cavernous angiomas.2,8
Based on these MR findings, although diagnosis is usually straightforward in typical cases of a cavernous angioma, lesions with unusual MR features may be misdiagnosed as primary or metastatic brain tumors.9–18 The atypical MR features of cavernous angiomas include variable or strong enhancement,2,9,10,19 a large perilesional vasogenic edema and mass effect,2,10,19,20 the cystic form of a cavernous angioma,9,20,21 and the manifestations of a recent hematoma.22,23 Cavernous angiomas that present with recent hemorrhage and with a surrounding edema may mimic a primary or secondary brain tumor with hemorrhage; these lesions may be frequently underestimated as a sole hematoma.
Recently, we encountered some cases that showed T1 hyperintensity in a perilesional edema around the acute or subacute hemorrhagic masses. As far as we know, the MR feature of T1 hyperintensity in a perilesional edema around a hemorrhagic mass has not yet been documented. The aim of this study was to determine in a retrospective study whether the MR sign of a T1 hyperintense perilesional signal intensity is useful for differentiating a cavernous angioma from other hemorrhagic cerebral masses.
Materials and Methods
Patients
We retrospectively reviewed 72 consecutive patients with acute or subacute hemorrhagic cerebral masses (29 cavernous angiomas, 13 glioblastomas, 1 oligodendroglioma, 16 hemorrhagic metastases, and 13 acute or subacute intracerebral hematomas). The primary tumors of 16 patients with hemorrhagic metastases were lung cancer (n = 8), liver cancer (n = 5), breast cancer (n = 1), thyroid cancer (n = 1), and chronic myeloid leukemia (n = 1). There were 43 males (age range, 11–71 years; mean age, 45.9 years) and 29 women (age range, 18–75 years; mean age, 46.2 years). The included patients with cavernous angiomas or brain tumors were selected from a total of 635 consecutive patients with cavernous angiomas (n = 65), glioblastomas (n = 192), and oligodendrogliomas (n = 77), which were histologically proven, and patients with surgically or clinically proven cerebral metastases (n = 301) between February 1999 and April 2004. The inclusion criteria for selection of patients were the presence of a perilesional vasogenic edema accompanying a hemorrhagic mass on a T2-weighted image and the presence of a hemorrhagic mass with a large amount of acute or subacute hemorrhage covering more than half of the mass area on 2D axial T1, T2, or susceptibility MR images. The presence of hemorrhage was determined by CT (the presence of an obvious high attenuation suggestive of an acute clot) or by MR imaging (the presence of typical signal intensity of an acute or subacute hematoma including hypointensity on T2- or T2* gradient-echo images and/or the presence of hyperintensity on a T1-weighted image).
This study also included 13 consecutive patients with an acute or subacute spontaneous intracerebral hematoma during a period of 2 years in which the presumed etiologies were hypertensive hemorrhage (n = 11) or undetermined (n = 2) by final clinical diagnoses. The institutional review board (Seoul National University) approved this study, and informed consent was waived for the retrospective study.
MR Imaging
MR imaging was performed by using two 1.5T MR imaging systems (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany and Signa; GE Medical Systems, Milwaukee, Wis). In all of the patients, unenhanced T1-weighted images (axial or sagittal) and fast spin-echo T2-weighted axial images were obtained. Enhanced T1-weighted images with gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) at a dose of 0.1 mmol/kg were also obtained in all of the patients except for 3 patients with cavernous angiomas. The MR imaging parameters used were as follows: 450–500/8–12/1–2 ms (TR/TE/NEX) for spin-echo T1-weighted images and 3666–5000/96–105/1–2/7 ms (TR/TEeff/NEX/echo train) for fast spin-echo T2-weighted images. Other parameters were 5-mm section thickness, 2-mm gap, 210 × 240 mm FOV, and a matrix of 256 × 192.
Assessment of the MR Sign of the T1 Hyperintense Perilesional Signal Intensity
Two experienced neuroradiologists (D.G.N. and B.J.K., with 10 years and 6 years of neuroradiology experience, respectively) independently reviewed all of the MR images; disagreements were decided by consensus, and both clinicians were unaware of the information concerning the clinical history, the findings of other modalities, and final diagnoses. The interpreters decided on the presence of a T1 hyperintense perilesional signal intensity in each case, which was determined when an obvious hyperintensity showing a higher signal intensity than the normal white matter was present within the vasogenic edema of a hemorrhagic mass on unenhanced T1-weighted images. In 37 patients in whom the unenhanced T1-weighted axial images were not available, the presence of a T1 hyperintense perilesional signal intensity was determined on unenhanced T1-sagittal images by using the 3D localization method in a PACS console (Maroview; Marotech, Seoul, Korea), and it was also reevaluated on enhanced T1-axial and -sagittal images.
