Abstract
BACKGROUND AND PURPOSE: Functional MR imaging (fMRI) is used to determine preoperatively the laterality of cortical language representation along with the relationship of language areas to adjacent brain tumors. The purpose of this study was to determine whether changing the statistical threshold for different language tasks influences the language laterality index (LI) for a group of controls, patients with tumor without prior surgery, and patients with tumor and prior surgery.
MATERIALS AND METHODS: Seven controls, 9 patients with tumor without prior surgery, and 4 patients with tumor and prior surgery performed verb-generation, phonemic fluency, and semantic fluency language tasks during fMRI. Interhemispheric activation differences between the left and right Broca regions of interest were determined by calculating language LIs. LIs were compared within each group, between groups, and between language tasks. Intraoperative electrocortical mapping or the presence of aphasia during postoperative neurology examinations or both were used as ground truth.
RESULTS: The language LI varied as a result of statistical thresholding, presence of tumor, prior surgery, and language task. Although patients and controls followed a similar shape in the LI curve, there was no optimal P value for determining the LI. Three patients demonstrated a shift in the LI between hemispheres as a function of statistical threshold. Verb generation was the least variable task both between tasks and across groups.
CONCLUSION: For preoperative patients with tumor, the LI should be examined across a spectrum of P values and a range of tasks to ensure reliability. Our data suggest that the LI may be threshold- and task-dependent, particularly in the presence of adjacent tumor.
Quality of life is an important factor in the decision to undergo neurosurgery. The ability to produce and comprehend language is a significant part of postoperative quality of life. Therefore, the potential of losing language function postoperatively may preclude surgical resection of a tumor.
The preoperative assessment of language localization can be useful in planning surgical resection and deciding whether to perform awake cortical mapping. To this end, functional MR imaging (fMRI) has been used to determine the areas of the cortex involved in language functioning in preoperative patients with tumor.1–5 fMRI is noninvasive and repeatable; and by showing the relationship between functional language cortex and a tumor, it may tailor a surgeon's approach to the tumor. Currently, intraoperative cortical mapping remains the gold standard for localizing language in patients with tumor, and several studies have demonstrated significant concordance between fMRI and intraoperative electrocorticography.1,6–9 Additionally, fMRI may reduce overall surgical time by guiding direct cortical stimulation and is particularly useful in cases in which the awake mapping procedure fails due to seizures or issues with the patient's tolerance for anesthesia.
The language laterality index (LI) is a ratio measure using the number of active fMRI voxels that determines hemispheric dominance for language and has been used to determine surgical candidacy for patients with brain tumor.7,10–14 One potential issue with the LI calculations in fMRI is that the specificity and sensitivity to true neural activity change with the application of different statistical thresholds. As a result, it is expected that language dominance as measured by the LI will vary as the statistical confidence threshold is changed. Theoretically, it is possible for the LI to transfer from 1 hemisphere to the other, depending on the threshold chosen. It has been shown in control subjects that the LI strengthens toward the dominant hemisphere as the statistical threshold increases.12 No prior studies have examined the LI across a spectrum of P values in patients with tumor. This issue is especially important in evaluating preoperative patients for whom the determination of language dominance is relevant to surgical planning.
Using fMRI in patients with tumor presents unique challenges. First, prior studies in angiography and MR imaging have shown that vessels within a glioma lose their ability to autoregulate normally.15–17 This, in turn, may limit the ability of the blood oxygen level–dependent (BOLD) signal intensity to detect true neural activity in patients with tumor. Previous studies have shown that the volume of BOLD activation is significantly reduced in the tumor hemisphere in comparison with the contralateral control hemisphere in the motor strip.18–21 Second, the mass effect of large tumors may affect the blood flow of adjacent circulation by compressing veins, thereby causing oxygenated blood to drain from the activated region more quickly, possibly truncating the BOLD signal intensity.22,23 Finally, susceptibility artifacts from surgical staples, metal used to secure skull flaps, and blood products may also compromise the detection of the BOLD signal intensity, given prior neurosurgery.24
In addition to physiologic and technical parameters affecting the measure of laterality, studies suggest that the degree of lateralization may depend on the language task.7,9,25 In general, paradigms such as verb generation tend to lateralize language better than paradigms like picture naming.7,11 The verb-generation task has shown the most concordance with intraoperative mapping overall.7,12,26
Accordingly, the goal of our study was multifactorial. We aimed to characterize the LIs measured over a range of statistical thresholds in control subjects and in patients with brain tumors, and we compared the relative distribution of fMRI activity seen by using several paradigms known to lateralize language. We predicted that laterality indices would be more variable in patients with tumor than in control subjects and that the verb-generation paradigm would most consistently lateralize language across groups.
