Abstract
BACKGROUND AND PURPOSE: The brain stem in patients with periventricular leukomalacia (PVL) appears smaller than normal on MR imaging, but few reports have described this feature, and the number of patients has been relatively small. The present study was conducted to examine the hypothesis that the pons in patients with PVL is smaller than normal.
MATERIALS AND METHODS: Using MR imaging, we examined 80 children (43 boys and 37 girls) with PVL and 80 age-matched control children (41 boys and 39 girls). The control children were diagnosed as neurologically and developmentally normal by pediatric neurologists and also showed normal MR imaging findings. MR imaging was performed at a corrected age range of 0–5 years in both groups. We measured the anteroposterior diameter of the whole pons, the tegmentum and the basis, and the corpus callosal length by using midline T1-weighted sagittal images and compared each parameter between the PVL groups and the control groups.
RESULTS: Pontine diameters in all of the regions were significantly smaller in the PVL group than in the control group (mean ± SD, whole pontine diameters, 1.66 ± 0.21 and 1.87 ± 0.23 cm [P < .001]; basis diameters, 0.42 ± 0.10 and 0.51 ± 0.14 [P < .001]; tegmentum diameters, 1.23 ± 0.20 and 1.36 ± 0.19 [P < .001], respectively). The respective corpus callosal lengths were 5.02 ± 0.90 and 5.51 ± 0.76 (P < .001). There was no significant difference in the basis/tegmentum ratio between the PVL group and the control group. When the age-related pontine diameter differences were examined, there was already a significant difference at 0 years of age between the 2 groups. There was a significant correlation between whole pontine diameter and corpus callosal length in the PVL group (correlation coefficient, 0.52; P < .001) and the control group (correlation coefficient, 0.63; P < .001).
CONCLUSION: We have proven that pontine diameter in patients with PVL is significantly smaller than that in normal control subjects, including each diameter of basis and tegmentum.
Periventricular white matter injury, that is, periventricular leukomalacia (PVL), is the dominant form of brain injury in premature infants that is later manifested as spastic cerebral palsy (spastic diplegia and spastic quadriplegia), with cognitive impairment and seizures in some children.1 The pathogenesis of this lesion is considered to be related to ischemia of the white matter, infection, hypotension, and hypocarbia or a combination of these factors.2 PVL consists of 2 components, that is, focal necrosis with loss of all cellular elements deep in the periventricular white matter and diffuse white matter involvement due to injury to glial cells, presumed to be oligodendroglia progenitors (preoligodendroglia).2 On MR imaging, the characteristic findings of PVL include volume loss of the white matter, associated with ventriculomegaly with irregular margin, cystic change, periventricular hyperintensity on T2-weighted images, and a thin corpus callosum.3,4 These are the results of white matter cavitation, loss, and gliosis.5
Although PVL is a form of deep white matter injury leading to loss of periventricular and deep white matter volume,2 its relationship to the development of white matter tracts is still unclear. A neuropathologic study has reported that the PVL lesions (spongy change with astrogliosis) in extremely low birth weight infants affect fibers involved in motor, sensory, visual, and higher cerebral function, predominantly in the descending corticospinal tract and in the thalamocortical tract, that is, descending motor tracts.5 On the other hand, a study using tractography has shown that the fibers connected to the sensory cortex are markedly reduced in comparison with the corticospinal tract in some patients with severe PVL.6 Recently, Nagae et al7 reported that the sensory fibers were affected in PVL patients more severely than motor fibers by using diffusion tensor imaging (DTI) of 24 children diagnosed with cerebral palsy.
In the brain stem, motor tracts descend through the ventral site, and sensory tracts ascend through the dorsal site.8,9 On conventional MR imaging, the brain stem in patients with PVL appears smaller than normal. With regard to the size of the whole pons on MR imaging, however, only one article has demonstrated a smaller pontine diameter in PVL children, and the number of patients that they examined was relatively small.10 The purpose of the present study is to examine the hypothesis that the pons in patients with PVL is smaller than in normal control subjects by using a larger series.