Measurement of the T1 Signal Intensity of a Perilesional Edema
A radiologist (T.J.Y.) who was unaware of final diagnoses performed a quantitative measurement of signal intensity. For the measurement of the T1 signal intensity of a perilesional edema, manually drawn regions of interest (ROIs) of perilesional edemas on T2-weighted axial images were copied to same locations on unenhanced T1-weighted axial images on a PACS console. Because the T1 signal intensity of a perilesional edema can be heterogeneous, we used an ROI drawn along the boundary of the whole perilesional edema on a T2-weighted axial image at 1 image level showing the largest perilesional vasogenic edema. The T1 signal intensities of contralateral normal white matter (corona radiata) were also measured using ROIs (mean size, 200 mm2). Quantitative measurements were performed in 32 of the 72 patients (cavernous angioma in 17 patients and other hemorrhagic mass in 15 patients). In the other 40 patients, a quantitative measurement was not performed, because the unenhanced T1-weighted axial images were either unavailable (n = 37) or available only as hard copy images (n = 3).
Comparison of Cavernous Angiomas With and Without the T1 Hyperintense Perilesional Signal Intensity
In all of the patients with cavernous angiomas, the locations of the lesions, the presence of contrast enhancement, the volumes of hemorrhagic masses and the perilesional edemas, and the time intervals between symptom onset and MR imaging were also assessed and were compared between cavernous angiomas with and without the T1 hyperintense perilesional signal intensity sign. One radiologist (T.J.Y.), unaware of the final diagnoses, measured the volumes of the hemorrhagic masses and the perilesional edemas. After the areas of the hemorrhagic masses and perilesional edemas were measured on T2-weighted axial images at each section level, the volumes of the hemorrhagic masses and the perilesional edemas were calculated by multiplying the lesion size by the section thickness.
Assessment of the Profoundly Hypointense Signal Intensity or Rim on the T2-Weighted Image
Two radiologists (D.G.N. and T.J.Y.) evaluated MR images to determine the presence of profoundly hypointense signal intensity or rim in the hemorrhagic mass on T2-weighted images. The interpreters were blinded to the final diagnoses of hemorrhagic masses and determined the presence of profoundly hypointense signal intensity or rim by consensus when a hypointense signal intensity or rim darker than the white matter was found within or at the margin of hemorrhagic masses on T2-weighted images.
Histologic Examination of the Surgical Specimens
In 18 cavernous angiomas that showed the MR sign of a T1 hyperintense perilesional signal intensity, the resected brain specimens were retrospectively evaluated. All of the specimens taken from patients with a cavernous angioma were routinely processed for the histopathologic examination after formalin fixation, and the resected brain specimens were stained with hematoxylin-eosin. The histologic features of white matter around the cavernous angioma were evaluated by 2 neuropathologists (Y.-L.S. and S.-H.P.) to investigate the histologic changes of the white matter showing the MR sign of a T1 hyperintense perilesional signal intensity around the cavernous angioma.
Statistical Analysis
Statistical analysis was performed by using commercially available software (Version 11.5 for Windows; SPSS, Chicago, Ill). The unpaired t test was used for the comparison of the T1 signal intensity of a perilesional edema between patients with and without the MR feature of a T1 hyperintense perilesional signal intensity. The unpaired t test was also used to assess differences in mass volumes and vasogenic edema and the intervals between symptom onset and MR imaging between cavernous angiomas with the T1 hyperintense perilesional signal intensity sign and lesions without the sign. Fisher exact test was used to assess differences in the presence of hemorrhagic mass enhancement and the presence of the T2 hypointense signal intensity or rim. For all of the statistical analysis, the significance level for differences was set at P < 0.05. κ values were interpreted as suggested by Landis and Koch,24 and the value used for determining the presence of the T1 hyperintense perilesional signal intensity sign was 0.77.
