Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to review CT imaging findings of soft tissue mucosal ulceration in patients following radiation treatment for head and neck malignancies and to correlate these with patient outcomes.
MATERIALS AND METHODS: The CT examinations in 20 patients with soft tissue ulceration after radiation therapy for treatment of head and neck cancer were reviewed. External beam radiation therapy was completed between 3 and 61 months (mean, 11.5 months) before the initial diagnosis of soft tissue ulceration. In all 20 patients, the initial diagnosis was made or confirmed on CT examination.
RESULTS: Of the 20 ulcerations, 12 did not demonstrate enhancement, and the results of biopsy in 9 of these 12 were negative. Of the 12 nonenhancing ulcerations, biopsy was not performed in 3, but they have been followed clinically and radiologically for 15.7 months without evidence of recurrence. Of the 20 ulcerations, 8 demonstrated adjacent enhancement, and the results of a biopsy in 4 were positive for recurrent cancer and negative in 2; these 2 have been followed for 16.3 months without evidence of recurrence. Biopsy was not performed in 2 ulcerations, but they have been followed for 15.0 months without evidence of recurrence.
CONCLUSION: For soft tissue ulceration occurring after radiation treatment, if there is no enhancement or clinical evidence of recurrence, it is likely benign and follow-up without biopsy seems warranted. If the ulceration is associated with adjacent enhancement, then differentiation between radiation necrosis and recurrent tumor is difficult. In these cases, correlation with clinical examination with close interval follow-up is necessary if a biopsy is not performed.
Squamous cell carcinoma of the head and neck occurs in approximately 40,000 patients annually in the United States1 and is often treated with radiation therapy. The radiation treatment causes endothelial damage and fibrosis, leading to impairment of vascular and lymphatic flow.2 This impairment produces hypoxic, hypocellular, and hypovascular tissue, which is unable to maintain normal tissue turnover3,4 resulting in tissue necrosis, infection, and ulceration.4–6 Ulceration is defined as a defect, or excavation, of the surface of a tissue or organ, which is produced by the sloughing of inflammatory necrotic tissue.7 On CT, soft tissues within the treatment field may demonstrate inflammatory swelling, fragmentation, and ulceration.8 However, recurrent tumor may also present clinically as a soft tissue ulceration,9–11 leading to difficulty in the diagnosis and management of these patients.
To our knowledge, no previous reports on the imaging appearance of soft tissue ulcerations that occur in patients after radiation treatment of head and neck cancer have been published in the radiology literature. The purpose of this study was to review the CT imaging findings of benign and malignant soft tissue ulceration in 20 patients who had undergone radiation therapy for head and neck cancer and to correlate with the patients’ outcomes.
Materials and Methods
The institutional review board approved this study and waived the requirement for informed consent. We reviewed the clinical records and imaging findings of 20 patients who underwent radiation therapy as part of the treatment of head and neck cancer and were diagnosed with a mucosal ulceration between September 2001 and August 2005.
Of 20 patients, 13 were men and 7 were women with an age range of 46 to 78 years (mean, 61 years). Primary tumors included squamous cell carcinoma in 19 patients of the oropharynx (n = 12), oral tongue (n = 3), larynx (n = 3), and hypopharynx (n = 1). One patient had poorly differentiated carcinoma of the nasopharynx. A total of 17 patients underwent radiation therapy at our institution (60–72 Gy; mean, 68.5 Gy, in 30–42 fractions), and 3 patients underwent radiation therapy at an outside institution, where the radiation dose was not available in the clinical notes. The radiation therapy was completed between 3 and 61 months (mean, 11.5 months; median, 5.5 months) before the initial diagnosis of soft tissue radiation necrosis in all 20 patients.
A total of 20 CT examinations with evidence of a mucosal ulceration, obtained as part of routine posttreatment follow-up between January 2001 and January 2006, were evaluated in the 20 patients for initial characterization of the soft tissue ulcerations. The ulcerations were originally detected on both clinical and radiologic examinations, 13 of them on radiologic examination alone because 3 of these were not clinically evident; 4 were not available on clinical examination and later on radiologic examination between 1 and 3 months (mean, 1.8 months) after the clinical diagnosis. The CT examinations were performed on scanners (Light Speed; GE Healthcare, Milwaukee, Wis) after intravenous administration of contrast material. Imaging in the axial plane was performed with the following section thicknesses: 1.25 mm (n = 11), 2.5 mm (n = 3), 3.75 mm (n = 1), and 5.0 mm (n = 5). Soft tissue and bone windows were evaluated in all patients. In 15 of the 20 patients, a biopsy was performed on the ulceration, results confirming either radiation necrosis or recurrent tumor. The remaining 5 patients were followed up clinically and radiologically between 8 and 30 months (mean, 15.4 months). Five positron-emission tomography (PET)/CT examinations were obtained in 4 patients, 2 before (1–2 weeks; mean, 1.5 weeks) and 3 after (1–2.5 months; mean, 1.7 months) biopsy.