Materials and Methods
Subjects
We examined 80 patients with PVL (43 boys and 37 girls) diagnosed on the basis of MR imaging undertaken at a corrected age range of 0–5 years retrospectively. The diagnosis of PVL was performed by 2 radiologists (S.Y. and K.H.), who had enough experience of pediatric neuroradiology on the basis of MR imaging findings, including T2 prolongation in the periventricular white matter, diminishment of myelinated white matter volume, ventricular dilation with irregular margin, and thinning of corpus callosum. The corrected age distribution of the 80 patients with PVL was as follows: 20 patients (12 boys and 8 girls) in the 0-year group, 20 patients (11 boys and 9 girls) in the 1-year group, 20 patients (11 boys and 9 girls) in the 2-year group, 16 patients (6 boys and 10 girls) in the 3-year group, and 4 patients (3 boys and one girl) in the 4- and 5-year group. The mean gestational age of the patients with PVL was 30.6 weeks (±3.2 weeks; range, 22–42 weeks), and the mean gestational weight was 1554 g (±468 g; range, 572–2980 g). The PVL children with psychomotor delay or with risk of cerebral palsy have been clinically followed by pediatric neurologists and have received physiotherapy as early as possible. The clinical assessments on their outcomes were performed by pediatric neurologists at 3 to 5 years of age during their follow-up physiotherapy, including those with spastic diplegia (n = 56), spastic tetraplegia (n = 14), mixed cerebral palsy (n = 3), tetratripleplegia (n = 1), and children unable to be followed (n = 4). Two children were less than 3 years old on their last visits to an outpatient clinic and were diagnosed to have a high risk of cerebral palsy at that time by pediatric neurologists. We selected 80 age-matched healthy children (41 boys and 39 girls) as a control group. They received an MR imaging examination due to headache, transient febrile convulsion, and larger (or smaller) cephalic presentation. All were diagnosed as normal neurologically and developmentally by pediatric neurologists and had normal MR imaging findings. The mean gestational age of the control group was 38.9 weeks (±1.7 weeks; range, 34–41 weeks), and the mean gestational weight was 3034 g (±408 g; range, 2192–3912 g). The study was approved by the institutional review board of Kyoto City Hospital, and informed consent was obtained from the parents of all of the children.
MR Imaging
All of the MR imaging examinations were performed on a 1.5T MR machine. All of the children were sedated during MR imaging examinations. MR images included sagittal spin-echo (SE) T1-weighted images (TR, 560; TE, 15; section thickness, 5 mm; section spacing, 1.5 mm; 256 × 256 matrix); axial SE T1-weighted images; axial fast SE T2-weighted images; coronal fast SE T2-weighted images; axial diffusion-weighted images using single-shot, SE-type echo-planar sequence, along 3 independent axes (b-value = 0, 500, 1000); and T2*-weighted images. In all of the sequences, the FOV was 200 × 200 mm. We used the midline T1-weighted sagittal image for measurement.