Results
MR Sign of the T1 Hyperintense Perilesional Signal Intensity
The MR sign of the T1 hyperintense perilesional signal intensity was observed for 18 (62%) of 29 cavernous angiomas (Figs 1 and 2) and in 1 (6.3%) of 16 hemorrhagic metastases but was not observed in glioblastomas, oligodendroglioma, and intracerebral hemorrhages (Figs 3 and 4). T1 hyperintensity was more obvious at the deep region, around the hemorrhagic mass, than at the peripheral region of the edema in all of the cavernous angiomas. In 1 patient with a hemorrhagic metastasis who had an MR finding of the T1 hyperintense perilesional signal intensity, there was a different pattern of T1 hyperintensity in the perilesional edema; namely, a subtle T1 hyperintensity was detected in the peripheral region of the peritumoral edema and spared the deep area of the edema around the hematoma. The accuracy, sensitivity, specificity, and positive and negative predictive values of the MR sign for a diagnosis of a cavernous angioma were 80%, 62%, 98%, 95%, and 79%, respectively, in patients with hemorrhagic cerebral masses.
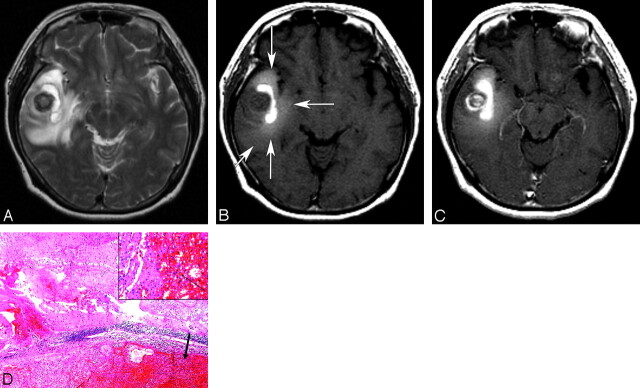
Fig 1.
A 54-year-old woman with a cavernous angioma. A, An axial T2-weighted image (TR/TE/NEX, 3700/104/2 ms) shows a hemorrhagic mass with heterogeneous signal intensity and a severe surrounding edema in the right temporal lobe. B, An unenhanced T1-weighted image (TR/TE/NEX, 467/8/1 ms) demonstrates obvious hyperintensity within the vasogenic edema (T1 hyperintense perilesional signal intensity sign; long arrows). The T1 hyperintense perilesional signal intensity sign is not obviously observed in the peripheral area of the vasogenic edema (short arrow). C, An enhanced T1-weighted image (TR/TE/NEX, 467/8/1 ms) shows heterogeneous enhancement of the round mass at the center of hemorrhagic mass. D, Surgery was performed 1 day after MR imaging. A histologic photomicrograph shows a relatively well-demarcated cavernous angioma with hematoma formation (arrow) and microscopic diffuse perilesional hemorrhage in the surrounding white matter (hematoxylin-eosin, original magnification, ×40). The inset shows hemorrhage with infiltration of siderophages (arrow; hematoxylin-eosin, original magnification, ×200).
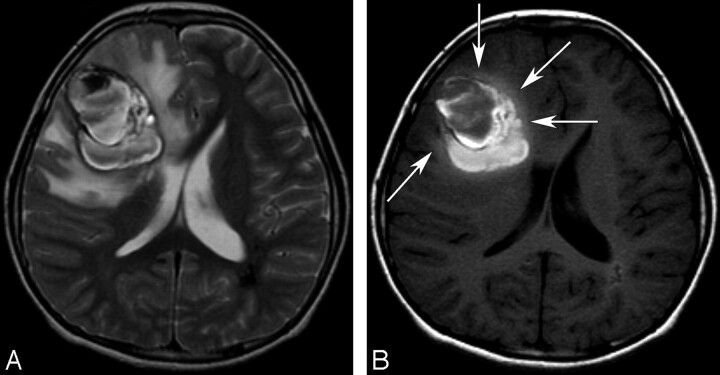
Fig 2.