Results
Sixteen patients complained of pain or odynophagia, or both. Two patients did not complain of pain but did report weight loss; one had a percutaneous endoscopic gastrotomy tube and the other was receiving a liquid diet. The remaining 2 patients were asymptomatic. Eighteen of the ulcerations occurred at the same location as the primary tumor. One patient was treated with a total laryngectomy followed by radiation therapy and developed an ulceration in the base of the tongue. The other patient had a squamous cell carcinoma of the left false vocal cord and developed ulceration in the epiglottis.
The types of ulcerations with their enhancement characteristics (enhancing or nonenhancing) and results of the biopsy for recurrent tumor (positive, negative, not performed) have been divided into the following 5 categories. Patient data are provided in the accompanying Table.
Summary of clinical data of patients with soft tissue ulceration
| Patient No. | Category | Age/Sex | Rad Dose (Gy) | Months After XRT | Location of Ulceration | Enhancement | Biopsy Results |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 55/M | 66 | 4.5 | Left palatine tonsil | None | Negative |
| 2 | 1 | 53/M | 66 | 8.5 | Left palatine tonsil | None | Negative |
| 3 | 1 | 46/M | 72 | 7 | Left oropharyngeal wall | None | Negative |
| 4 | 1 | 58/M | 70 | 6.5 | Left vocal cords | None | Negative |
| 5 | 1 | 59/F | 60 | 3.5 | Right oral tongue | None | Negative |
| 6 | 1 | 50/M | OSH | 5 | Left base of tongue | None | Negative |
| 7 | 1 | 61/F | OSH | 12 | Epiglottis | None | Negative |
| 8 | 1 | 59/M | 70 | 3.5 | Epiglottis | None | Negative |
| 9 | 1 | 51/F | 70 | 3 | Right base of tongue | None | Negative |
| 10 | 2 | 70/M | 60 | 3.5 | Left lateral nasopharynx | None | N/A |
| 11 | 2 | 75/F | 70 | 5 | Left oropharyngeal wall | None | N/A |
| 12 | 2 | 64/M | 70 | 5 | Right floor of mouth | None | N/A |
| 13 | 3 | 46/M | 70 | 7 | Left pyriform sinus | Submucosal | Positive |
| 14 | 3 | 63/F | 76 | 9 | Midline oral tongue | Submucosal | Positive |
| 15 | 3 | 70/M | 66 | 6.5 | Right oral cavity | Nodular | Positive |
| 16 | 3 | 54/M | 66 | 5 | Left palatine tonsil | Submucosal | Positive |
| 17 | 4 | 78/F | 72 | 10.5 | Right retromolar trigone | Heterogeneous | Negative |
| 18 | 4 | 70/M | OSH | 61 | Base of tongue | Peripheral/Nodular | Negative |
| 19 | 5 | 64/M | 70 | 5 | Left oropharyngeal wall | Peripheral | N/A |
| 20 | 5 | 74/F | 70 | 6 | Left oropharynx | Peripheral | N/A |
Note:—Rad indicates radiation; OSH, outside hospital; XRT, external beam radiation therapy; N/A, biopsy not performed.
N.B.—Categoric description of ulcerations is author's terminology.
Category 1: Nonenhancing Ulcerations, Negative Biopsy Results (n = 9)
Nine ulcerations did not demonstrate enhancement, and results of the biopsy were negative (Fig 1). Eight were followed clinically and radiologically between 5 and 28 months (mean, 16.2 months) without evidence of recurrence. The final patient in this group underwent a total laryngectomy for airway compromise related to the radiation therapy. Pathologic examination reported no evidence of recurrent tumor within the ulceration and in the other submitted specimens.
Fig 1.
