Abstract
BACKGROUND AND PURPOSE: Functional outcome in patients with minor head injury with neurocranial traumatic findings on CT is largely unknown. We hypothesized that certain CT findings may be predictive of poor functional outcome.
Materials and METHODS: All patients from the CT in Head Injury Patients (CHIP) study with neurocranial traumatic CT findings were included. The CHIP study is a prospective, multicenter study of consecutive patients, ≥16 years of age, presenting within 24 hours of blunt head injury, with a Glasgow Coma Scale (GCS) score of 13–14 or a GCS score of 15 and a risk factor. Primary outcome was functional outcome according to the Glasgow Outcome Scale (GOS). Other outcome measures were the modified Rankin Scale (mRS), the Barthel Index (BI), and number and severity of postconcussive symptoms. The association between CT findings and outcome was assessed by using univariable and multivariable regression analysis.
RESULTS: GOS was assessed in 237/312 patients (76%) at an average of 15 months after injury. There was full recovery in 150 patients (63%), moderate disability in 70 (30%), severe disability in 7 (3.0%), and death in 10 (4.2%). Outcome according to the mRS and BI was also favorable in most patients, but 82% of patients had postconcussive symptoms. Evidence of parenchymal damage was the only independent predictor of poor functional outcome (odds ratio = 1.89, P = .022).
CONCLUSION: Patients with neurocranial complications after minor head injury generally make a good functional recovery, but postconcussive symptoms may persist. Evidence of parenchymal damage on CT was predictive of poor functional outcome.
Head injury is one of the main causes of disability, especially in the younger population. In patients with severe head injury, long-term outcome in terms of disability has been studied extensively and is consequently well documented.1–4 In clinical practice, however, most patients with head injury presenting to emergency departments have sustained minor head injury, which is commonly defined as a presenting Glasgow Coma Scale (GCS) score of 13–15, with or without a brief history of loss of consciousness (maximum of 15 minutes) or posttraumatic amnesia (maximum of 60 minutes) after blunt trauma to the head.5 Generally, these patients make a full functional recovery, though it is not uncommon to see patients with minor head injury with long-term sequelae after the injury.6–12
With the advent of routine head CT scanning of virtually all patients with head injury, it has become clear that a substantial number (6%–10%) of patients with minor head injury have evidence of neurocranial traumatic complications.13–18 Functional outcome in these patients with so-called “complicated” minor head injury has been shown to be significantly poorer than that in patients without neurocranial traumatic complications after minor head injury.19,20 Long-term outcome in terms of functional disability or postconcussive symptoms in patients with complicated minor head injury specifically, however, is still largely unknown.12,20 Also, outcome may not be the same for different traumatic CT findings. Traumatic findings may range from an isolated linear skull fracture, which is commonly considered to be clinically insignificant and would thus be expected to be associated with favorable functional outcome, to acute extra-axial hematoma requiring neurosurgical intervention, possibly associated with poorer functional outcome and an increased prevalence of posttraumatic complaints.18,21
The purpose of our study was to assess functional outcome in terms of disability and postconcussive symptoms in patients with neurocranial complications as established with CT after minor head injury. We hypothesized that certain CT findings may be predictive of poor long-term outcome in these patients with complicated minor head injury.
Methods
Study Population
This follow-up study was an extension of the CT in Head Injury Patients (CHIP) study, in which data were prospectively collected in 4 Dutch university hospitals on 3364 consecutively included patients between February 11, 2002, and August 31, 2004 (Fig 1).22 Inclusion criteria for the CHIP study were the following: presentation within 24 hours of blunt head injury, 16 years of age or older, a GCS score of 13 or 14 on presentation or a GCS score of 15 and a minimum of 1 risk factor. Risk factors were a history of loss of consciousness, short-term memory deficit, amnesia for the traumatic event, posttraumatic seizure, vomiting, headache, clinical evidence of intoxication with alcohol or drugs, anticoagulant treatment or history of coagulopathy, external evidence of injury above the clavicles, or neurologic deficit. Exclusion criteria were contraindications for CT scanning or concurrent injuries precluding head CT within 24 hours of injury. After assessment by a neurologist or by a neurologist-in-training under supervision of a neurologist, all patients underwent head CT in accordance with local hospital policies and the guidelines set out by the Dutch Neurologic Society and the European Federation of Neurologic Societies.23,24 Non-contrast-enhanced head CTs were performed in all included patients, by using a maximal section thickness of 5 mm infra- and 8 mm supratentorially.25,26 All CT scans were evaluated by a trauma radiologist or neuroradiologist.
Fig 1.