Methods
Participants
Seven control subjects and 13 patients were studied. Control subjects included 5 women and 2 men with an average age of 26.14 years (SD, 2.79 years). All controls were right-handed as determined by the Edinburgh Handedness Inventory, with a laterality quotient of >80,27 and were native speakers of English. Controls had no known neurologic impairments. Eight female patients and 5 male patients participated in the study, with an average age of 48.77 years (SD, 14.05 years). All patients had pathologically confirmed tumors located in or adjacent to the left or right inferior frontal gyrus. Pathology included 4 glioblastoma multiformes, 3 anaplastic astrocytomas, 2 oligodendrogliomas, 1 low-grade glioma, and 3 metastases (On-line Table). Nine patients did not have prior surgery, and 4 patients had prior neurosurgery. Both controls and patients performed identical behavioral paradigms. The study was approved by the institutional review board.
Data Acquisition
Scanning was performed on a 1.5T TwinSpeed Excite scanner (GE Healthcare, Milwaukee, Wis) by using a standard quadrature head coil. Functional images were gathered by using a T2*-weighted gradient-echo echo-planar imaging sequence (TE = 40 ms, TR = 4000 ms, section thickness = 4.5 mm with no gap, matrix size = 128 × 128, FOV = 240 mm, 21 sections). T1-weighted spin-echo images (TR = 600 ms, TE = 8 ms, 256 × 256 matrix, 90° flip angle, 4.5 mm in thickness with no gap, 240-mm FOV, 21 sections) were also acquired. 3D T1-weighted anatomic images were acquired with a spoiled gradient-recalled-echo sequence (TR = 22 ms, TE = 4 ms, 256 × 256 matrix, 30° flip angle, 1.5 mm in thickness, 24-mm FOV). The subject's head motion was minimized by using straps and foam padding.
fMRI Activation Tasks
Subjects performed 3 language tasks presented visually through liquid crystal display goggles. Tasks included verb generation (verb), phonemic fluency (letter), and semantic fluency (category). Subjects were asked to perform the tasks silently, avoiding mouth and tongue movement. Patients performed 1, 2, or all 3 tasks, depending on clinical necessity and their ability to perform the task correctly (On-line Table). In verb generation, subjects were presented with a noun and asked to generate action words associated with the noun. For the letter task, a letter appeared on the screen, and subjects were asked to silently generate words that began with that letter. During the category task, subjects were given a supraordinate category and were told to generate subordinate words that fit the category. Patients were carefully pretested on each task outside the scanner to assure that they could perform the task correctly. The baseline was resting and consisted of fixation on a cross-hair. The paradigm was presented as a block design and consisted of 60 images, with 5 images of paradigm execution alternating with 5 images of rest.
fMRI Data Analysis
Images were processed and analyzed by using Analysis of Functional NeuroImages (AFNI) software (available at http://afni.nimh.nih.gov/afni).28 3D motion correction was performed to correct for both in-plane and out-of-plane head motion. Voxels where the SD of the acquired time series exceeded 8% of the mean signal intensity were set to zero to reduce false-positive activity. Cross-correlation was used to generate statistical maps. Each pixel was compared with a modeled boxcar waveform.
Regions of interest were defined on the basis of the T1-weighted or T2-weighted anatomic images. The region of interest included the inferior frontal gyrus up to the precentral gyrus. The entirety of the inferior frontal gyrus was included on the inferior margin and was delimited superiorly by the appearance of the middle frontal gyrus. The number of activated voxels included in each region of interest was determined by an automated AFNI script in the left and right hemispheres. LIs were calculated in each region of interest by using the formula: LI = (L − R) / (L + R). LIs were calculated at 15 different statistical thresholds (r = 0.25–0.95, corresponding to P values of 0.164–4.92 × 10−29, uncorrected). The LI ranged from −1 (completely right-lateralized) to +1 (completely left-lateralized). In concordance with prior studies, bilateral language representation was defined in the −0.2 to 0.2 range.29
The LI is expected to vary across the range of statistical thresholds. Therefore, to compare the variability in LI between groups, we calculated the median LI (MLI) value obtained at all thresholds between 2 bounds: P values between P < .008 and the least stringent P value, where either LI equaled ±1 or there was no activation. We used the median LI of this range to calculate the variability (median absolute deviation [MAD]) for each subject, group, and task.