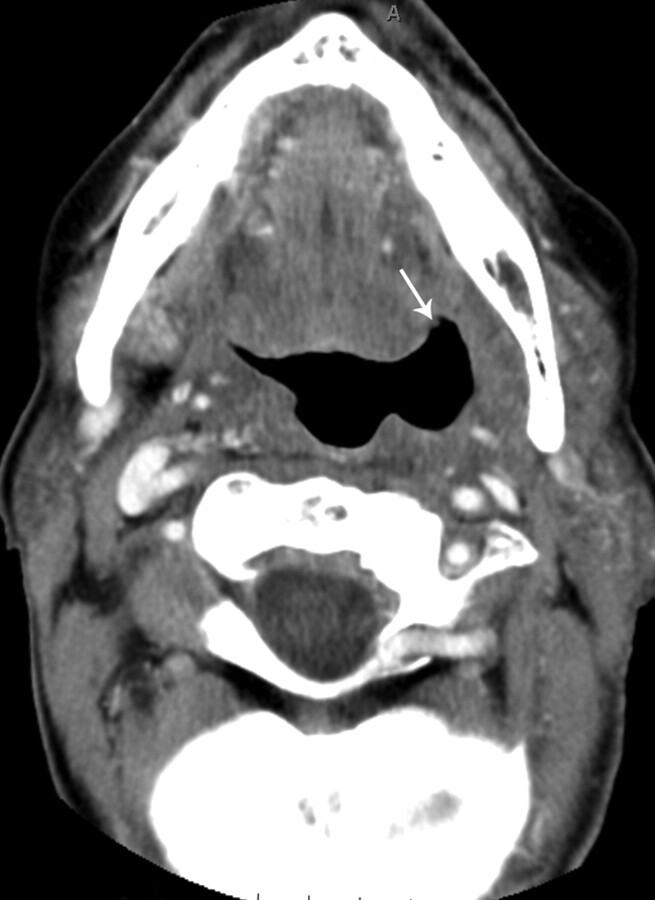
Patient 3, a 46-year-old man status post 7 months of radiation therapy (72 Gy) for treatment of a squamous cell carcinoma of the left oropharynx, with a nonenhancing ulceration with negative biopsy results (category 1) in the left oropharynx (arrows). This patient has been followed for 11.5 months since initial diagnosis without evidence of tumor recurrence.
Two of the nonenhancing ulcers with negative biopsy results underwent a PET/CT examination, which demonstrated [18F]fluorodeoxyglucose (18F-FDG) avidity. The first patient had a negative biopsy result 1 week before an 18F-FDG PET/CT (standardized uptake value [SUV], 5.5 mg/mL) and a second negative biopsy result 1 month after the PET/CT. This patient has subsequently been followed up for 14 months clinically and radiologically, with CT examinations demonstrating no evidence of tumor recurrence. The second patient (Fig 2A and B) underwent biopsy of a nonenhancing ulceration of the right lateral part of the oral tongue because of increase in pain and clinical suspicion of a recurrent tumor. The results of this biopsy were negative, and a PET/CT scan obtained 2.5 months later was suspicious for a recurrent tumor (SUV, 12 mg/mL). The results of a clinical examination were consistent with a healing ulcer, and the patient was observed. Although the ulcer was nonenhancing and seemed to be regressing on imaging, because of clinical persistence of the ulceration and associated pain, a second biopsy (13 months after the first biopsy) was performed, the results of which were also negative for a recurrent tumor.
Fig 2.
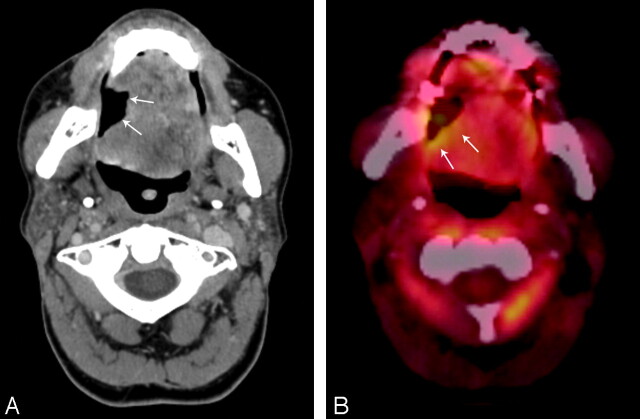
Patient 5, a 59-year-old woman status post 3.5 months of radiation therapy (60 Gy) for treatment of squamous cell carcinoma of the oral tongue, with a nonenhancing ulceration with negative biopsy results (category 1). This patient has been followed for 16 months since initial diagnosis without evidence of tumor recurrence. A, Contrast-enhanced CT with ulceration in the right lateral part of the oral tongue (arrows). B, PET/CT with 18F-FDG activity (SUV, 12 mg/mL) along the posterior border (arrows) on the ulceration, suspicious for a recurrent tumor.
Category 2: Nonenhancing Ulcerations, Biopsy Not Performed (n = 3)
There were 3 ulcerations that were without enhancement and in which a biopsy was not performed. These patients were followed up clinically and radiologically for 8 to 30 months (mean, 15.7 months) without evidence of recurrence (Fig 3). One of these patients developed osteoradionecrosis (ORN) of the hyoid bone that was separate from an ulceration of the right base of the tongue.
Fig 3.
Patient 11, a 75-year-old woman status post 5 months of radiation therapy (70 Gy) for treatment of squamous cell carcinoma of the left oropharyngeal wall, with a nonenhancing ulceration of the left oropharynx (arrow) in which a biopsy was not performed (category 2). This patient has been followed for 30 months since initial diagnosis without evidence of tumor recurrence.