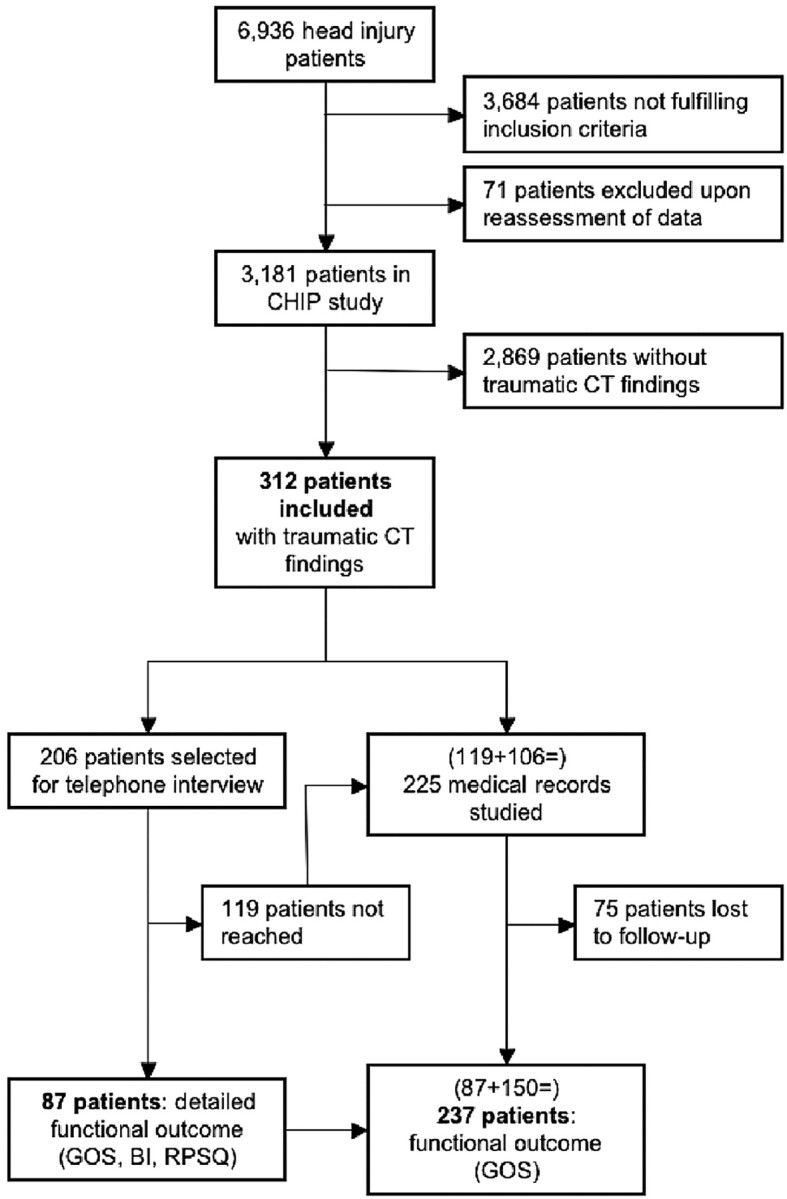
The number of patients presenting with head injury (6936) is an estimate based on the proportion of patients included out of the total number of patients with trauma seen by a neurologist or neurologist-in-training in the emergency department of the participating center that included most patients. Of the 3181 patients included in the CHIP study, 2869 did not have any evidence of a neurocranial traumatic findings on CT, leaving 312 patients eligible for inclusion in the current follow-up study.
For the current follow-up study, all patients with neurocranial traumatic findings on CT were included (Fig 1). The study protocol was approved by the Internal Review Board, and written informed consent was obtained from all patients participating in the assessment of outcome by telephone interview.
Assessment Procedure
A detailed assessment of outcome was performed by telephone interview in a large sample of patients (subpopulation) from our study population. This sample consisted of all included patients in the center in which most patients had been included. In all patients who had been included in the remaining 3 participating centers, as well as in patients who could not be reached for telephone interview, global functional outcome was assessed by careful review of the patients' medical records.27 Every attempt was made to reach all surviving patients from the subpopulation and to obtain their current addresses and telephone numbers from the hospital information system, patients' family doctors, telephone registry, and the local citizens' registry. Patients willing to participate were contacted by telephone, and a structured interview was conducted by a single trained researcher (D.A.v.R.). In patients who were unable to answer the questionnaire, the interview was performed with the patient's relative or caregiver as a proxy.
Mortality
We evaluated all-cause 30-day and disease-specific 1-year mortality in all patients, to avoid errors due to adjudication, which is consistent with the cardiologic and surgical literature. In patients who had died within 1 year, patient records were reviewed to establish whether death was related to the head injury, including remote mortality. In patients who had died of a cause unrelated to head injury, and in patients who had died more than 1 year after the head injury, death was considered not to be related to the head injury. In these patients, functional outcome from before death was derived from the patients' medical records.
Outcome Measures
Primary outcome measure was the Glasgow Outcome Scale (GOS), which was assessed in all patients reached for telephone interview or was derived from the patients' medical records. The GOS is a 5-point scale to assess disability after head injury or other neurologic events.27 The clearly defined categories are as follows: 5, full recovery (ie, the resumption of normal life even though there may be minor neurologic or psychological deficits); 4, moderate disability (ie, disabled, but independent in daily life); 3, severe disability (ie, conscious but disabled with the patient being dependent for daily support [for >8 hours per day] due to mental or physical disability); 2, vegetative state (ie, the patient being unresponsive and speechless for weeks or months after the injury); and 1, dead, which included 30-day all-cause mortality and 1-year disease-specific mortality. Although the categories of disability are rather crude and may therefore not be very sensitive to subtle differences or changes in disability, it is the most widely used scale to assess functional outcome after head injury. It has been extensively validated and has been shown to correlate well with other measures of disability.2,28–30
In patients reached for telephone interview, a more detailed assessment of functional outcome was made according to the modified Rankin scale (mRS), the Barthel Index (BI), and the Rivermead Postconcussion Symptoms Questionnaire (RPSQ).
The mRS is a 7-point scale that is also used to assess functional disability after neurologic events such as head injury or stroke. It is more sensitive than the GOS for subtle differences in outcome, and ranges from zero (no symptoms) to 6 (death, all-cause 30-day and disease-specific 1-year mortality).31,32
The BI is a 10-item questionnaire of daily functioning, assessing the patient's independence or dependence for each item on a scale from zero (fully dependent) to 2, 3, or 4 (fully independent, maximal score varies per item).33 It covers the following items: eating, getting dressed, transferring from bed to chair, ambulating, negotiating stairs, managing personal care, bathing, toileting, and controlling bowel and bladder. A maximal score (score = 20) indicates full independence for all items, whereas a minimal score (score = 0) indicates that the patient is fully dependent for all items.
The RPSQ is a 5-point scale of 16 commonly reported symptoms after head injury, with a high test-retest and inter-rater agreement for the assessment of the presence and severity of postconcussive symptoms.34 Patients are asked to rate the severity for each symptom in comparison with preinjury levels on a scale from zero (no symptoms) to 4 (severe symptoms). Additional symptoms resulting from the head injury may also be recorded and rated. The higher the sum score, the more (severely) symptoms are present after the injury.