An 11-year-old boy with a cavernous angioma. A, An axial T2-weighted image (TR/TE/NEX, 3683/104/2 ms) shows a large hemorrhagic mass with heterogeneous signal intensity and peripheral hypointense rims in the right frontal lobe. A perilesional massive edema and mass effect is seen. There is a hypointense lesion in the periventricular white matter of the left parietal lobe and a few small hypointense lesions that were also found in both hemispheres on a T2* gradient-echo image (data not shown), which indicate possible multiple cavernous angiomas. B, An axial T1-weighted image (TR/TE/NEX, 500/9/2 ms) shows a T1 hyperintense perilesional signal intensity sign and mild hyperintensity of a perilesional edema at the deep area abutting the hemorrhagic mass (arrows). Note the centripetal pattern of the T1 hyperintense perilesional signal intensity sign in which T1 hyperintensity within the perilesional edema is observed only in the deep area around the hemorrhagic mass; it is not observed at the periphery of edema.
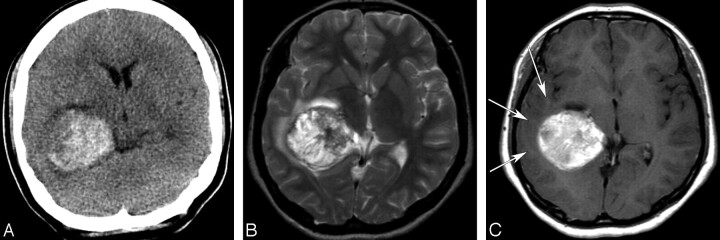
Fig 3.
A 17-year-old girl with a glioblastoma. A, An unenhanced CT image shows a hyperattenuated acute hemorrhagic mass in the right temporal lobe. B, An axial T2-weighted image (TR/TE/NEX, 5000/96/2 ms) shows a hemorrhagic mass with heterogeneous signal intensity and a mild peritumoral edema. C, An axial enhanced T1-weighted image (TR/TE/NEX, 500/12/1 ms) demonstrates mild hypointensity or isointensity of the peritumoral edema (arrows) and mild heterogeneous enhancement of the hemorrhagic mass, which was hyperintense on an unenhanced T1-weighted image (data not shown).
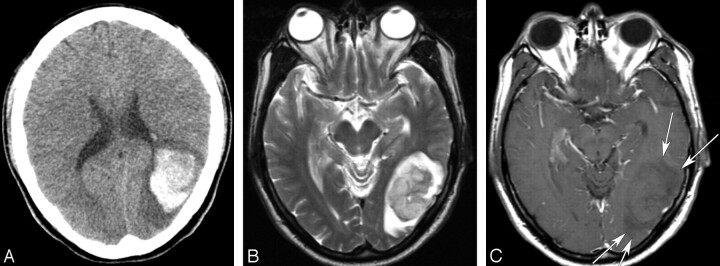
Fig 4.
A 48-year-old man with a cerebral metastasis from a hepatocellular carcinoma. A, An unenhanced CT image shows an acute hemorrhagic mass with a surrounding edema in the left temporooccipital lobe. B, An axial T2-weighted image (TR/TE/NEX, 5000/99/2 ms) demonstrates an acute hematoma with a profound perilesional edema and mass effect. C, An axial enhanced T1-weighted image (TR/TE/NEX, 500/12/1 ms) demonstrates hypointensity of the perilesional edema (arrows).
T1 Signal Intensity of a Perilesional Edema
Table 1 shows a summary of the quantitative analysis of the T1 hyperintense perilesional signal intensity sign in 32 patients. The mean T1 signal intensity ratio of the perilesional edema was significantly higher for cavernous angiomas (n = 17) than for other hemorrhagic masses (n = 15; P < .001). In 17 patients with cavernous angiomas, the mean T1 signal intensity of the edema was significantly higher than that of the contralateral white matter (P = .045), but in other hemorrhagic masses, the T1 signal intensity of the perilesional edema was significantly lower than that of the contralateral white matter (P < .001).
Table 1:
The T1 Signal Intensity Ratio of a Perilesional Edema in Patients with Cerebral Acute or Subacute Hemorrhagic Masses (n = 32)
| Diagnosis of Hemorrhagic Masses | T1 Signal Intensity Ratio of the Perilesional Edema* | P† |
|---|---|---|
| Cavernous angioma (n = 17) | 1.06 ± 0.15 | .045 |
| MR sign (+) (n = 12) | 1.13 ± 0.10 | |
| MR sign (−) (n = 5) | 0.91 ± 0.13 | |
| Other hemorrhagic mass (n = 15) | 0.91 ± 0.06 | <.001 |
| Glioblastoma (n = 5) | 0.89 ± 0.01 | |
| Metastasis (n = 4) | 0.89 ± 0.08 | |
| Acute or subacute ICH (n = 6) | 0.92 ± 0.06 |
Note:—Data are mean ± SD. MR sign indicates the MR sign of a T1 hyperintense perilesional signal. ICH indicates intracerebral hemorrhage.