Category 3: Enhancing Ulcerations, Positive Biopsy Results (n = 4)
Of the 20 ulcerations, 4 demonstrated adjacent enhancement (submucosal [n = 3], nodular [n = 1]), biopsy results were positive for recurrent disease (Fig 4A), and these patients underwent further treatment of recurrent tumor. Two of these patients, both with oral cavity carcinoma, developed ORN of the mandible. One of the areas of ORN involved the anterior midline part of the mandible, separate from the ulceration of the oral tongue, and the other in the right mandibular ramus adjacent to an ulceration of the right oral cavity.
Fig 4.
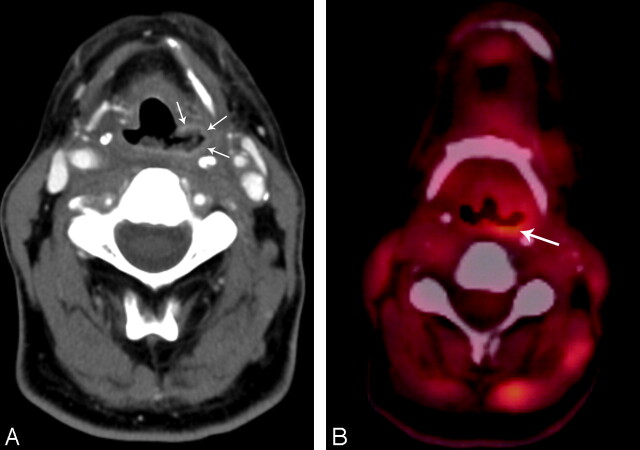
Patient 13, a 46-year-old man status post 7 months of radiation therapy (70 Gy) for treatment of a squamous cell carcinoma of the left oropharynx and hypopharynx, with an enhancing ulceration with positive biopsy results (category 3) of the left pyriform sinus. A, Contrast-enhanced CT with an enhancing ulceration of the pyriform sinus (arrows). B, PET/CT with 18F-FDG activity (SUV, 4.6 mg/mL) along the posterior border of the ulceration (arrow).
A PET/CT examination was also performed in 2 of the enhancing (submucosal [n = 2]) ulcerations with positive biopsy results at approximately the same time of biopsy (2 weeks before and 1.5 months after biopsy), which demonstrated 18F-FDG avidity (SUV, 6 and 4.6 mg/mL, respectively) (Fig 4B).