Definitions
Neurocranial complications as identified on CT included all traumatic findings of the neurocranium. Intracranial lesions included all neurocranial complications except for isolated linear skull or skull base fractures. Traumatic subarachnoid hemorrhage and epidural and subdural hematomas were recorded as present or absent. Intraparenchymatous contusions included both hemorrhagic and nonhemorrhagic lesions. Diffuse axonal injury was defined as multiple small focal traumatic lesions in the typical locations of shearing injury. Depressed fractures included all fractures of the skull vault in which inward displacement of at least 1 of the bone fragments was seen. Linear skull fractures included all fractures of the skull vault, with no evidence of displacement of bone fragments. Skull base fractures included all fractures of the skull base. If multiple findings were present, the number of findings was recorded. Bilaterally occurring lesions were counted as 2 separate lesions. Diffuse axonal injury was counted as 1 finding. Each intraparenchymatous contusion was counted as 1 finding.
Data Analysis
We assessed our study population for patient and clinical characteristics, including the presence of risk factors and findings on physical and neurologic examination, as well as neurocranial traumatic CT findings. We tested differences between the entire study population and the subpopulation for significance (P < .05) with respect to patient and clinical characteristics as well as CT findings, by using the independent samples 2-tailed t test for continuous, the Pearson χ2 test for nominal, and the Mann-Whitney U test for ordinal variables. Similarly, we tested for significant differences (P < .05) between the patients who had been reached for the telephone interview and those who could not be reached within the subpopulation. We also assessed potential differences in the distribution of the GOS obtained by telephone interview and the GOS derived from the patients' medical records, with the Mann-Whitney U test (P < .05 for significance). All analyses, by using complete cases only, were performed with the Statistical Package for the Social Sciences (Version 12.0; SPSS, Chicago, Ill).
The distribution of functional disability according to the GOS, mRS, and BI was assessed, and the number and severity of postconcussive symptoms according to the RPSQ was recorded. We tested the association of each of the neurocranial traumatic CT findings with disability and symptoms for significance by using the Mann-Whitney U test for ordinal variables (GOS, mRS, and BI) and the independent samples t test for continuous variables (RPSQ). We assessed the association between the number of neurocranial traumatic findings and outcome by using linear regression analysis. A P value <.05 was used as a threshold for statistical significance. Results not reaching this level of significance but with a P value <.10 were considered near-significant.
To assess whether any of the neurocranial traumatic CT findings were independently predictive of long-term outcome according to the primary outcome measure, we performed multivariable logistic regression analysis after dichotomizing GOS into good (GOS = 5) and poor (GOS = 1–4) outcome.35 We used a stepwise backward procedure by using the likelihood ratio criterion with P < .05 for inclusion and P > .10 for removal of variables. To reduce the number of variables,36 we grouped variables that were similar as follows: Epi- and subdural hematoma were combined as extra-axial hematoma; intraparenchymatous contusions and diffuse axonal injury, as parenchymal damage; and linear and skull base fractures, as nondepressed skull fracture. The other variables entered into the model were traumatic subarachnoid hemorrhage and depressed skull fracture.
Results
Study Population
Of the 3181 patients originally included in the CHIP study, 312 had at least 1 neurocranial traumatic finding on CT and were thus included in the current follow-up study (Fig 1).
The subpopulation selected for telephone interview consisted of 206 (66%) patients. There were no significant differences (P < .05) between the subpopulation and the entire study population with respect to patient and clinical characteristics or neurocranial traumatic findings on CT. Telephone interview was successfully performed in 87 (42%) patients at a mean of 2.8 years after the injury (range, 1.7–4.3 years). No telephone interview was performed in 119 patients for the following reasons: They died (n = 14), lived abroad (n = 8), were homeless or had no permanent address (n = 7), moved without a forwarding address (n = 16), did not speak Dutch (n = 5), refused to participate (n = 23), or had no telephone number available (n = 46). In these 119 patients, as well as in the 106 patients not selected for telephone interview, medical records were reviewed (n = 225) to assess functional outcome according to the GOS (Fig 1).
Patient characteristics are shown in Table 1. Within the subpopulation, patients who were reached for telephone interview were older than those who were not reached and were more commonly female (Table 1). Of the neurocranial traumatic findings on CT, depressed fracture was seen less and subdural hematoma more frequently in patients who were reached for telephone interview than those who were not reached (Table 1). Overall, there were no differences in the frequency of intracranial traumatic complications on CT. Multiple findings on CT were present in 198 patients (67%; range, 2–16).