Results
General Shape of the LI Curve
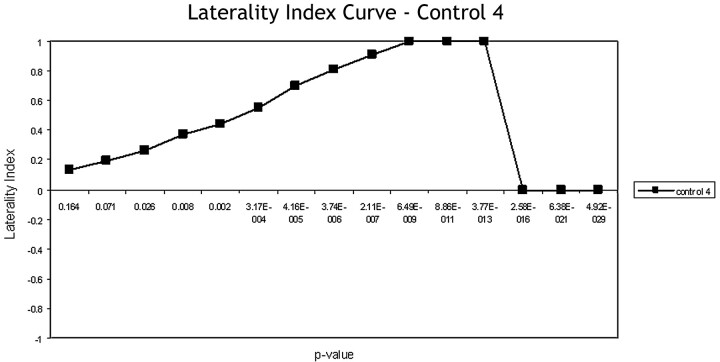
At a low correlation coefficient (high P value), more noise-related rather than task-associated voxels survived the statistical threshold. Therefore, at low correlation coefficients, the LI approached zero because noise affects both hemispheres approximately equally. Increasing the statistical threshold diminished the effects of noise and allowed more true-positive voxels to survive. Accordingly, as the statistical significance increased, increasing lateralization was seen, with the LI approaching +1 (left dominance) or −1 (right dominance). This “maximal” LI is expected to over-represent true functional laterality if excessively high stringency excludes voxels from the nondominant hemisphere when there is a quantitatively weaker, though still significant, level of activation. At a P value unique to each individual subject, the statistical threshold became too high for any activated voxels to survive, and the LI dropped back to zero. (Figs 1 and 2). This pattern we termed the “expected LI curve.”
Fig 1.
The general LI curve starts near zero and rises as the significance (P value) increases as statistical stringency reveals the dominant hemisphere. The LI peaks at a value of +1 or −1 when either the left or the right hemisphere is dominant. At peak statistical stringency, no activated voxels survive and the curve drops back to zero.
Fig 2.
Lateralization changes as a function of statistical thresholding in patient 1. At r = 0.35, P < .026, language would be considered bilateral, but as the statistical threshold increases, the language task indicates left lateralization in the Broca area.
Controls
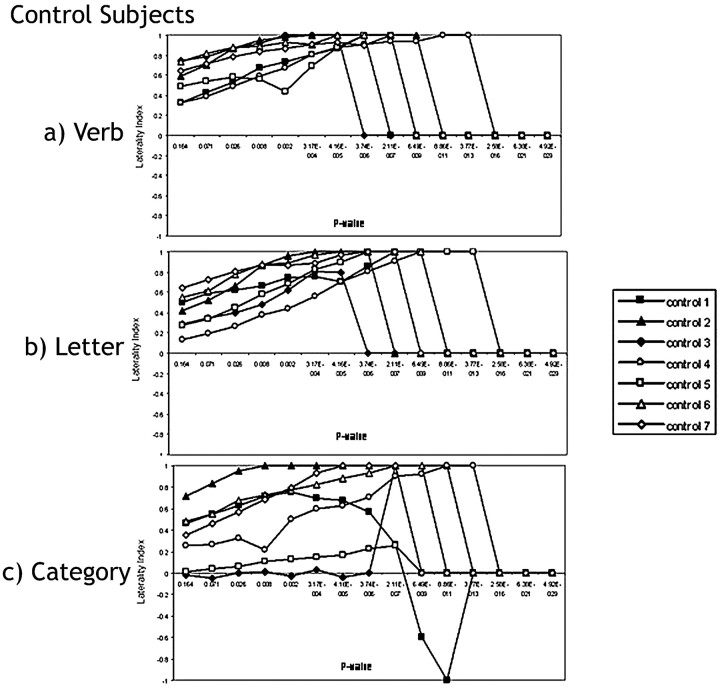
A sample LI curve for a single healthy control subject is shown in Fig 1. The curve demonstrates the expected shift in laterality as voxel stringency is increased. Most control subjects followed this expected LI curve shape across all 3 language paradigms (Fig 3A–C), though the tasks showed differences in variability among healthy subjects.
Fig 3.
Laterality index curves of controls performing the following: A, the verb-generation task. In all the control subjects, LI indicates complete left language lateralization in the Broca area across all P values. B, the letter task. All control subjects indicate left-hemispheric dominance in the Broca area except for control 4, whose fMRI suggests bilateral language representation at the highest 2 P values (P < .164 and P < .071), which are clinically insignificant. C, the category task. The individual laterality curves follow the expected pattern, except for control 1 and control 3. In control 1, the LI follows the normal trajectory from r = 0.25, P < .164 until r = 0.45, P < .002, where the LI curve then changes direction and becomes right-lateralized until it hits a peak of LI = −1 at r = 0.75, P < 8.86 × 10−11. fMRI in control 3 indicates bilaterality for language in the Broca area across the entire range of P values measured except for at an r value of 0.65 (P < .3.74 × 10−6) when this subject's fMRI suggests left dominance for language.