Category 4: Enhancing Ulcerations, Negative Biopsy Results (n = 2)
Results of a biopsy on 2 ulcerations with enhancement (peripheral and nodular [n = 1], heterogeneous [n = 1]) were negative for recurrent tumor (Fig 5) and were subsequently followed clinically and radiologically for 13 and 20 months (mean, 16.5 months) without evidence of recurrence. One of these patients developed ORN of the mandibular ramus, adjacent to an ulceration, which extended into the right retromolar trigone.
Fig 5.
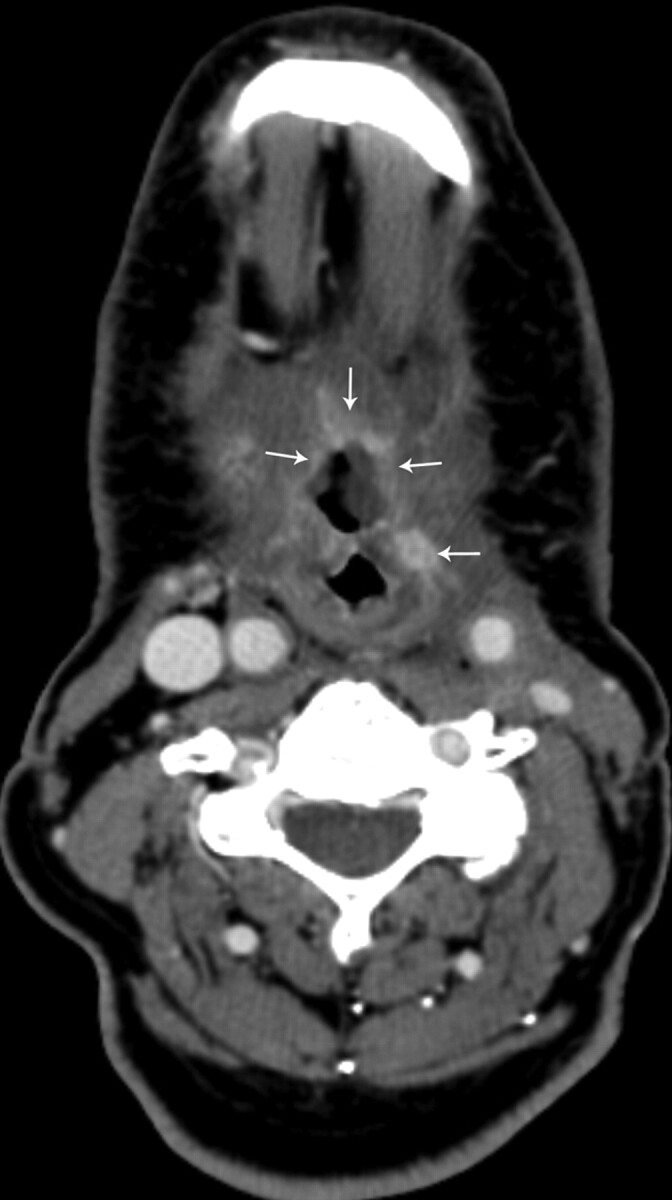
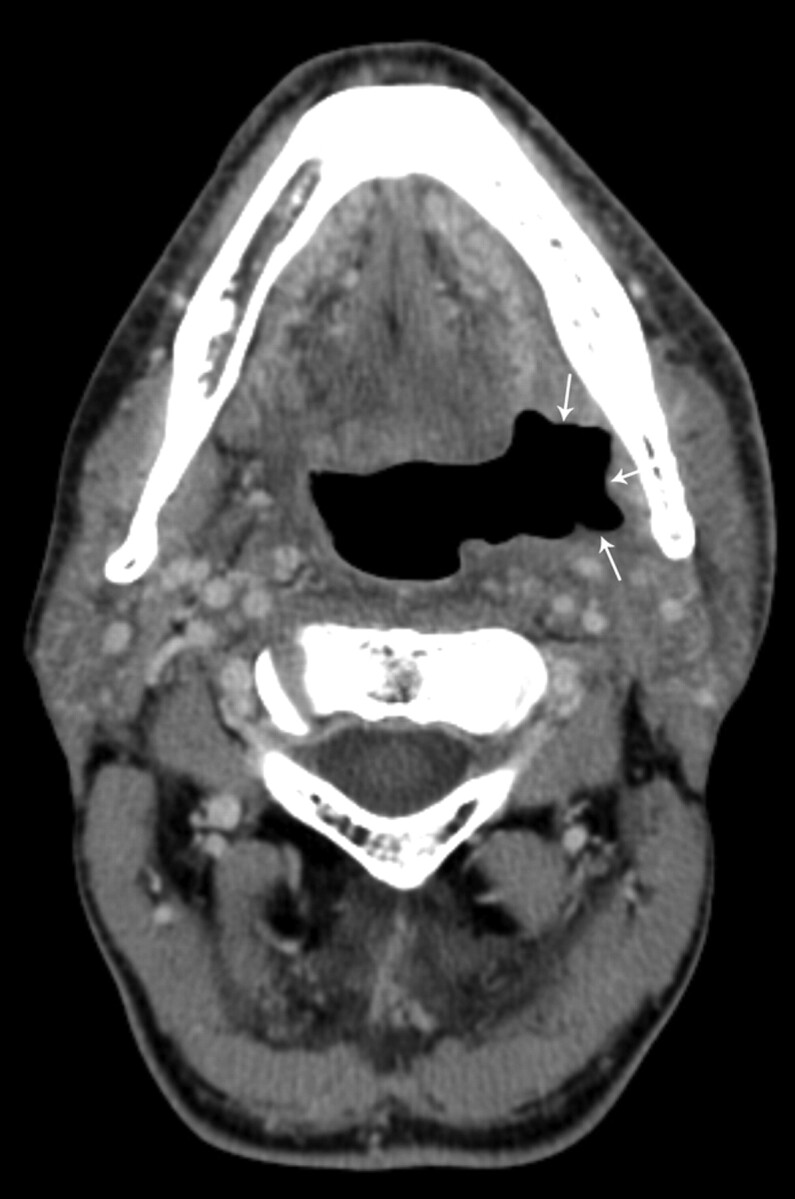
Patient 18, a 70-year-old men status post 61 months of radiation therapy for treatment of a squamous cell carcinoma of the larynx, followed by total laryngectomy with an enhancing ulceration with negative biopsy results (category 4) of the base of the tongue. There is adjacent peripheral and nodular enhancement (arrows). This patient has been followed for 19.5 months since initial diagnosis without evidence of tumor recurrence.
Category 5: Enhancing Ulcerations, Biopsy Not Performed (n = 2)
In 2 of the ulcerations with enhancement (peripheral [n = 2]), a biopsy was not performed because these were not suspicious on clinical examination. They were followed clinically and radiologically for 14 and 20 months (mean, 17 months) without evidence of recurrence (Fig 6).
Fig 6.