Table 1:
Patient characteristics and CT findings in the entire study population and in the subpopulation selected for telephone interview
| Findings | Entire Population* (n = 312) |
Subpopulation (n = 206) |
P Value† | ||||
|---|---|---|---|---|---|---|---|
| Reached (n = 87) |
Not reached (n = 119) |
||||||
| No. | % | No. | % | No. | % | ||
| Patient | |||||||
| Mean age‡ (range) | 47.2 (17.0–93.3) | 50.5 (17.5–86.6) | 45.1 (17.7–93.3) | .043 | |||
| Male sex | 238 | 76 | 59 | 68 | 106 | 89 | .000 |
| Died | 22 | 7.1 | 0 | 0 | 14 | 12 | .001 |
| Intoxication | 118 | 38 | 33 | 38 | 57 | 48 | .154 |
| Clinical | |||||||
| GCS score = 15 | 185 | 59 | 49 | 56 | 71 | 60 | .631 |
| GCS score = 14 | 90 | 29 | 27 | 31 | 33 | 28 | .606 |
| GCS score = 13 | 37 | 12 | 11 | 13 | 15 | 13 | .993 |
| LOC | 227 | 73 | 60 | 69 | 89 | 75 | .356 |
| PTA | 239 | 77 | 66 | 76 | 90 | 76 | .969 |
| Persistent amnesia | 83 | 27 | 24 | 28 | 26 | 22 | .343 |
| Seizure§ | 6 | 1.9 | 0 | 0 | 3 | 2.5 | .136 |
| Headache | 212 | 68 | 67 | 77 | 87 | 73 | .524 |
| Vomiting | 69 | 22 | 23 | 26 | 24 | 20 | .290 |
| Neurologic deficit | 48 | 15 | 14 | 16 | 21 | 18 | .769 |
| Infraclavicular injury | 121 | 23 | 19 | 22 | 27 | 23 | .885 |
| CT | |||||||
| Intracranial lesions | 243 | 78 | 63 | 72 | 92 | 77 | .421 |
| Linear fracture | 114 | 37 | 41 | 47 | 44 | 37 | .144 |
| Skull base fracture | 82 | 26 | 29 | 33 | 26 | 22 | .066 |
| Depressed fracture | 19 | 6.1 | 3 | 3.4 | 14 | 12 | .032 |
| Subdural hematoma | .033 | ||||||
| Mild | 58 | 19 | 23 | 26 | 16 | 14 | |
| Severe | 9 | 2.9 | 2 | 2.3 | 3 | 2.6 | |
| Epidural hematoma | .332 | ||||||
| Mild | 31 | 9.9 | 8 | 9.2 | 14 | 12 | |
| Severe | 4 | 1.3 | 0 | 0.0 | 2 | 1.7 | |
| SAH | 86 | 28 | 30 | 34 | 30 | 25 | .148 |
| Contusion | 142 | 46 | 28 | 32 | 51 | 43 | .120 |
| Diffuse axonal injury | 14 | 4.5 | 2 | 2.3 | 7 | 5.9 | .214 |
Note:—LOC indicates loss of consciousness; PTA, posttraumatic amnesia; SAH, traumatic subarachnoid hemorrhage.
Multiple symptoms and clinical and CT findings may be present in 1 patient.
P values <.05 indicate differences between patients who were reached compared with those who were not reached for telephone interview (independent samples t test for continuous, Pearson χ2 test for nominal, and Mann-Whitney U test for ordinal variables).
Age in years.
Post-traumatic seizure.
Mortality
Twenty-two patients died (7.1%) at an average of 199 days (median, 78 days; range, 0–702 days). All-cause 30-day mortality was 2.9% (n = 9). A further 7 patients died within 1 year of injury, resulting in an all-cause 1-year mortality of 5.1%. In 1 of these patients, death was determined by the investigators to be secondary to complications from the head injury. One-year disease-specific mortality was thus 3.2% (n = 10). The remaining 6 patients died more than 1 year after the head injury.
Functional Outcome
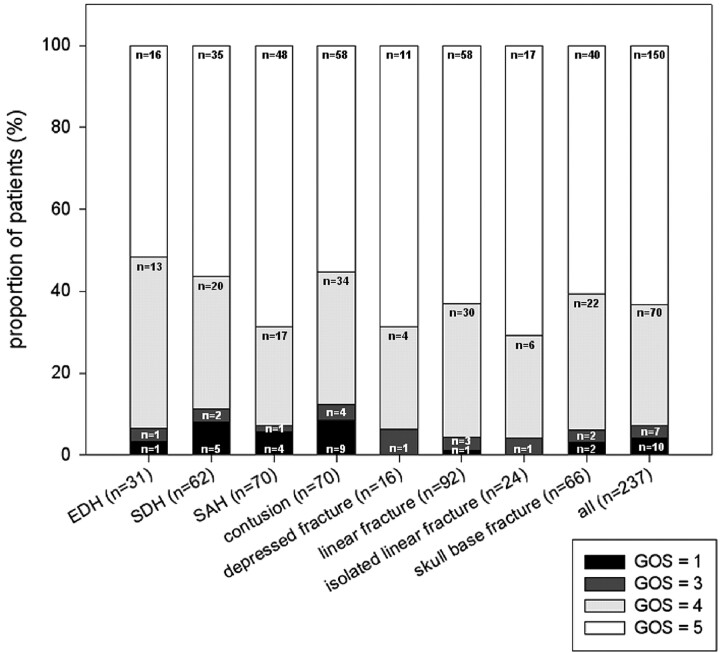
GOS data were obtained in 237 of 312 (76%) patients. There was no difference in the distribution of GOS obtained from telephone interview (87 patients, 37%) and GOS derived from medical records (Mann-Whitney U test, P = .173). Mean duration between the latest available follow-up data and time of injury was 15.1 months (median, 3.6 months; range, 0–56 months). Most patients had a good functional outcome (Fig 2), 150 (63%) patients having made full recovery. Seventy patients (30%) had minor disability. Severe disability was present in 7 (3.0%) patients, and 10 (4.2%) patients died as a consequence of head injury (9 patients within 30 days and 1 patient within 1 year of head injury).
Fig 2.
Distribution (percentage) of the GOS in patients with epidural hematoma (EDH), subdural hematoma (SDH), traumatic subarachnoid hemorrhage (SAH), intraparenchymatous contusion, depressed fracture, linear fracture isolated, linear fracture, skull base fracture, and in all patients (n = 233). GOS = 1 indicates dead; GOS = 2, vegetative state; GOS = 3, severe disability; GOS = 4, moderate disability; GOS = 5, full recovery.
In the subpopulation, data on mRS were available on 87 surviving patients as well as 3 patients who had died as a result of head injury (all within 30 days of injury). Most patients had no (n = 16, 18%) or minor (n = 43, 48%) symptoms. Minor disability was present in 15 (17%) patients, moderate disability in 10 (11%), and moderately severe disability in 3 (3.3%).
BI could be assessed in 87 patients. Most patients were independent for all activities (n = 73, 84%). All patients were independent with respect to self-care. Dependency was highest for walking stairs, with 4 (4.6%) patients not being able to walk stairs and another 4 (4.6%) needing assistance.