Healthy control subjects demonstrated the strongest LI while performing the verb-generation task (Fig 3A). The MLI during verb generation indicated strong left-lateralization (MLI = 0.84). The control subjects’ individual LI curves showed the least variability (smallest MAD) during the verb-generation task as compared with the letter and category tasks. The LI curves were the following: MAD verb = 0.06, MAD letter = 0.11, MAD category = 0.19 (Fig 3A–C). None of the controls’ fMRI examinations indicated a switch in hemispheric dominance across the spectrum of statistical thresholds during the verb-generation task.
The category task was the most variable (Fig 3C). Although most of the individual laterality curves followed the expected LI curve pattern, there were a few exceptions. Specifically, the measurement during the category task in control 1 indicated different hemispheric language dominance depending on the statistical threshold applied. Although the laterality followed the expected trajectory from r = 0.25, P < .164 until r = 0.45, P < .002, the LI curve then changed direction and suggested right-lateralization at a peak LI of −1 (completely right-lateralized) at r = 0.75, P < 8.86 × 10−11. Consequently, the subject would be considered left-lateralized for language in the Broca area at P < .16 to P < .0003, but right-lateralized at more stringent P values. fMRI suggested bilaterality in control 3 in Broca area across the entire range of P values measured except for an r value of 0.65 (P < 2.11 × 10−7), where this subject would be considered left-dominant for language. Most important, both of these of these subjects’ fMRIs suggested strong left-dominance (LIs never <0.48) on both the letter and the verb tasks. Overall, the category task revealed the largest variability (the greatest median absolute deviation) when compared with the verb and letter tasks.
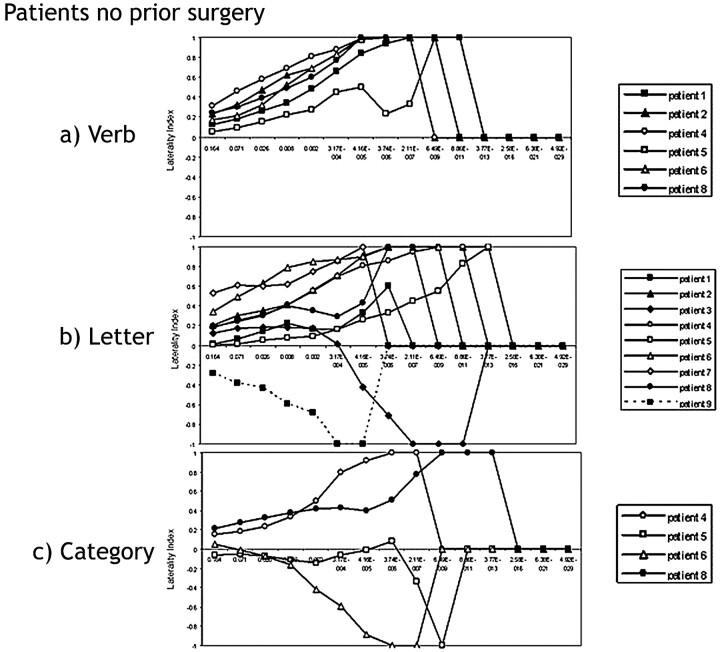
Patients with Tumor Without Prior Surgery
Patients with tumor without prior surgery demonstrated the least variability on the verb-generation task, in comparison with the letter and category tests (MAD verb = 0.07, MAD letter = 0.35, MAD category = 0.37; Fig 4A–C). However, patients with tumor without prior surgery demonstrated greater variability than that seen in the control subjects. In the verb-generation task, patient 5 showed threshold-dependent laterality. Between P values 4.16 × 10−5 (LI = 0.5) and 3.74 × 10−6 (LI = 0.23), the LI measurement shifted from left-dominant to bilateral (−0.2 < LI < +0.2; Fig 4A). In the letter fluency task, 6 patients of 9 demonstrated bilateral language representation in the Broca area at the 3 least significant P values (Fig 4B). fMRI in patient 9 indicated lateralization of language to the right. The fMRI measurement in patient 3 was bilateral for language at less significant P values and lateralized to the right at more stringent P values (Figs 4B and 5). Of the 4 patients with tumor who performed the category task, 2 patients’ fMRI maps suggested left-lateralization in the Broca area, and 2 patients’ fMRI maps suggested right-lateralization (Fig 4C). These same 2 patients exhibited the expected left-lateralization for language in the Broca area during the letter and verb tasks.
Fig 4.