T1 signal intensity ratio indicates the ratio of signal intensity of the edema to that of the contralateral white matter.
Comparison of the T1 signal intensity between the perilesional edema and the contralateral white matter.
Of 17 patients with cavernous angiomas, the mean perilesional edema T1 signal intensity was significantly higher in patients (n = 12) with the MR sign of the T1 hyperintense signal intensity than in patients (n = 5) without the MR sign (P < .001). No significant difference was found between cavernous angiomas without the MR sign of the T1 hyperintense signal intensity and other hemorrhagic masses in terms of the perilesional T1 signal intensity (P = .895).
Cavernous Angiomas With and Without the T1 Hyperintense Perilesional Signal Intensity
Table 2 shows a summary of data for 29 patients with cavernous angiomas. The mean volume of a vasogenic edema was significantly larger for patients with a T1 hyperintense perilesional signal intensity sign than for those without the sign (P < .001). However, the mean volume of hemorrhagic masses and the presence of enhancement were not found to be significantly associated with presence of the MR sign of the T1 hyperintense perilesional signal intensity (P > .2). The T1 hyperintense perilesional signal intensity sign was found in the white matter of cerebral hemispheres in 16 (89%) of the 18 patients with this sign. The time interval between symptom onset and MR imaging showed a tendency to be shorter for patients with the T1 hyperintense perilesional signal intensity than in patients without the sign, but this was not statistically significant (P = .06).
Table 2:
MR Features of the Cavernous Angiomas (n = 29)
| MR features | MR Sign of T1 Hyperintense Perilesional Signal |
P | |
|---|---|---|---|
| Positive (n = 18) | Negative (n = 11) | ||
| Volume of vasogenic edema, cm3 | 32.3 ± 22.0 | 12.4 ± 9.6 | .009 |
| Volume of mass, cm3 | 12.3 ± 7.6 | 9.1 ± 5.5 | .220 |
| Presence of enhancement* | 9 | 3 | .248 |
| Time interval between symptom onset and MR imaging, d | 10.4 ± 9.7 | 18.3 ± 11.2 | .060 |
| Location | |||
| Cerebral hemisphere | 16 | 5 | |
| Cerebellar hemispheres | 1 | 3 | |
| Brain stem | 1 | 3 | |
Note:—Volumes of masses and edemas are mean ± SD.
Enhanced T1-weighted images were not available in 2 patients with positive MR signs of a T1 hyperintense perilesional signal and in 1 patient with no MR sign.
Profoundly Hypointense Signal Intensity or Rim on the T2-Weighted Image
The MR finding of profoundly hypointense signal intensity or rim in the hemorrhagic mass on the T2-weighted image was significantly more common in cavernous angiomas than those in other hemorrhagic masses (P = .02 and P < .0001, respectively). The accuracy, sensitivity, specificity, and positive and negative predictive values of the profoundly hypointense signal intensity for a diagnosis of cavernous angioma were 60%, 83%, 44%, 50%, and 79%, and those of the profoundly hypointense rim were 74%, 76%, 72%, 65%, and 82%.
Histologic Findings of White Matter Showing the T1 Hyperintense Perilesional Signal Intensity
Histologic changes of white matter around a cavernous angioma were evaluated on surgical specimens in 14 of 18 patients who had MR signs of the T1 hyperintense signal intensity. In 4 patients, surgical specimens of white matter around a cavernous angioma were not available or not optimal for determining the presence of hemorrhage unrelated to surgery. Histologic examinations of the surgical specimens in 14 patients with cavernous angiomas revealed cavernous angiomas with hematoma formation and recent microscopic hemorrhage in the surrounding white matter tissue in all of the patients. The perilesional hemorrhage in the white matter was stained yellow or brown by hemosiderin pigmentation and was associated with infiltration of siderophages or inflammatory cells (n = 11) and gliosis (n = 13; Fig 1).