RPSQ data were available on 87 patients (Table 2). Seventy-one (82%) patients had at least 1 postconcussive symptom. If present, symptoms were more frequently moderate to severe than mild. Most frequently reported symptoms were fatigue (40%), depression (30%), dizziness (30%), irritability (28%), sleep disturbances (25%), and cognitive symptoms such as poor memory (38%) and concentration (32%) and slowness of thinking (28%).
Table 2:
Postconcussive symptoms as assessed with the RPSQ in 87 patients
| Symptom | No or No More (%) | Mild (%) | Moderate (%) | Severe (%) |
|---|---|---|---|---|
| Headache | 68 (78) | 2 (2.3) | 4 (4.6) | 13 (15) |
| Dizziness | 61 (70) | 7 (8.0) | 6 (6.9) | 13 (15) |
| Nausea | 82 (94) | 1 (1.1) | 1 (1.1) | 3 (3.4) |
| Noise hypersensitivity | 80 (92) | 2 (2.3) | 1 (1.1) | 4 (4.6) |
| Sleep disturbance | 65 (75) | 6 (6.9) | 8 (9.2) | 8 (9.2) |
| Fatigue | 52 (60) | 8 (9.2) | 4 (4.6) | 23 (26) |
| Irritability | 63 (72) | 7 (8.0) | 9 (10) | 8 (9.2) |
| Depression | 61 (70) | 7 (8.0) | 9 (10) | 10 (11) |
| Frustration | 66 (76) | 6 (6.9) | 7 (8.0) | 8 (9.2) |
| Poor memory | 54 (62) | 7 (8.0) | 6 (6.9) | 20 (23) |
| Poor concentration | 59 (68) | 4 (4.6) | 6 (6.9) | 18 (21) |
| Slow thinking | 63 (72) | 7 (8.0) | 5 (5.7) | 12 (14) |
| Blurred vision | 72 (83) | 5 (5.7) | 7 (8.0) | 3 (3.4) |
| Light hypersensitivity | 82 (94) | 2 (2.3) | 1 (1.1) | 2 (2.3) |
| Double vision | 79 (91) | 5 (5.7) | 0 (0.0) | 3 (3.4) |
| Restlessness | 79 (91) | 3 (3.4) | 2 (2.3) | 3 (3.4) |
Note:—RPSQ scores of 0 and 1 indicate no or no more complaints; 2, mild; 3, moderate; 4, severe postconcussive symptoms.
Association between Neurocranial Traumatic CT Findings and Functional Outcome
Patients with diffuse axonal injury had a significantly or near significantly poorer outcome on all functional outcome measures than those without diffuse axonal injury (BI, P = .000; mRS, P = .018; GOS, P = .096; Table 3). Patients with intraparenchymatous lesions had a significantly poorer GOS outcome (P = .008, Table 3), and patients with subdural hematoma had a significantly poorer BI outcome (P = .050, Table 3). Patients with epidural hematoma had near significantly more or more severe symptoms than those without epidural hematoma (mean RPSQ score, 23 versus 13, respectively; P = .054; Table 3), as did patients with a skull base fracture (mean RPSQ score 18 versus 12, P = .070; Table 3). No association with outcome was found in patients with traumatic subarachnoid hemorrhage, isolated linear or linear skull fracture, or depressed skull fracture. The number of neurocranial traumatic findings on CT was significantly associated with poorer functional outcome (P = .001) according to GOS and near significantly associated with more or more severe symptoms (P = .069) according to RPSQ.
Table 3:
Mean scores and SDs on the GOS, BI, mRS, and RPSQ*
| Traumatic CT Finding | GOS |
BI |
mRS |
RPSQ |
||||
|---|---|---|---|---|---|---|---|---|
| Score (SD) | P Value | Score (SD) | P Value | Score (SD) | P Value | Score (SD) | P Value | |
| Linear fracture | .820 | .433 | .419 | .619 | ||||
| Absent | 4.4 (1.0) | 20 (1.2) | 1.3 (1.1) | 13 (15) | ||||
| Present | 4.6 (0.7) | 19 (2.0) | 1.4 (0.9) | 15 (12) | ||||
| Skull base fracture | .690 | .602 | .105 | .070 | ||||
| Absent | 4.5 (0.9) | 19 (1.9) | 1.2 (1.0) | 12 (12) | ||||
| Present | 4.5 (0.8) | 20 (0.6) | 1.6 (1.0) | 18 (15) | ||||
| Depressed fracture | .633 | .445 | .230 | .302 | ||||
| Absent | 4.5 (0.9) | 19 (1.6) | 1.4 (1.0) | 14 (13) | ||||
| Present | 4.6 (0.6) | 20 (0.0) | 0.7 (1.2) | 6 (10) | ||||
| Subdural hematoma | .137 | .050 | .573 | .552 | ||||
| Absent | 4.5 (0.8) | 20 (1.6) | 1.3 (1.0) | 13 (13) | ||||
| Present | 4.3 (1.1) | 19 (1.5) | 1.4 (1.0) | 15 (13) | ||||
| Epidural hematoma | .200 | .705 | .313 | .054 | ||||
| Absent | 4.5 (0.9) | 19 (1.7) | 1.3 (1.0) | 13 (13) | ||||
| Present | 4.4 (0.8) | 20 (0.4) | 1.6 (0.9) | 23 (16) | ||||
| SAH | .330 | .238 | .502 | .681 | ||||
| Absent | 4.5 (0.9) | 19 (1.9) | 1.4 (1.1) | 14 (14) | ||||
| Present | 4.5 (1.0) | 20 (0.8) | 1.2 (0.7) | 13 (12) | ||||
| Contusion | .008 | .140 | .449 | .643 | ||||
| Absent | 4.7 (0.6) | 20 (1.6) | 1.3 (1.1) | 13 (14) | ||||
| Present | 4.3 (1.1) | 19 (1.5) | 1.4 (0.9) | 15 (11) | ||||
| Diffuse axonal injury | .096 | .000 | .018 | .803 | ||||
| Absent | 4.5 (0.9) | 20 (1.5) | 1.3 (1.0) | 14 (13) | ||||
| Present | 3.8 (1.5) | 16 (2.1) | 3.5 (0.7) | 12 (16) | ||||
Note:—SAH indicates traumatic subarachnoid hemorrhage.