Methods
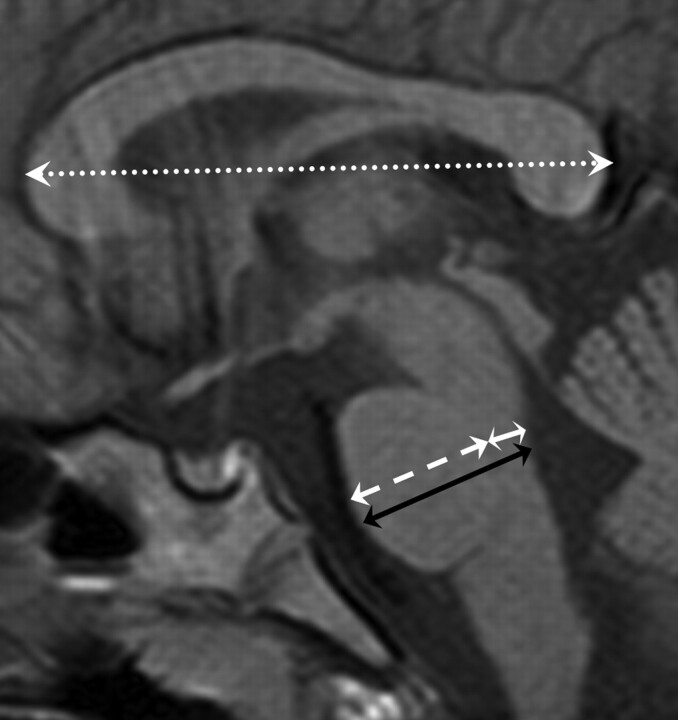
We measured the anteroposterior (AP) diameter of the whole pons, basis, and tegmentum and the length of the corpus callosum using the midline T1-weighted sagittal image (Fig 1). The AP diameter of whole pons was the maximal transverse length, which was defined to be perpendicular to the long axis of the pons. The basis was clearly differentiated from the tegmentum along the margin of the medial lemniscus (linear low signal intensity on T1-weighted image). The maximal transverse lengths from the medial lemniscus to the anterior line of the midpons and from the medial lemniscus to the posterior line of the midpons were defined as the AP diameter of the basis and tegmentum, respectively, which was defined to be perpendicular to the long axis of the pons. All of the measurements were made by only 1 person (S.Y.). We compared the mean of each measurement between the PVL groups and the control groups.
Fig 1.
SE T1-weighted midsagittal image (TR, 560; TE, 15) from which measurements of the AP diameter of the whole pons (black arrow), basis (white broken arrow), tegmentum (white arrow), and corpus callosal length (white dotted arrow) were obtained.
Statistics
The unpaired 2-tailed Student t test was used for statistical analysis. Differences at P < .05 were regarded as significant.
Results
The pontine diameters in all of the regions, that is, the whole pons, basis, and tegmentum, and the corpus callosal lengths were significantly smaller in the PVL group versus the control group (P < .001).
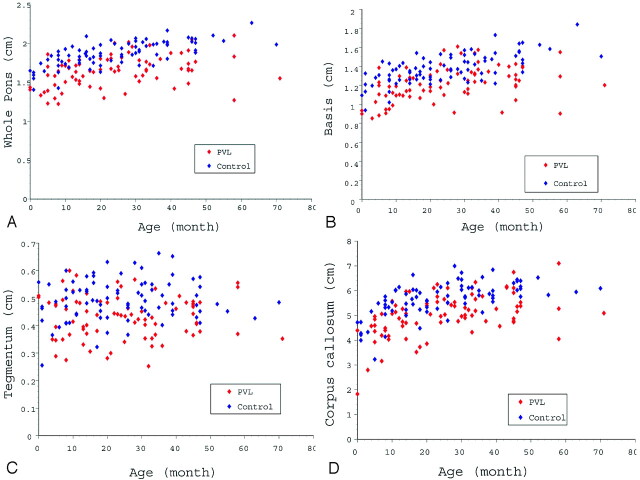
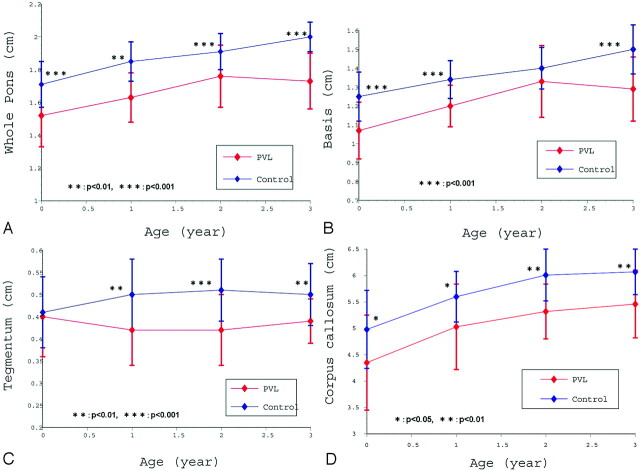
The whole pontine diameter in the PVL group (1.66 ± 0.21 cm) was significantly smaller than in the control group (1.87 ± 0.16; P < .001). A scattergram of whole pontine diameters in both groups is shown in Fig 2A. These measurements indicate the quantitative growth pattern in terms of the whole pontine diameter in the 2 groups. When the whole pontine diameters for each age group were calculated, there were significant differences for each age group between the 2 groups (Fig 3A).