Discussion
In the present study, it was found that the T1 hyperintense perilesional signal intensity sign was present in 62% of cavernous angiomas with recent hemorrhage and that it was highly specific (98%) and predictive (95%) for a cavernous angioma in patients manifesting an acute or subacute hemorrhagic cerebral mass. The perilesional edema produced by an intracerebral hemorrhage or tumor is typically a vasogenic edema manifested as T2 hyperintensity and T1 hypointensity due to an increase in water content of the brain tissue, which is usually most obvious in the white matter.25 The T1 hyperintensity of a perilesional edema in a cavernous angioma is an atypical MR feature of a vasogenic edema that cannot be explained by the mechanism of a typical vasogenic edema that is usually manifested in a brain tumor or intracerebral hemorrhage.
A brain edema is, by definition, an increase in the water content of brain tissue, and a vasogenic edema typically develops in patients with a brain tumor or intracerebral hematoma. A vasogenic edema results from an increase in the permeability of the blood-brain barrier (BBB). The vasogenic edema is characterized by BBB dysfunction and accumulation of plasma fluid within the brain tissue extracellular spaces.25 Although the mechanism of a perihematomal edema has not been fully established, edema formation is caused by BBB dysfunction of the cerebral vessels around a hematoma, which could be associated with many factors, including hydrostatic pressure and clot retraction,26,27 coagulation cascade activation and thrombin formation,28 red blood cell lysis, and hemoglobin toxicity.29,30 Peritumoral edema formation is caused by an increase in the capillary permeability of tumor vessels, which may be associated with tumor microvessel ultrastructural abnormalities, the underexpression of tight junction proteins, or the overexpression of the membrane water channel protein aquaporin-4.31–33
Although the presumed etiology for edema in a cavernous angioma is still unclear, a perilesional edema is often associated with the occurrence of overt hemorrhage and lesion expansion and may be associated with the occurrence of symptoms in patients with cavernous angiomas.19,20,34 The BBB in normal cerebral blood vessels is a specialized system of capillary endothelial cells with tight junctions and periendothelial structures (pericytes, basal lamina, and astrocytic foot processes). Recent electron microscopy and immunohistochemistry studies of cavernous angiomas35–37 have identified specific differences between the architectures of normal cerebral blood vessels and those in cavernous malformations. In contrast to the normal cerebral vessels, the vascular walls of a cavernous angioma show endothelial cell fenestrations, large endothelial cell junction gaps, the absence of basal lamina, no astrocytic foot process, and the rare presence of pericytes.35,37 Thus, these ultrastructural characteristics of cavernous angiomas create dysfunctional BBBs, which probably permit the chronic extravasation of red blood cells through the vessel walls and result in microhemorrhages and hemosiderin deposition in the surrounding parenchyma.36,37
Although the exact mechanism of T1 hyperintense perilesional signal intensity is unclear, the results of our study provide insights that may explain this MR finding for cavernous angiomas. First, our study shows that a perilesional edema is larger in cavernous angiomas with a T1 hyperintense perilesional signal intensity sign than in lesions without this sign, which suggests that this MR sign may be closely associated with more significant edema formation. Second, the results of the histologic examinations suggest that the T1 hyperintense perilesional signal intensity sign may be due to extravascular leakage of a large amount of red blood cells and plasma during edema formation. This finding is also supported by the observation that the T1 hyperintense perilesional signal intensity was more obvious at the area abutting the cavernous angioma. Third, the T1 hyperintense perilesional signal intensity may be related to overt hemorrhage within a cavernous angioma, because a perilesional edema is most commonly found in a cavernous angioma accompanying overt hemorrhage. A histopathologic study of cavernous angiomas36 showed that the BBB integrity loss was more obvious in cavernous angiomas with hemorrhage than in lesions without hemorrhage, which also suggests that a dysfunctional BBB in the vessels of a cavernous angioma appears to be closely associated with the occurrence of overt hemorrhage within cavernous angiomas.
The differential diagnosis of a focal cerebral hemorrhagic mass should include neoplastic hemorrhage, as well as a nonneoplastic hemorrhage, in patients with hemorrhagic stroke, because a glioblastoma or hemorrhagic metastasis may manifest as an acute intracerebral hemorrhage with no obvious enhancing mass as seen on MR images. Moreover, cavernous angiomas with overt hemorrhage may mimic malignant tumors, such as a high-grade glioma or a metastatic tumor manifesting overt hemorrhage, because a cavernous angioma may accompany a significant perilesional edema and variable enhancement. Although the T1 hyperintense perilesional signal intensity sign was found only in approximately two thirds of cavernous angiomas with overt hemorrhage and perilesional edema, this MR sign was found to be highly specific for a cavernous angioma and provides an additional confirmation for the differential diagnosis of cerebral hemorrhagic masses.