Higher scores on the GOS and BI indicate more favorable outcome, whereas higher scores on mRS and RPSQ indicate poorer outcome and more severe postconcussive complaints, respectively.
Multivariable logistic regression analysis to predict outcome in terms of GOS was performed with the independent variables of extra-axial hematoma, parenchymal damage, traumatic subarachnoid hemorrhage, and depressed and nondepressed skull fracture. Evidence of parenchymal damage on CT (which included diffuse axonal injury or intraparenchymatous contusions) was the only independent predictor of poor functional outcome (odds ratio = 1.86; 95% confidence interval, 1.09–3.18; P = .022).
Discussion
In this follow-up study of patients with evidence on CT of neurocranial traumatic complications after minor head injury, we found that most patients made a good functional recovery on long-term assessment. Evidence of parenchymal damage was the only independent factor significantly predictive of poor functional outcome. Despite generally good functional recovery, most of the patients we interviewed had 1 or more postconcussive symptoms.
A limitation of our study was the fact that a substantial number of patients were lost to follow-up. This limitation is inherent to the patient population we studied and the fact that we assessed outcome several years (2–4 years) after the injury had occurred. Patients with trauma tend to be a young actively employed population that often moves, is difficult to reach, and is less willing to participate than older patients. In our study, most patients who could not be reached had moved without forwarding addresses or had changed telephone numbers. Patients who had been reached were somewhat older and more frequently female, suggesting possible selection bias. Although these differences were statistically significant, patients from both groups were within the same middle-aged range, and in both groups male patients formed the majority. We expect that this difference in demographic characteristics will only have a very small effect on our results. More important, however, the overall frequency of intracranial traumatic findings between the 2 groups was not significantly different, suggesting that the injury severity was comparable for patients who were reached versus those who could not be reached.
A further limitation may be that we included only patients with neurocranial complications and not patients with uncomplicated minor head injury. This makes it impossible to establish whether complicated minor head injury has a poorer outcome than uncomplicated head injury. This question, however, has already been addressed in other studies.19,20 Outcome in patients with minor head injury without neurocranial complications is generally known to be good,37 and the additional effort involved in following up these patients in our study would not have been justifiable. Another limitation is that we did not evaluate the effect on outcome of potentially confounding factors, such as multiple injuries due to the injury, premorbid disability, chronic pain, or litigation. Although it would be interesting to include these potential confounders in the outcome assessment, it would still be difficult to disentangle the effect on outcome of neurocranial complications and those of confounders.
Finally, the fact that some of the data in our study were obtained retrospectively may be considered another limitation. Because there were no differences between the entire study population and the subpopulation or between patients in whom outcome was assessed prospectively or retrospectively, we believe that no significant bias has been introduced by this study design. Because follow-up rates in patients with trauma tend to be low,6,10,20 studies with prospectively included patients but retrospectively collected outcome data may be very valuable for these patient populations and may actually introduce less bias than an entirely prospective study with high rates of loss to follow-up.38
Follow-up studies of patients with traumatic neurocranial complications after minor head injury are in fact scarce. In a recent study by de Andrade et al,39 266 selected patients' medical records were reviewed to assess GOS after minor head injury in the presence of neurocranial traumatic CT findings. Williams et al19 prospectively assessed GOS at 6 months after minor head injury in 74 patients with radiographic evidence of neurocranial traumatic complications. In both studies, most patients had made a good functional recovery but outcome was significantly poorer than that in patients without radiographic evidence of neurocranial traumatic complications. In a third study, Fabbri et al40 reported favorable outcome in most (89%) of 491 patients with minor head injury with neurocranial traumatic CT findings, but not all patients included in their study had undergone CT. Because we assessed outcome in patients with neurocranial traumatic complications from a large cohort of unselected patients with minor head injury who had all undergone CT, our findings are a good reflection of outcome in the general population of patients with complicated minor head injury. Our study confirms these previous findings of a generally favorable functional outcome after complicated minor head injury.
Diffuse axonal injury was significantly associated with higher grades of functional disability on all outcome scales, whereas intraparenchymatous contusions and subdural hematoma were associated with poor outcome according to GOS and BI, respectively. There was also a significant association between the number of neurocranial traumatic finding on CT and poor functional outcome according to the GOS. None of the CT findings were significantly associated with postconcussive symptoms, though a near significant association was found for epidural hematoma and skull base fracture and with the number of neurocranial traumatic findings on CT. Evidence of parenchymal damage (diffuse axonal injury or intraparenchymatous contusions) was found to be the only independent predictor of poor functional outcome, with an odds ratio of 1.9. Most interesting, none of the neurocranial traumatic findings were significantly associated with a better outcome, suggesting that good outcome even for so-called clinically nonsignificant lesions may not be certain. One could argue that the CT imaging protocol we used for this study was relatively insensitive for the detection of small lesions, such as small intraparenchymatous contusions or small amounts of subarachnoid hemorrhage; this insensitivity may have influenced our findings. Potentially, sensitivity could be increased with the acquisition of thinner sections as is now common practice with the advent of multidetector CT scanners, though we are not aware of any published studies formally comparing head CTs of varying section thicknesses in a trauma setting. Even assuming that thinner sections increase the detection of small intraparenchymatous injury, it is unclear whether this would affect the ability to predict outcome.