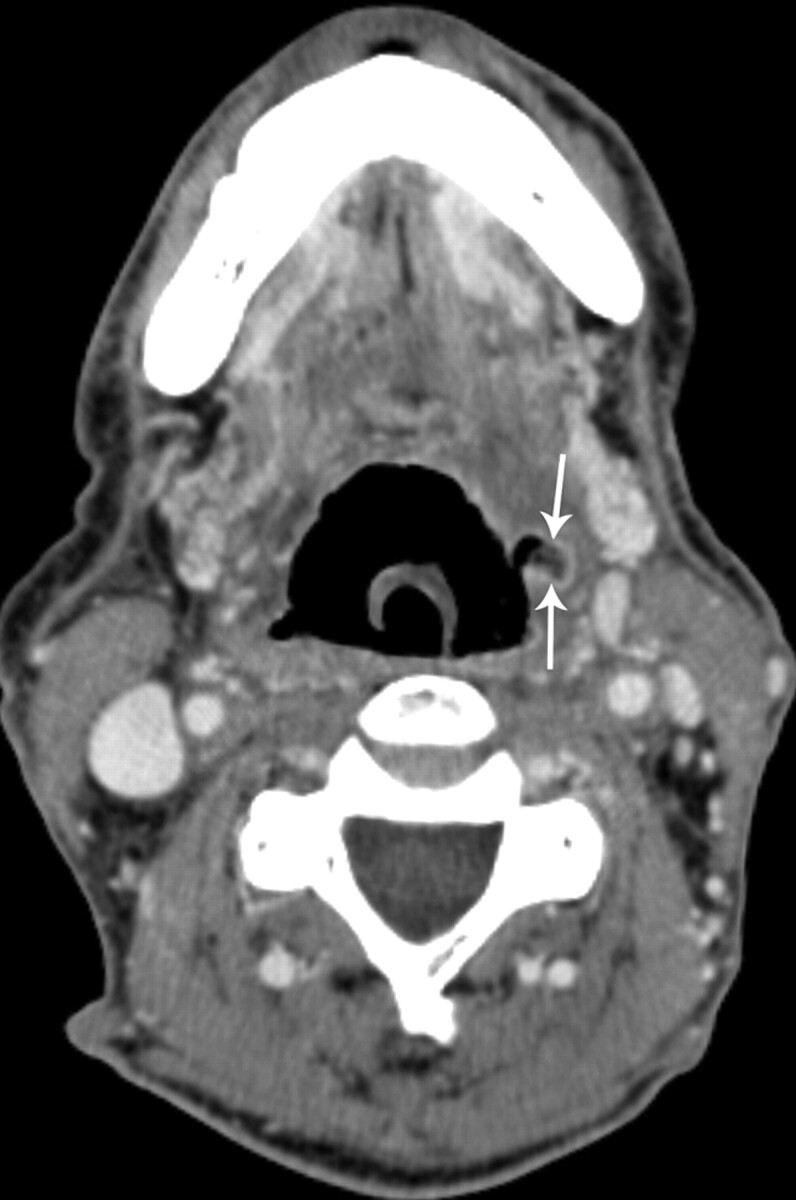
Patient 19, a 64-year-old man status post 5 months of radiation therapy (70 Gy) for treatment of a squamous cell carcinoma of the left tonsil, with an enhancing ulceration in the left oropharyngeal wall (arrows) in which biopsy was not performed (category 5). This patient has been followed for 20 months since initial diagnosis without evidence or tumor recurrence.
Discussion
Our findings that all 12 ulcerations without adjacent enhancement failed to demonstrate evidence of recurrent tumor, either with biopsy results or on follow-up imaging, suggest that an ulceration without adjacent enhancement is benign and is likely radiation induced. When ulcerations demonstrate adjacent enhancement, careful observation is required if biopsy is not performed because 4 of 8 ulcerations with adjacent enhancement demonstrated recurrent tumor, whereas the other 4 ulcerations were free of tumor on biopsy results or on follow-up clinical and radiologic evaluations.
The risk for injury related to radiation necrosis is greatest during the first 6 to 12 months after radiation therapy.12,13 Our results were similar, with radiation therapy completed between 3 and 61 months (mean, 11.5 months; median, 5.5 months) before the initial radiologic diagnosis of soft tissue ulceration. However, most necroses and many recurrences occur within 2 years after radiation therapy,14 so the time of onset of the ulceration is usually not helpful in distinguishing between radiation injury and recurrent tumor.
Imaging of the head and neck in patients treated for cancer is routinely performed to evaluate for recurrent tumor and is complementary to physical examination. CT examinations can evaluate the underlying soft tissues, which cannot be visualized on physical examination. Hermans et al15 found that CT examination detected treatment failure in 12 (41%) of 29 of patients at the primary site, a mean of 5.5 months before results of clinical examination in laryngeal and hypopharyngeal carcinoma.
Osteoradionecrosis is a known complication of radiation therapy for malignant tumors of the head and neck.16–18 Four of the patients in our series developed osteoradionecrosis, which was adjacent to the soft tissue ulceration in 2 patients (categories 2 and 3) and was remote from the ulceration in the other 2 patients (categories 3 and 4). Overall, we did not find the presence or absence of associated osteoradionecrosis to be beneficial in differentiating soft tissue ulcerations as benign or harboring a recurrent tumor.