Fig 2.
A, Whole pontine diameters in the PVL group and control group plotted with respect to age. The whole pontine diameter in the PVL group is smaller than in the control group. B, Basis diameters in the PVL group and control group plotted with respect to age. The basis diameter in the PVL group is smaller than in the control group. C, Tegmentum diameters in the PVL group and control group plotted with respect to age. Tegmentum diameter in the PVL group was smaller than in the control group. D, Corpus callosal lengths in the PVL group and control group plotted with respect to age. Corpus callosal length in the PVL group was smaller than in the control group.
Fig 3.
A, Relationship between each age group and mean of whole pontine diameter (with 1 SD). In each age group, the whole pontine diameter in the PVL group is significantly smaller than in the control group. B, Relationship between each age group and mean basis diameter (with 1 SD). In the 0-, 1-, and 3-year age groups, basis diameter in the PVL group was significantly smaller than in the control group. C, Relationship between each age group and mean tegmentum diameter (with 1 SD). In the 1-, 2-, and 3-year age groups, tegmentum diameter in the PVL group was significantly smaller than in the control group. D, Relationship between each age group and mean corpus callosal length (with 1 SD). In each age group, corpus callosal length in the PVL group was significantly smaller than in the control group.
The basis diameter in the PVL group (1.22 ± 0.19 cm) was also significantly smaller than in the control group (1.38 ± 0.16; P < .001). The quantitative growth patterns in terms of basis diameter for the 2 groups are shown in Fig 2B. When the basis diameters of the each age group were calculated, there were significant differences for the 0-, 1-, and 3-year age groups between the PVL group and the control group (Fig 3B).
The tegmentum diameter in the PVL group (0.43 ± 0.08 cm) was also significantly smaller than in the control group (0.49 ± 0.08; P < .001). The quantitative growth patterns in terms of tegmentum diameter in both groups are shown in Fig 2C. When the tegmentum diameters of each age group were calculated, there were significant differences for the 1-, 2-, and 3-year age groups between the PVL group and the control group (Fig 3C).
The corpus callosal length in the PVL group (5.04 ± 0.86 cm) was also significantly smaller than in the control group (5.66 ± 0.69; P < .001). The quantitative growth patterns in terms of corpus callosal length in the 2 groups are shown in Fig 2D. When the corpus callosal lengths of each age group were calculated, there were significant differences for all of the age groups between the PVL group and the control group (Fig 3D).
The basis/tegmentum ratio for each age group in the PVL patients and control children is shown in the Table. In the PVL group, the ratio of the basis to the tegmentum ranged from 0.71:0.29 (0-year group) to 0.75:0.25 (2- and 3-year groups), but the differences between the age groups were not significant. In the control group, the ratio of the basis to the tegmentum ranged from 0.73:0.27 (0-, 1-, and 2-year groups) to 0.78 to 0.22 (4- to 5-year groups), and none of the intergroup differences were significant. There was no statistically significant difference in the basis/tegmentum ratio between the PVL and control groups.