Thus far, the relationship between traumatic findings on CT and functional outcome has only been assessed in severely head-injured patients and not as yet in patients with complicated minor head injury. Only in 2 studies were less severely injured patients also included, but no distinction was made between patients with moderate and those with minor head injury.41,42 In line with our findings, intracranial hematoma and contusions were among the CT findings that were identified as independent predictors of poor outcome in their and previous studies of severely injured patients.41,43,44 We dichotomized the GOS classification specifically for our patient population into good (GOS = 5) and poor (GOS < 4) outcome, whereas in many studies, good functional outcome also included GOS of 4 (moderate disability).28 This dichotomization is indeed appropriate for the more severely injured patients, but after minor head injury, patients generally recover fully, and moderate disability would not qualify as a good functional outcome.35
We used several outcome measures to assess the patients' outcomes. Our primary outcome measure, the GOS, is often criticized for being too crude a measure of functional outcome, in particular for patients with minor head injury. As reported previously and confirmed in the present study, most patients with minor head injury were classified according to the GOS as having made a full recovery, which does not seem to reflect the high rate of symptoms many of these patients still experience. In the subpopulation of patients whom we interviewed, we used the mRS as a more sensitive measure of outcome and were indeed able to distinguish between patients who had fully recovered and those who still had symptoms, though without any disability. The low rate of disability and the high rate of postconcussive symptoms, as assessed by the BI and the RPSQ respectively, support these findings. Although the mRS thus seems to better reflect functional outcome, the absence of disability would generally be considered good functional outcome, with or without the presence of symptoms. The GOS, with its high test-retest and interobserver agreement,2,28,29 shows consistent relations with other outcome measures30 and is also more suitable for reliable outcome assessment in retrospective studies because medical records often do not contain sufficient detail for classification according to the mRS.
In the present follow-up study, we found a very high rate of postconcussive symptoms. Postconcussive symptoms are very common after head injury, especially in the first weeks to months after the injury.6,7,11,12,45–48 Because many of the reported symptoms, such as headache and fatigue, have a high base rate in the general population, patients with postconcussive symptoms are often considered malingerers, especially when no objective or imaging abnormalities can be found or, as in our study, no relationship between specific imaging findings and postconcussive symptoms can be determined. In a case-control study by Masson et al11 of patients with head injury versus those with lower limb injury, postconcussive symptoms, except for fatigue, were significantly more often present in patients with the head injury than in those with the lower limb injury. By using the RPSQ, we attempted to control for premorbid levels of symptoms. However, because the injury had occurred on average several years previously, some degree of recall bias was unavoidable. Despite the high reported rates, symptoms generally disappear in most patients after 3–6 months, persisting only in a minority of patients.7,49 To the best of our knowledge, we are the first to report postconcussive symptoms in patients with neurocranial traumatic complications after minor head injury. The high rate of symptoms in our patient population suggests that patients with minor head injury with neurocranial complications are at high risk of persistence of symptoms for years after the injury.
Conclusion
Patients with neurocranial traumatic complications after minor head injury generally make a good functional recovery, though postconcussive symptoms may persist for many years after the injury. Evidence of parenchymal damage on CT was predictive of poor functional outcome.
Acknowledgments
We thank the research nurses of the department of radiology at Erasmus MC, W.J. van Leeuwen, C.H. van Bavel-van Hamburg, and B. Tara-Prins, as well as J. Brauer, research nurse at the department of neurology at University Medical Center Nijmegen St. Radboud, for their invaluable contribution to patient data collection. Furthermore, we thank the research nurses of the department of neurology at Erasmus MC, E. van der Heijden, and N. el Ghannouti, for training one of the investigators (D.A.v.R.) in conducting telephone interviews for the assessment of functional outcome after neurologic events.
Footnotes
This research was supported by a grant from ZonMW (ZonMW DO: 945-06-309), from College voor Zorgverzekeringen (CVZ: VAZ 01-104), and from Radiologisch onderzoek Nederland (RADION). The authors' work was independent of the funding organizations. The funding organizations had no involvement in the study design, data collection, analysis, and interpretation or in the decision to approve publication of the finished manuscript.
References
- 1.Temkin NR, Machamer JE, Dikmen SS. Correlates of functional status 3–5 years after traumatic brain injury with CT abnormalities. J Neurotrauma 2003;20:229–41 [DOI] [PubMed] [Google Scholar]
- 2.Teasdale GM, Pettigrew LE, Wilson JT, et al. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma 1998;15:587–97 [DOI] [PubMed] [Google Scholar]
- 3.Vitaz TW, Jenks J, Raque GH, et al. Outcome following moderate traumatic brain injury. Surg Neurol 2003;60:285–91, discussion 291 [DOI] [PubMed] [Google Scholar]
- 4.King JT Jr, Carlier PM, Marion DW. Early Glasgow Outcome Scale scores predict long-term functional outcome in patients with severe traumatic brain injury. J Neurotrauma 2005;22:947–54 [DOI] [PubMed] [Google Scholar]
- 5.Carroll LJ, Cassidy JD, Holm L, et al. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;43(suppl):113–25 [DOI] [PubMed] [Google Scholar]
- 6.Rimel RW, Giordani B, Barth JT, et al. Disability caused by minor head injury. Neurosurgery 1981;9:221–28 [PubMed] [Google Scholar]
- 7.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;43(suppl):84–105 [DOI] [PubMed] [Google Scholar]