Our study showed a significantly higher incidence of profoundly hypointense signal intensity or rim on the T2-weighted images in cavernous angiomas compared with that in other hemorrhagic masses. Although the MR feature of the profoundly hypointense rim was more accurate for a diagnosis of cavernous angiomas than the MR feature of profoundly hypointense signal intensity, both MR features were less specific and less predictive of cavernous angiomas. This may be explained by the fact that the MR feature of profoundly hypointense signal intensity or rim by acute clot or hemosiderin deposits could be the result of other hemorrhagic lesions, as well as cavernous angiomas.
This study has some limitations. First, we included only patients with hemorrhagic tumors—glioblastomas, oligodendroglioma, and hemorrhagic metastases. Therefore, it may be difficult to generalize the diagnostic value of the T1 hyperintense perilesional signal intensity for other hemorrhagic tumors. Second, the T1 perilesional edema signal intensity was measured in only a limited number of patients, because source data were not available in some patients.
Conclusion
The T1 hyperintense perilesional signal intensity is a useful sign for differentiating cavernous angioma from other hemorrhagic masses, such as primary or secondary brain neoplasms and primary intracerebral hematomas. The MR sign of a T1 hyperintense perilesional signal intensity was highly specific and predictive of a cavernous angioma; therefore, when the T1 hyperintense perilesional signal intensity is present, a diagnosis of a cavernous angioma should be suggested with a high probability.
References
- 1.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 1994;80:422–32 [DOI] [PubMed] [Google Scholar]
- 2.Rigamonti D, Drayer BP, Johnson PC, et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg 1987;67:518–24 [DOI] [PubMed] [Google Scholar]
- 3.Dorsch NWC, McMahon JHA. Intracranial cavernous malformations—natural history and management. Crit Rev Neurosurg 1998;8:154–68 [DOI] [PubMed] [Google Scholar]
- 4.Moran NF, Fish DR, Kitchen N, et al. Supratentorial cavernous haemangiomas and epilepsy: a review of the literature and case series. J Neurol Neurosurg Psychiatry 1999;66:561–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandalcioglu IE, Wiedemayer H, Secer S, et al. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry 2002;72:351–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapacki TF, Brantley MJ, Furlow TW Jr, et al. Heterogeneity of cerebral cavernous hemangiomas diagnosed by MR imaging. J Comput Assist Tomogr 1990;14:18–25 [DOI] [PubMed] [Google Scholar]
- 7.Gomori JM, Grossman RI, Goldberg HI, et al. Occult cerebral vascular malformations: high-field MR imaging. Radiology 1986;158:707–13 [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson FH, Houser OW, Scheithauer BW, et al. Angiographically occult vascular malformations: a correlative study of features on magnetic resonance imaging and histological examination. Neurosurgery 1994;34:792–99 [DOI] [PubMed] [Google Scholar]
- 9.Gelal F, Feran H, Rezanko T, et al. Giant cavernous angioma of the temporal lobe: a case report and review of the literature. Acta Radiol 2005;46:310–13 [DOI] [PubMed] [Google Scholar]
- 10.Olivero WC, Deshmukh P, Gujrati M. Radiation-induced cavernous angioma mimicking metastatic disease. Br J Neurosurg 2000;14:575–78 [DOI] [PubMed] [Google Scholar]
- 11.Duong H, del Carpio-O'Donovan R, Pike B, et al. Multiple intracerebral cavernous angiomas. Can Assoc Radiol J 1991;42:329–34 [PubMed] [Google Scholar]
- 12.Rosso D, Lee DH, Ferguson GG, et al. Dural cavernous angioma: a preoperative diagnostic challenge. Can J Neurol Sci 2003;30:272–77 [DOI] [PubMed] [Google Scholar]
- 13.House P, Dunn J, Carroll K, et al. Seeding of a cavernous angioma with mycoplasma hominis: case report. Neurosurgery 2003;53:749–52 [DOI] [PubMed] [Google Scholar]