The basis/tegmentum ratio in each age group
| Variable, y | PVL Group |
Control Group |
||
|---|---|---|---|---|
| Basis | Tegmentum | Basis | Tegmentum | |
| 0 | 0.71 | 0.29 | 0.73 | 0.27 |
| 1 | 0.74 | 0.26 | 0.73 | 0.27 |
| 2 | 0.75 | 0.25 | 0.73 | 0.27 |
| 3 | 0.75 | 0.25 | 0.75 | 0.25 |
| 4–5 | 0.73 | 0.27 | 0.78 | 0.22 |
Note:—PVL indicates periventricular leukomalacia.
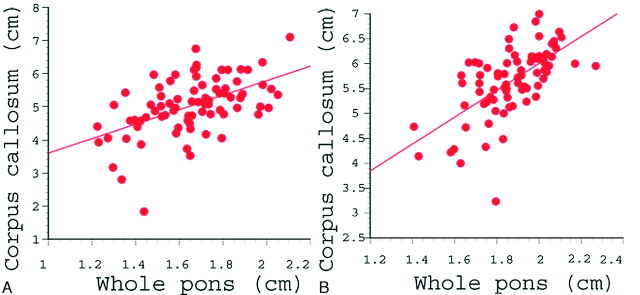
The correlation between the whole pontine diameter and corpus callosal length is shown in Fig 4. In the PVL group, the correlation coefficient was 0.52, which was strongly statistically significant (P < .001). In the control group, there was also a significantly strong correlation between the whole pontine diameter and the corpus callosal length (correlation coefficient, 0.63; P < .001).
Fig 4.
Correlation between whole pontine diameter and corpus callosal length in the PVL group (A) and the control group (B). In the PVL group (A), the correlation coefficient was 0.52, which was statistically significant (P < .001). The correlation coefficient in the control group (B) was 0.63, which was also statistically significant (P < .001).
Discussion
Pontine white matter tracts continue to the cerebral white matter tracts and transmit motor and sensory fibers to the face and body. The motor fibers descend through the basis, and the sensory fibers ascend through the tegmentum.8,9 With regard to the size of the whole pons on MR imaging, Argyropoulou et al10 examined 33 premature infants with PVL and reported that the AP diameter of the whole pons was significantly smaller than in a control group. However, the number of patients that they examined was relatively small, and there were no other studies regarding PVL patients evaluating the size of pons using conventional MR imaging.
In the present study, using a large series of 80 patients, we demonstrated that the diameters of the whole pons, basis, and tegmentum are significantly smaller in PVL patients than in control subjects. Scattergrams of quantitative growth patterns showed that the whole pons, basis, and tegmentum are reduced in volume in PVL patients compared with control subjects. In both groups, we proved that there was a significant correlation between whole pontine diameter and corpus callosal length. Furthermore, when the pontine region was compared among age groups, the whole pons and basis at 0 years of age in the PVL group were already significantly smaller than in the control group.
We consider 2 pathogenetic mechanisms for pontine hypoplasia, including the basis and tegmentum, in patients with PVL. First, focal necrotic change due to hypoxia and ischemia may interfere with development of the pons. On the basis of neuropathologic studies, Skullerud and Skjaeraasen11 and Mito et al12 reported that pontosubicular necrosis was associated with PVL in some patients. Although pontosubicular necrosis cannot explain the tegmentum hypoplasia because it involves neuronal karyorrhexis and gliosis in the basal pontine nuclei, it may occur in PVL and cause pontine basis hypoplasia.