- 8.af Geijerstam JL, Oredsson S, Britton M. Medical outcome after immediate computed tomography or admission for observation in patients with mild head injury: randomised controlled trial. BMJ 2006;333:465 . Epub 2006 Aug 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Naalt J. Prediction of outcome in mild to moderate head injury: a review. J Clin Exp Neuropsychol 2001;23:837–51 [DOI] [PubMed] [Google Scholar]
- 10.Thornhill S, Teasdale GM, Murray GD, et al. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000;320:1631–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masson F, Maurette P, Salmi LR, et al. Prevalence of impairments 5 years after a head injury, and their relationship with disabilities and outcome. Brain Inj 1996;10:487–97 [DOI] [PubMed] [Google Scholar]
- 12.van der Naalt J, van Zomeren AH, Sluiter WJ, et al. One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J Neurol Neurosurg Psychiatry 1999;66:207–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.af Geijerstam JL, Britton M. Mild head injury: mortality and complication rate–meta-analysis of findings in a systematic literature review. Acta Neurochir (Wien) 2003;145:843–50, discussion 850 [DOI] [PubMed] [Google Scholar]
- 14.Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;43(suppl):61–75 [DOI] [PubMed] [Google Scholar]
- 15.Fabbri A, Servadei F, Marchesini G, et al. Prospective validation of a proposal for diagnosis and management of patients attending the emergency department for mild head injury. J Neurol Neurosurg Psychiatry 2004;75:410–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000;343:100–05 [DOI] [PubMed] [Google Scholar]
- 17.Miller EC, Holmes JF, Derlet RW. Utilizing clinical factors to reduce head CT scan ordering for minor head trauma patients. J Emerg Med 1997;15:453–57 [DOI] [PubMed] [Google Scholar]
- 18.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet 2001;357:1391–96 [DOI] [PubMed] [Google Scholar]
- 19.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery 1990;27:422–28 [DOI] [PubMed] [Google Scholar]
- 20.Hsiang JN, Yeung T, Yu AL, et al. High-risk mild head injury. J Neurosurg 1997;87:234–38 [DOI] [PubMed] [Google Scholar]
- 21.Atzema C, Mower WR, Hoffman JR, et al. Defining “therapeutically inconsequential” head computed tomographic findings in patients with blunt head trauma. Ann Emerg Med 2004;44:47–56 [DOI] [PubMed] [Google Scholar]
- 22.Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA 2005;294:1519–25 [DOI] [PubMed] [Google Scholar]
- 23.Twijnstra A, Brouwer O, Keyser A, et al. Guidelines for diagnosis and management of patients with minor head injury [in Dutch]. Available at: http://www.neurologie.nl/richtlijnen. Accessed December 15,2006
- 24.Vos PE, Battistin L, Birbamer G, et al. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol 2002;9:207–19 [DOI] [PubMed] [Google Scholar]
- 25.Udstuen GJ, Claar JM. Imaging of acute head injury in the adult. Semin Ultrasound CT MR 2001;22:135–47 [DOI] [PubMed] [Google Scholar]
- 26.McDaniel T. Head and brain trauma. In: Zimmerman R, Gibby W, Carmody R, eds. Neuroimaging: Clinical and Physical Principles. New York: Springer-Verlag;2000. :700 [Google Scholar]
- 27.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–84 [DOI] [PubMed] [Google Scholar]
- 28.Wilson JT. Assessing outcome in head injury trials. Curr Pharm Des 2001;7:1537–52 [DOI] [PubMed] [Google Scholar]
- 29.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998;15:573–85 [DOI] [PubMed] [Google Scholar]
- 30.Wilson JT, Pettigrew LE, Teasdale GM. Emotional and cognitive consequences of head injury in relation to the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 2000;69:204–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–07 [DOI] [PubMed] [Google Scholar]
- 32.Bamford JM, Sandercock PA, Warlow CP, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20:828. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–65 [PubMed] [Google Scholar]
- 34.King NS, Crawford S, Wenden FJ, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995;242:587–92 [DOI] [PubMed] [Google Scholar]
- 35.Murray GD, Barer D, Choi S, et al. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma 2005;22:511–17 [DOI] [PubMed] [Google Scholar]
- 36.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman & Hall;1999. :349 [Google Scholar]
- 37.Norlund A, Marke LA, Geijerstam JL, et al. Immediate computed tomography or admission for observation after mild head injury: cost comparison in randomised controlled trial. BMJ 2006;333:455–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenbroucke JP. Prospective or retrospective: what's in a name? BMJ 1991;302:249–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Andrade AF, de Almeida AN, Bor-Seng-Shu E, et al. The value of cranial computed tomography in high-risk, mildly head-injured patients. Surg Neurol 2006;65 Suppl 1:S10–1:13 [DOI] [PubMed] [Google Scholar]
- 40.Fabbri A, Servadei F, Marchesini G, et al. Clinical performance of NICE recommendations versus NCWFNS proposal in patients with mild head injury. J Neurotrauma 2005;22:1419–27 [DOI] [PubMed] [Google Scholar]
- 41.Rudehill A, Bellander BM, Weitzberg E, et al. Outcome of traumatic brain injuries in 1,508 patients: impact of prehospital care. J Neurotrauma 2002;19:855–68 [DOI] [PubMed] [Google Scholar]
- 42.Wardlaw JM, Easton VJ, Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry 2002;72:188–92, discussion 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fearnside MR, Cook RJ, McDougall P, et al. The Westmead Head Injury Project outcome in severe head injury: a comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg 1993;7:267–79 [DOI] [PubMed] [Google Scholar]
- 44.Liu HM, Tu YK, Su CT. Changes of brainstem and perimesencephalic cistern: dynamic predictor of outcome in severe head injury. J Trauma 1995;38:330–33 [DOI] [PubMed] [Google Scholar]
- 45.Ingebrigtsen T, Waterloo K, Marup-Jendsen S, et al. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol 1998;245:609–12 [DOI] [PubMed] [Google Scholar]
- 46.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995;45:1253–60 [DOI] [PubMed] [Google Scholar]
- 47.Bazarian JJ, Wong T, Harris M, et al. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj 1999;13:173–89 [DOI] [PubMed] [Google Scholar]
- 48.Bohnen N, Jolles J. Neurobehavioral aspects of postconcussive symptoms after mild head injury. J Nerv Ment Dis 1992;180:683–92 [DOI] [PubMed] [Google Scholar]
- 49.Evans RW. The postconcussion syndrome and the sequelae of mild head injury. Neurol Clin 1992;10:815–47 [PubMed] [Google Scholar]