- 14.Jabbour P, Gault J, Murk SE, et al. Multiple spinal cavernous malformations with atypical phenotype after prior irradiation: case report. Neurosurgery 2004;55:1431. [PubMed] [Google Scholar]
- 15.Arismendi Morillo GJ, Fernandez Abreu MC, Cardozo Sosa DP, et al. Nonneoplastic space occupying lesions mimicking central nervous system tumors. Rev Neurol 2004;38:427–30 [PubMed] [Google Scholar]
- 16.Tamburrini G, Iannelli A, Caldarelli M, et al. Large cerebral cavernoma mimicking a brain tumor. Pediatr Neurosurg 2002;37:105–06 [DOI] [PubMed] [Google Scholar]
- 17.Harada S, Niimi M, Murakami K, et al. Cavernous angioma of the corpus callosum mimicking an astrocytic tumor–case report. Neurol Med Chir 2001;41:349–51 [DOI] [PubMed] [Google Scholar]
- 18.Chicani CF, Miller NR, Tamargo RJ. Giant cavernous malformation of the occipital lobe. J Neuroophthalmol 2003;23:151–53 [DOI] [PubMed] [Google Scholar]
- 19.Sage MR, Brophy BP, Sweeney C, et al. Cavernous haemangiomas (angiomas) of the brain: clinically significant lesions. Australas Radiol 1993;37:147–55 [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Kubota T. Large calcified cystic cavernous angioma in the thalamus–case report. Neurol Med Chir 1995;35:100–03 [DOI] [PubMed] [Google Scholar]
- 21.Yagi K, Kageji T, Nagahiro S, et al. Multiple cystic cavernous angiomas associated with hemorrhage. Acta Neurochir 2005;147:201–03 [DOI] [PubMed] [Google Scholar]
- 22.Duffau H, Capelle L, Sichez JP, et al. Early radiologically proven rebleeding from intracranial cavernous angiomas: report of 6 cases and review of the literature. Acta Neurochir 1997;139:914–22 [DOI] [PubMed] [Google Scholar]
- 23.Gottfried ON, Gluf WM, Schmidt MH. Cavernous hemangioma of the skull presenting with subdural hematoma. Case report. Neurosurg Focus 2004;17:ECP1. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 25.Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am 2002;13:371–83 [DOI] [PubMed] [Google Scholar]
- 26.Betz AL, Iannotti F, Hoff JT. Brain edema: a classification based on blood-brain barrier integrity. Cereb Brain Metab Rev 1989;1:133–54 [PubMed] [Google Scholar]
- 27.Wagner KR, Xi G, Hua Y, et al. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke 1996;27:490–97 [DOI] [PubMed] [Google Scholar]
- 28.Xi G, Wagner KR, Keep RF, et al. The role of blood clot formation on early edema development following experimental intracerebral hemorrhage. Stroke 1998;29:2580–86 [DOI] [PubMed] [Google Scholar]
- 29.Huang FP, Xi G, Keep RF, et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg 2002;96:287–93 [DOI] [PubMed] [Google Scholar]
- 30.Xi G, Hua Y, Bhasin RR, et al. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke 2001;32:2932–38 [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos MC, Saadoun S, Binder DK, et al. Molecular mechanisms of brain tumor edema. Neuroscience 2004;129:1011–20 [DOI] [PubMed] [Google Scholar]
- 32.Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Brain and nervous system. Curr Opin Oncol 2004;16:593–600 [DOI] [PubMed] [Google Scholar]
- 33.Arismendi-Morillo G, Castellano A. Tumoral micro-blood vessels and vascular microenvironment in human astrocytic tumors. A transmission electron microscopy study. J Neurooncol 2005;73:211–17 [DOI] [PubMed] [Google Scholar]
- 34.Maraire JN, Awad IA. Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery 1995;37:591–605 [DOI] [PubMed] [Google Scholar]
- 35.Clatterbuck RE, Eberhart CG, Crain BJ, et al. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry 2001;71:188–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu J, Stoodley MA, Morgan MK, et al. Ultrastructural characteristics of hemorrhagic, nonhemorrhagic, and recurrent cavernous malformations. J Neurosurg 2005;103:903–09 [DOI] [PubMed] [Google Scholar]
- 37.Wong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery 2000;46:1454–59 [DOI] [PubMed] [Google Scholar]