Laterality index curves of patients with tumor without prior surgery performing the following: A, the verb-generation task. Some patients show bilateral language lateralization in the Broca area at clinically insignificant P values, but all the patients lateralize language to the left hemisphere in the Broca area at clinically significant P values (P < .05). B, the letter task. Six patients show bilateral language representation in the Broca area at less significant P values. Patient 9 lateralizes language to the right. Patient 3 lateralizes to the left at less significant P values and to the right at more stringent P values, C, the category task. Two patients have LI curves that lateralize to the left, and 2 patients lateralize language to the right in the Broca area. Both patients who show right-hemispheric dominance for language (patients 5 and 6) have tumors in the right hemisphere.
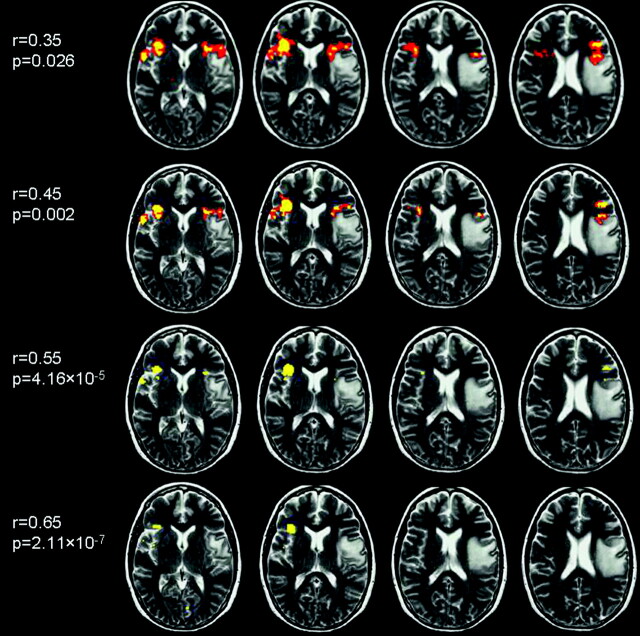
Fig 5.
Axial T2-weighted images with coregistered fMRI data in patient 3, from the patients with tumor without prior surgery category, performing the letter fluency task. The fMRI data are presented at different thresholds. At less stringent thresholds (r < 0.55), the language LI is bilateral, whereas at more stringent (r > 0.55) thresholds, the LI shifts to the right side. This right-handed patient showed signs of moderate aphasia preoperatively and mild aphasia and dysarthria postoperatively, clinically suggesting a significant left language component.
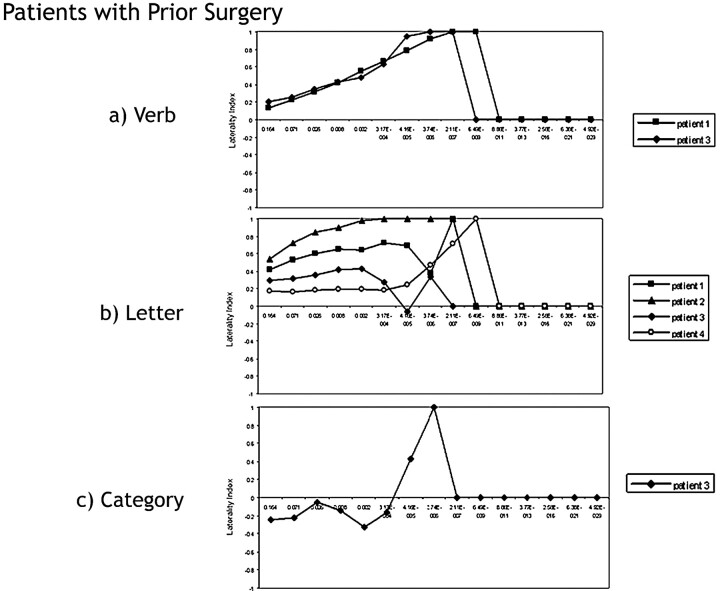
Patients with Tumor and Prior Surgery
We examined 4 patients with tumor and prior surgery (Fig 6A–C). Patients with prior surgery demonstrated the least variability in the fMRI laterality measurement when performing the verb task (MAD verb generation = 0.05, MAD letter fluency = 0.11; Fig 6A, -B). Both patients who performed the verb task did not demonstrate threshold-dependent laterality measurements. In the letter task, LI curves in the patients with prior surgery were more variable than those of the patients with tumor without prior surgery and the control subjects performing the same task (Fig 6B). Patient 4 elicited an LI consistent with bilateral representation until P values fell below P < 4.16 × 10−5, when the LI suggested left-dominance. Patient 3 had a left-dominant LI for all thresholds except P = 4.16 × 10−5, where the LI was in the bilateral range. Only 1 patient with prior surgery performed the category task. However, depending on the statistical threshold used, this patient could be classified as demonstrating right language laterality (from P < .164–.071), bilateral language laterality (P < .025–.008), or left language laterality (from P < 4.16 × 10−5 to 3.74 × 10−6) (Fig 6C).