Second, pontine hypoplasia may result from secondary degeneration of the white matter tracts associated with cerebral white matter injury. Because the pontine white matter tracts connect motor and sensory fibers to the cerebral white matter tracts, any interruption with secondary degeneration of the cerebral white matter tracts could result in pontine hypoplasia. Using MR imaging, we demonstrated a significant correlation between whole pontine diameter and corpus callosal length. It is generally accepted that the size and shape of the corpus callosum are sensitive indicators of the volume of the white matter and its myelination status.13,14 In addition, in sonography studies regarding very premature infants, the length of the corpus callosum at term equivalency was significantly shorter than that in control infants, and the growth rate of corpus callosal length has significant correlation with clinical outcome.15,16 We then consider that the length of the corpus callosum, as well as its size and shape, reflects the volume of the myelinated white matter. Consequently, the significant correlation between whole pontine diameter and corpus callosal length may reflect the volume of the cerebral myelinated white matter. Thus, there may be a correlation between pontine hypoplasia and other MR findings reflecting PVL severity, though we did not examine these parameters in the present study. Because in PVL patients at 0 years of age, the whole pons and basis were already significantly hypoplastic in comparison with the control subjects of the same age, it is considered that the developmental delay of the pons may occur as early as the antenatal or early postnatal stage.
In addition, we demonstrated that each of the pons, basis, and tegmentum are significantly smaller in PVL patients than in age-matched control subjects. Each part of the pons, basis, and tegmentum in PVL appears hypoplastic on MR imaging, but a literature survey reveals no study that has documented this. It has been believed that the corticospinal tract is predominantly injured in PVL. That is, the clinical abnormalities in PVL, which include spastic diplegia or quadriplegia, reflect the anatomic location of the lesion in the fibers from the motor cortex that descend medially in the periventricular white matter in proximity to the lateral ventricles, an area that is most vulnerable to PVL.17 However, in this study, we found that the tegmentum, as well as the basis, was also significantly smaller in PVL patients than in control subjects and that there was no significant difference in the ratio of the basis to the tegmentum between the PVL and the control groups. This suggests that in PVL, not only the motor tracts but also the sensory tracts may be disturbed. In a study using tractography, Hoon et al6 reported that the fibers connected to the sensory cortex were markedly reduced in comparison with those of the corticospinal tract in some patients with severe PVL and suggested that the posterior thalamocortical fibers might be affected. In addition, Nagae et al7 demonstrated by using DTI that the corticospinal tract was often affected in PVL children, though the sensory tract has been disturbed more severely. Regardless of simple methods using conventional MR imaging, our results consist of studies using DTI and show that both the basis and tegmentum are significantly affected in PVL children compared with normal controls. DTI, including tractography, is a very useful method for demonstrating tracts projecting to the cerebral white matter and brain stem,18 and we expect that the etiology of the pontine hypoplasia in PVL will be evaluated by using DTI in future.
In addition, the present study demonstrated that the length of the corpus callosum was significantly smaller in PVL patients than in age-matched control subjects. Thinning of the corpus callosum is commonly seen in preterm children with PVL and is thought to be a secondary manifestation due to white matter loss.1 In reports on a bedside sonography study about very premature infants, the lengths of the corpus callosum at term equivalency were significantly shorter than those in control infants, and the growth rate of corpus callosal length to term equivalent age has significant correlation with clinical outcome.16,17 Our study proved that the quantitative growth patterns of the corpus callosal length in the PVL group are significantly different from the control groups after the infant period. We consider that this result supports the possible explanation that the growth pattern of corpus callosal length may reflect growth and maturation of white matter itself. In addition to the length, measurement of the corpus callosal thickness might have the reflected growth pattern of the white matter volume more appropriately. In this study, we measured corpus callosum in length, but did not measure the thickness or whole area. As the child brain is small and the child corpus callosum is very thin, the slice thickness of our protocol (5 mm) can induce unacceptable error because of the partial volume effect. It is one of the limitations in this study.
There are also other limitations in our study. We measured only AP diameter of the pons by using the midline T1-weighted sagittal image. This is quite a simple, convenient method, without the need for any special tool, and is not time consuming. So this method may be useful for busy pediatric clinicians or to measure the large series. However, the AP diameter is only a 1D parameter, and the measurement and calculation of area and/or volume are needed to evaluate pons more precisely. Moreover, the correlation between pontine size and other MR imaging and clinical findings reflecting PVL severity could not be demonstrated in this study. It might be useful for clinicians to predict the children's outcomes and the necessity for the early physiotherapy.