PET/CT is now often used to follow patients after treatment of malignant tumors of the head and neck, with a reported specificity of 18F-FDG-PET ranging between 67% and 93% for recurrent squamous cell carcinoma.19–22 An SUV after therapy of more than 323,24 or 425 mg/mL is regarded as positive for a recurrent tumor. In our series, 4 patients underwent a PET/CT examination, and all 4 studies were suspicious for a recurrent tumor. The results of 2 of these studies were true-positives (SUV, 4.6 and 6 mg/mL) for recurrent tumor, both in cases in which the ulceration had adjacent enhancement. The results of 2 cases were false-positives (SUV, 5.5 and 12 mg/mL), and in both cases, there was no adjacent enhancement around the ulceration. The false-positive result was likely related to radiation-induced pharyngitis,26 in which therapy causes an inflammatory or leukocytic infiltrate with 18F-FDG uptake in macrophages, fibroblasts, and granulation tissue.27–29 Therefore, a positive PET/CT examination must correlate with information from the physical examination and cross-sectional imaging because nonspecific inflammation may often be 18F-FDG avid, as illustrated by Quon et al.21 Our results further suggest that PET/CT may not be necessary if no enhancement around a soft tissue ulceration develops after radiation therapy. It should also be noted that the results of a biopsy obtained before a PET/CT examination may lead to a false-positive study.30
Before biopsy of a soft tissue ulceration is performed, information from the CT, PET/CT, and physical examination should be compared because deep biopsies can aggravate existing necrosis,31 further complicating imaging findings. Our results suggest that ulceration without enhancement is benign and can be followed without the need for biopsy.
Conclusion
On CT examination, soft tissue radiation necrosis may have the appearance of an ulceration, possibly with adjacent enhancement. From a radiologic prospective, if the ulceration is free of adjacent enhancement, then it is likely radiation induced and benign, and continued monitoring without biopsy or PET/CT examination seems warranted. If the ulceration is associated with adjacent enhancement, then differentiation between radiation necrosis and recurrent tumor is difficult. In these cases, correlation with clinical examination with close interval follow-up is necessary if biopsy is not performed. The radiologist should be aware of the CT finding of a benign ulceration as a side effect of radiation treatment of malignant tumors of the head and neck to avoid misinterpreting this finding as a recurrent tumor or other process. Knowledge of recent radiation treatment, surgical history, and clinical examination will assist the radiologist in making the correct diagnosis of benign soft tissue radiation necrosis.
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 3, 2006; San Diego, Calif.
References
- 1.Carvalho AL, Nishimoto IN, Califano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer 2005;114:806–16 [DOI] [PubMed] [Google Scholar]
- 2.Alexander FW. Micropathology of radiation reaction in the larynx. Ann Otol Rhinol Laryngol 1963;72:831–41 [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Osteoradionecrosis: a new concept for its pathophysiology. J Oral Maxillofac Surg 1983;41:283–88 [DOI] [PubMed] [Google Scholar]
- 4.Mathes SJ, Alexander J. Radiation injury. Surg Oncol Clin N Am 1996;5:809–24 [PubMed] [Google Scholar]
- 5.Ben-Yosef R, Kapp DS. Persistent and/or late complications of combined radiation therapy and hyperthermia. Int J Hyperthermia 1992;8:733–45 [DOI] [PubMed] [Google Scholar]
- 6.Gibbs IC, Le QT, Shah RD, et al. Long-term outcomes after external beam irradiation and brachytherapy boost for base-of-tongue cancers. Int J Radiat Oncol Biol Phys 2003;57:489–94 [DOI] [PubMed] [Google Scholar]
- 7.Dorland's Illustrated Medical Dictionary. Philadelphia: W.B. Saunders;1988. .