Fig 6.
Laterality index curves of patients with tumor with prior surgery performing the following: A, the verb-generation task. Both fMRIs in surgical patients who performed the verb task suggest left-lateralized language. B, the letter task. Patients with prior surgery lateralize language to the left. Patient 4 exhibits bilateral language until P < 4.16 × 10−5. C, the category task. Depending on the statistical threshold used, this patient could be considered right-dominant (from P < .164–3.17 × 10−4) or left-dominant (from P < 4.16 × 10−5 to 3.74 × 10−6).
Discussion
We examined LIs across a range of P values for control subjects and patients with brain tumor. We showed that the LI was influenced by statistical thresholding. Additionally, we demonstrated that the LI varied as a function of the presence or absence of a tumor and the choice of language paradigm. Patients showed more variability than control subjects. Verb generation was the least variable between tasks and across groups, whereas category was the most variable.
A few subjects demonstrated shifts from bilateral representations to left or right dominance. Bilaterality or codominance for language is beyond the methodology of this study because the ground truth for laterality in these subjects as a function of task is not known. There is likely some true bilaterality that is task-dependent. However, at the highest statistical stringencies, the LI measurement will be saturated at ±1. This saturation can be spurious or artifactual, even if it is based on a large number of voxels being counted. Baciu et al30 have described an “average magnitudes technique” in a study that compared the average signal-intensity variation in predefined language regions of interest across hemispheres with the standard laterality index ratio technique. The laterality index was found to be a more accurate representation of true laterality than the technique that relied on signal intensity. Additionally, Lohmann et al31 showed that task repetition and learning can artifactually elicit bilaterality for language, making the issue of bilaterality measurement by fMRI a complicated one.
The presence of adjacent and/or infiltrative tumor confounds the interpretation of the LI measurement. Previous studies suggest that tumor neovasculature interferes with the BOLD signal intensity, leading to an attenuation of BOLD fMRI activation in the tumor hemisphere.18–21 The 2 patients who demonstrated right-hemispheric dominance in the letter task had tumors in the left Broca area. The patient's true laterality may be altered if invasion of the functional language cortex by a tumor resulted in interhemispheric reorganization.9,32 Alternatively, tumor-induced neurovascular uncoupling, in which the tumor alters the coupling between neuronal activity and blood flow, may have occurred. Reduced signal intensity from the left hemisphere increases the weight of the right-hemispheric activation in the LI calculation, resulting in an LI that mistakenly indicates right-hemispheric language dominance. The concept of neurovascular uncoupling could make laterality index a less useful measure in patients with tumor. However, the fact that other patients with tumors showed consistency in language lateralization, performing 3 tasks, supports the idea that the laterality index does reliably predict language dominance. In addition, the 2 patients with right-hemispheric tumors mentioned previously who showed right-lateralization in the category task (Fig 4C) showed left-lateralization in both the letter and verb tasks (Fig 4A, -B), suggesting that category may just be a less reliable task because the other tasks correctly lateralized language to the dominant hemisphere.
In keeping with this finding, our data provide evidence that the language LI is dependent on the task that the subject performs. Language lateralization was the least variable during the verb task, followed by letter and then category. This is in agreement with prior studies that showed verb generation as the most reliable in lateralizing language.7,12,26 fMRI studies have shown that the categoric retrieval and object-naming tasks activate a distributed network extending from inferior frontal regions to posterior temporal regions in healthy subjects.33–35 It has been proposed that the temporal lobes are critical for retrieving information about objects in a hierarchical fashion from generic categories to unique objects.36,37 Patients with temporal lesions often have category-naming deficits, suggesting that posterior regions are involved in categoric retrieval.38–41 Consequently, we may have found greater reliability with the verb task because our region of interest included only the Broca area, and we may have obtained better results with category had we included a region of interest in the Wernicke area.
In recalling the 2 patients who exhibited right-hemispheric dominance in the category task, we found that in both cases, fMRI suggested left-lateralization when performing the letter and verb tasks. This suggests that a combination of tasks should be used to maximize accuracy in language-dominance determination. In terms of analysis-dependent ways to bolster the accuracy of the fMRI laterality measurement, a combined task analysis whose output is only those areas commonly activated by all language tasks has been suggested. This approach may reveal cortical areas essential for language function rather than regions that are supportive and variable among different language tasks.4,12,14,42
Although there were a very small number of patients with prior surgery, our results suggest that prior surgery further complicates the calculation of laterality. Susceptibility artifact may be a detriment to the reliable detection of the BOLD signal intensity.24 The LI curves in the patients with prior surgery varied more than those of healthy controls and patients with tumor without prior surgery (MAD letter patients with prior surgery = 0.22, MAD letter controls = 0.11). The verb-generation task in patients with prior surgery showed a similar variability compared with that of controls (MAD verb patients with prior surgery = 0.05, MAD verb controls = 0.06); however, further inference is limited because there were only 2 patients available for comparison in the prior surgery group.