In conclusion, we have demonstrated quantitatively that the diameter of the whole pons is significantly smaller in PVL patients than in age-matched normal control subjects using a large series. There was a significant correlation between whole pontine diameter and corpus callosal length. When comparisons were made among age groups, there were already significant differences at 0 years of age between PVL patients and control subjects. In addition, we demonstrated for the first time that each of the pons, basis, and tegmentum is significantly smaller in PVL patients than in control subjects.
References
- 1.Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia: WB Saunders;2001;307–15, 362–63
- 2.Blumenthal I. Periventricular leukomalacia: a review. Eur J Pediatr 2004;163:435–42 [DOI] [PubMed] [Google Scholar]
- 3.Olsen P, Paakko E, Vainionpaa L, et al. Magnetic resonance imaging of periventricular leukomalacia and its clinical correlation in children. Ann Neurol 1997;41:754–61 [DOI] [PubMed] [Google Scholar]
- 4.Melhem ER, Hoon AH, Ferrucci JT, et al. Periventricular leukomalacia: relationship between lateral ventricular volume on brain MR images and severity of cognitive and motor impairment. Radiology 2000;214:199–204 [DOI] [PubMed] [Google Scholar]
- 5.Okoshi Y, Ito M, Takashima S. Characteristic neuropathology and plasticity in periventricular leukomalacia. Pediatr Neurol 2001;25:221–26 [DOI] [PubMed] [Google Scholar]
- 6.Hoon AH, Lawrie WT, Melhem ER, et al. Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology 2002;59:752–56 [DOI] [PubMed] [Google Scholar]
- 7.Nagae LM, Hoon AH, Stashinko E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol 2007;28:1213–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman RI, Yousem MD. Neuroradiology. The Requisites. 2nd ed. Philadelphia: Elsevier;2003. :55–66
- 9.Takahashi A. Brain MRI. Volume 1. Normal Anatomy. 2nd ed. Tokyo: Shujun-sha;2005. :174–84
- 10.Argyropoulou MI, Xydis V, Drougia A, et al. MRI measurement of the pons and cerebellum in children born preterm; associated with the severity of periventricular leukomalacia and perinatal risk factors. Neuroradiology 2003;45:730–34 [DOI] [PubMed] [Google Scholar]
- 11.Skullerud K, Skjaeraasen J. Clinicopathological study of germinal matrix hemorrhage, pontosubicular necrosis, and periventricular leukomalacia in stillborn. Child's Nerv Syst 1988;4:88–91 [DOI] [PubMed] [Google Scholar]
- 12.Mito T, Kamei A, Takashima S, et al. Clinicopathological study of pontosubicular necrosis. Neuropediatrics 1993;24:204–07 [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa K, Kanda T, Hashimoto K, et al. MR imaging of spastic diplegia. The importance of corpus callosum. Acta Radiol 1996;37:830–36 [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa K, Konishi Y, Matsuda T, et al. Development and aging of brain midline structures: assessment with MR imaging. Radiology 1989;172:171–77 [DOI] [PubMed] [Google Scholar]
- 15.Anderson NG, Laurent I, Cook N, et al. Growth rate of corpus callosum in very premature infants. AJNR Am J Neuroradiol 2005;26:2685–90 [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson NG, Laurent I, Cook N, et al. Detection of impaired growth of the corpus callosum in premature infants. Pediatrics 2006;118:951–60 [DOI] [PubMed] [Google Scholar]
- 17.Swaiman FK, Ashwal S, Ferriero MD. Pediatric Neurology. Principles & Practice. 4th ed. Philadelphia: Elsevier;2006;288–89, 318–20
- 18.Lee SK, Kim DI, Kim F, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 2005;25:53–65 [DOI] [PubMed] [Google Scholar]