- 8.Becker M, Schroth G, Zbären P, et al. Long-term changes induced by high-dose irradiation of the head and neck region: imaging findings. Radiographics 1997;17:5–26 [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, deSilva BW, Buckley BM, et al. Role of the physician versus the patient in the detection of recurrent disease following treatment for head and neck cancer. Laryngoscope 2004;114:232–35 [DOI] [PubMed] [Google Scholar]
- 10.Quillin SP, Balfe DM, Glick SN. Pharyngography after head and neck irradiation: differentiation of postirradiation edema from recurrent tumor. AJR Am J Roentgenol 1993;161:1205–08 [DOI] [PubMed] [Google Scholar]
- 11.Epstein JB, Feldman R, Dolor RJ, et al. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck 2003;25:911–21 [DOI] [PubMed] [Google Scholar]
- 12.Epstein JB, Wong FL, Stevenson-Moore P. Osteoradionecrosis: clinical experience and a proposal for classification. J Oral Maxillofac Surg 1987;45:104–10 [DOI] [PubMed] [Google Scholar]
- 13.Beumer J, Harrison R, Sanders B, et al. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck Surg 1984;6:819–27 [DOI] [PubMed] [Google Scholar]
- 14.Mancuso AA, Hanafee WN. Oral cavity and oropharynx including tongue base, floor of mouth and mandible. In: Mancuso AA, Hanafee WN, eds. Computed Tomography and Magnetic Resonance Imaging of the Head and Neck. 2nd ed. Baltimore: Williams and Wilkins;1985. :371–75
- 15.Hermans R, Pameijer FA, Mancuso AA, et al. Laryngeal or hypopharyngeal squamous cell carcinoma: can follow-up CT after definitive radiation therapy be used to detect local failure earlier than clinical examination alone? Radiology 2000;214:683–87 [DOI] [PubMed] [Google Scholar]
- 16.Bedwinek JM, Shukovsky LJ, Fletcher GH, et al. Osteonecrosis in patients treated with definitive radiotherapy for squamous cell carcinomas of the oral cavity and naso-and oropharynx. Radiology 1976;119:665–67 [DOI] [PubMed] [Google Scholar]
- 17.Beumer J 3rd, Curtis T, Harrison RE. Radiation therapy of the oral cavity: sequelae and management, part 2. Head Neck Surg 1979;1:392–408 [DOI] [PubMed] [Google Scholar]
- 18.Morrish RB Jr, Chan E, Silverman S Jr, et al. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer 1981;47:1980–83 [DOI] [PubMed] [Google Scholar]
- 19.Kunkel M, Förster GJ, Reichert TE, et al. Detection of recurrent oral squamous cell carcinoma by [18F]-2-fluorodeoxyglucose-positron emission tomography: implications for prognosis and patient management. Cancer 2003;98:2257–65 [DOI] [PubMed] [Google Scholar]
- 20.Fogarty GB, Peters LJ, Stewart J, et al. The usefulness of fluorine 18-labelled deoxyglucose positron emission tomography in the investigation of patients with cervical lymphadenopathy from an unknown primary tumor. Head Neck 2003;25:138–45 [DOI] [PubMed] [Google Scholar]
- 21.Quon A, Fischbein NJ, McDougal IR, et al. Clinical role of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med 2007;48:58S–67S [PubMed] [Google Scholar]
- 22.Farber LA, Benard F, Machtay M, et al. Detection of recurrent head and neck squamous cell carcinomas after radiation therapy with 2–18F-fluoro-2-deoxy-D-glucose positron emission tomography. Laryngoscope 1999;109:970–75 [DOI] [PubMed] [Google Scholar]
- 23.Kapoor V, Fukui MB, McCook BM. Role of 18FFDG PET/CT in the treatment of head and neck cancers: principles, technique, normal distribution, and initial staging. AJR Am J Roentgenol 2005;184:579–87 [DOI] [PubMed] [Google Scholar]
- 24.Sakamato H, Nakai Y, Ohashi Y, et al. Monitoring of response to radiotherapy with fluorine-18 deoxyglucose FDG PET of head and neck squamous cell carcinomas. Acta Otolaryngol 1998;538:254–60 [PubMed] [Google Scholar]
- 25.Kitagawa Y, Sadato N, Azuma H, et al. FDG PET to evaluate combined intra-arterial chemotherapy and radiotherapy of head and neck neoplasms. J Nucl Med 1999;40:1132–37 [PubMed] [Google Scholar]
- 26.Goerres GW, von Schulthess GK, Hany TF. Positron emission tomography and PET CT of the head and neck: FDG uptake in normal anatomy, in benign lesions, and in changes resulting from treatment. AJR Am J Roentgenol 2002;179:1337–43 [DOI] [PubMed] [Google Scholar]
- 27.Kostakoglu L, Goldsmith SJ. PET in assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. J Nucl Med 2004;45:56–58 [PubMed] [Google Scholar]
- 28.Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972–80 [PubMed] [Google Scholar]
- 29.Reinhardt MJ, Kubota K, Yamada S, et al. Assessment of cancer recurrence in residual tumors after fractionated radiotherapy: a comparison of fluorodeoxyglucose, L-methionine and thymidine. J Nucl Med 1997;38:280–87 [PubMed] [Google Scholar]
- 30.Terhaard CH, Bongers V, van Rijk PP, et al. F-18-fluoro-deoxy-glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/pharyngeal cancer. Head Neck 2001;23:933–41 [DOI] [PubMed] [Google Scholar]
- 31.Hermans R, Pameijer FA, Mancuso AA, et al. CT findings in chondroradionecrosis of the larynx. AJNR Am J Neuroradiol 1998;19:711–18 [PMC free article] [PubMed] [Google Scholar]