As a final note, intraoperative mapping of tumor patient 1 elicited speech arrest in the right hemisphere, whereas fMRI predicted left-lateralization for language. This discrepancy could have occurred because the size of this tumor (2.8 cm anterior to posterior and 3.5 cm medial to lateral and 4.1 cm superior to inferior) may have limited the sensitivity of fMRI in the right hemisphere.
Overall, our study was limited by a small number of controls and patients with tumor and patients with tumor and prior surgery. Hence, further studies will be needed to confirm our results and further confirm the clinical implications of looking at a range of thresholds when using LI as a tool to determine preoperative language dominance in patients with tumor.
Conclusion
Our results show that the language LI measurement varies with changes in the statistical threshold. A spectrum of P values should be used to evaluate language dominance in patients with tumor. Furthermore, because the LI measurement varies on the basis of the language task, a variety of tasks should be used to determine language laterality. Our results suggest that fMRI reliably predicts language laterality across a spectrum of P values during the verb task, even when other confounding factors are present.
Supplementary Material
Footnotes
This work was supported by the Memorial Sloan-Kettering Cancer Summer Student Fellowship Program for the first author from the National Institutes of Health/National Cancer Institute.
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 3, 2006; San Diego, Calif.
Indicates article with supplemental on-line tables.
References
- 1.Maldjian JA, Schulder M, Liu WC, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assist Tomogr 1997;21:910–12 [DOI] [PubMed] [Google Scholar]
- 2.Schulder M, Maldjian JA, Liu WC, et al. Functional MRI-guided surgery of intracranial tumors. Stereotact Funct Neurosurg 1997;68:98–105 [DOI] [PubMed] [Google Scholar]
- 3.Hirsch J, Ruge MI, Kim KH, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery 2000;47:711–21 [DOI] [PubMed] [Google Scholar]
- 4.Roux FE, Boulanouar K, Lotterie JA, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery 2003;52:1335–45 [DOI] [PubMed] [Google Scholar]
- 5.Moritz C, Haughton V. Functional MR imaging: paradigms for clinical preoperative mapping. Magn Reson Imaging Clin N Am 2003;11:529–42 [DOI] [PubMed] [Google Scholar]
- 6.Ojemann GA. Functional mapping of cortical language areas in adults: intraoperative approaches. Adv Neurol 1993;63:155–63 [PubMed] [Google Scholar]
- 7.Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 1999;52:798–809 [DOI] [PubMed] [Google Scholar]
- 8.Brannen JH, Badie B, Moritz CH, et al. Reliability of functional MR imaging with word-generation tasks for mapping Broca's area. AJNR Am J Neuroradiol 2001;22:1711–18 [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovich NM, Holodny AI, Brennan CW, et al. Isolated translocation of Wernicke's area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. AJNR Am J Neuroradiol 2004;25:130–33 [PMC free article] [PubMed] [Google Scholar]
- 10.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 1996;46:978–84 [DOI] [PubMed] [Google Scholar]
- 11.Lehericy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 2000;54:1625–33 [DOI] [PubMed] [Google Scholar]
- 12.Rutten GJ, Ramsey NF, van Rijen PC, et al. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang 2002;80:421–37 [DOI] [PubMed] [Google Scholar]
- 13.Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 2003;61:699–701 [DOI] [PubMed] [Google Scholar]
- 14.Gaillard WD, Balsamo L, Xu B, et al. fMRI language task panel improves determination of language dominance. Neurology 2004;63:1403–08 [DOI] [PubMed] [Google Scholar]
- 15.Huber P. Functional tests in angiography of brain tumors. Neuroradiology 1970;1:132–41 [Google Scholar]
- 16.Bradac GB, Simon RS, Heidieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence on different CO2 tensions. Neuroradiology 1976;10:257–62 [DOI] [PubMed] [Google Scholar]
- 17.Pronin IN, Holodny AI, Kornienko VN, et al. The use of hyperventilation in contrast-enhanced MR of brain tumors. AJNR Am J Neuroradiology 1997;18:1705–08 [PMC free article] [PubMed] [Google Scholar]
- 18.Holodny AI, Schulder M, Liu WC, et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 1999;20:609–12 [PMC free article] [PubMed] [Google Scholar]
- 19.Holodny AI, Schulder M, Liu WC, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000;21:1415–22 [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber A, Hubbe U, Ziyeh S, et al. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol 2000;21:1055–63 [PMC free article] [PubMed] [Google Scholar]
- 21.Ulmer JL, Krouwer HG, Mueller WM, et al. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol 2003;24:213–17 [PMC free article] [PubMed] [Google Scholar]
- 22.Boxerman JL, Bandettini PA, Kwong KK, et al. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med 1995;34:4–10 [DOI] [PubMed] [Google Scholar]
- 23.Gao JH, Miller I, Lai S, et al. Quantitative assessment of blood inflow effects in functional MRI signals. Magn Reson Med 1996;36:314–19 [DOI] [PubMed] [Google Scholar]
- 24.Kim MJ, Holodny AI, Hou BL, et al. The effect of prior surgery on blood oxygen level-dependent functional MR imaging in the preoperative assessment of brain tumors. AJNR Am J Neuroradiol 2005;26:1980–85 [PMC free article] [PubMed] [Google Scholar]
- 25.Binder JR. Functional MRI of the language system. In: Moonen CT, Bandettini PA, eds. Functional MRI. Berlin, Germany: Springer-Verlag;1999. :407–19
- 26.Puccini S, Prothmann S, Dalitz B, et al. Preoperative Mapping of the Speech-Eloquent Areas with fMRI: Comparison of Different Task Designs—Proceedings of the Twelfth Scientific Meeting and Exhibition of the International Society of Magnetic Resonance in Medicine, Kyoto, Japan, 15–21 October, 2004. 2004. :11
- 27.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 28.Cox RW. AFNI software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73 [DOI] [PubMed] [Google Scholar]
- 29.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically healthy and epilepsy subjects: a functional MRI study. Brain 1999;122:2033–46 [DOI] [PubMed] [Google Scholar]
- 30.Baciu MV, Watson JM, Maccotta L, et al. Evaluating functional MRI procedures for assessing hemispheric language dominance in neurosurgical patients. Neuroradiology 2005;47:835–44 [DOI] [PubMed] [Google Scholar]
- 31.Lohmann H, Deppe M, Jansen A, et al. Task repetition can affect functional magnetic resonance imaging-based measures of language lateralization and lead to pseudoincreases in bilaterality. J Cereb Blood Flow Metab 2004;24:179–87 [DOI] [PubMed] [Google Scholar]
- 32.Holodny AI, Schulder M, Ybasco A, et. al. Translocation of Broca's area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr 2002;26:941–43 [DOI] [PubMed] [Google Scholar]
- 33.Martin A, Wiggs CL, Ungerleider LG, et al. Neural correlates of category-specific knowledge. Nature 1996;379:649–52 [DOI] [PubMed] [Google Scholar]
- 34.Grabowski TJ, Damasio H, Damasio AR. Premotor and prefrontal correlates of category-related lexical retrieval. Neuroimage 1998;7:232–43 [DOI] [PubMed] [Google Scholar]
- 35.Vitali P, Abutalebi J, Tettamanti M, et al. Generating animal and tool names: an fMRI study of effective connectivity. Brain Lang 2005;93:32–45 [DOI] [PubMed] [Google Scholar]
- 36.Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition 1989;33:25–62 [DOI] [PubMed] [Google Scholar]
- 37.Damasio H, Grabowski TJ, Tranel D, et al. A neural basis for lexical retrieval. Nature 1996;380:499–505 [DOI] [PubMed] [Google Scholar]
- 38.Tippett LJ, Glosser G, Farah MJ. A category-specific naming impairment after temporal lobectomy. Neuropsychologia 1996;34:139–46 [DOI] [PubMed] [Google Scholar]
- 39.Strauss E, Semenza C, Hunter M, et al. Left anterior lobectomy and category-specific naming. Brain Cogn 2000;43:403–06 [PubMed] [Google Scholar]
- 40.Luckhurst L, Lloyd-Jones TJ. A selective deficit for living things after temporal lobectomy for relief of epileptic seizures. Brain Lang 2001;79:266–96 [DOI] [PubMed] [Google Scholar]
- 41.Pouratian N, Bookheimer SY, Rubino G, et al. Category-specific naming deficit identified by intraoperative stimulation mapping and postoperative neuropsychological testing: case report. J Neurosurg 2003;99:170–76 [DOI] [PubMed] [Google Scholar]
- 42.Ramsey NF, Sommer IE, Rutten GJ, et al. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage 2001;13:719–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.