Abstract
BACKGROUND AND PURPOSE: Cerebral sinovenous thrombosis (CSVT) is increasingly encountered in children, including neonates. The purpose of this study was to assess the frequency and topographic distribution of parenchymal brain lesions associated with CSVT in children and to compare these with the known anatomic venous drainage pathways.
MATERIALS AND METHODS: Brain CT/CT venograms and/or MR imaging/MR venograms of 71 consecutive patients with CSVT were reviewed retrospectively. The patients were grouped into neonates, infants, and older children. The site of CSVT, the location and size of the brain lesions, and the presence of hemorrhage were documented. The frequency of the brain lesions was calculated.
RESULTS: There were 34 neonates, 10 infants, and 27 older children with CSVT who were included. The most common sites of CSVT were the transverse sinuses, the superior sagittal sinus, and the straight sinus. Overall, 37 of 71 children with CSVT had parenchymal brain lesions. There were 21 of 34 neonates, 4 of 10 infants, and 12 of 27 older children who had brain lesions. The most common locations were in the frontal and parietal lobes. The topographic distribution of lesions correlated with the corresponding venous drainage territory in 16 of 21 neonates, all infants, and all older children. The neonates had smaller-sized lesions. Brain lesions were hemorrhagic in 76% of neonates, 75% of infants, and 33% of older children.
CONCLUSION: The topographic distribution of brain lesions associated with CSVT correlates with the known drainage territories of the dural venous sinus in children.
Cerebral sinovenous thrombosis (CSVT) in children is increasingly diagnosed mainly because of improved neuroimaging techniques and increasing awareness of the disease. The true incidence of CSVT is also probably increasing because of more aggressive treatment and improved survival in various childhood diseases (eg, childhood cancers). The incidence of CSVT in children is estimated at 0.67 per 100,000 children, occurring most commonly in neonates.1
Focal brain abnormalities have been identified in approximately 50% to 60% of patients with sinus venous thrombosis despite the reported protective role of the venous collateral pathways in preventing parenchymal injury during sinus occlusion.2-4 The pathophysiologic mechanism of parenchymal injuries related to CSVT is not fully understood. The severity of parenchymal injuries in experimental CSVT models has been considered proportional to the degree of venous occlusion.5 The lack of correlation between the extent and site of thrombosis in the dural sinuses and location of brain lesions has been suggested in adults.6 In this study, our aims were to evaluate the frequency and topographic distribution of brain lesions associated with CSVT in children and to try and compare the topographic distribution of brain lesions with known normal venous drainage and anatomy.
Materials and Methods
After the study was approved by the institutional research ethics board, brain CT/CT venography (CTV) and/or MR imaging/MR venography (MRV) examinations of all consecutive patients with radiographically diagnosed CSVT between January 1999 and November 2006 were evaluated by 2 neuroradiologists (M.S. and M.T.) retrospectively. Each neuroradiologist read studies independently, and discrepancies were later resolved by consensus. Patients whose brain abnormalities were attributed to head trauma, brain surgery, tumor, vascular malformation, arteriovenous malformation, arterial infarction, hypoxic-ischemic encephalopathy, germinal matrix hemorrhage, and sickle cell disease were excluded to eliminate the confounding factors for cerebral edema or hemorrhage, or both. The patients were divided into 3 groups according to their age (neonate, 0–28 days; infant, 29 days–12 months; older children, 1–18 years). The age distribution of the older children was 1 to 17 years (mean age, 9 years). The site of the CSVT, the location of the brain lesion (brain parenchymal attenuation, signal intensity change, or hemorrhage), the size of the brain lesion (the largest diameter of the lesion was measured and classified as <1 cm, small; 1–3 cm, medium; >3 cm, large), and the presence of hemorrhage were recorded. Hemorrhage was defined as a focal hyperattenuated parenchymal abnormality with CT attenuation values between 40 and 80 HU on noncontrast CT images. On MR imaging, hemorrhage was documented by detection of blood products on multi-planar gradient-echo sequences where available. T2*-weighted echo-planar imaging from diffusion-weighted images was used to evaluate for blood products when multi-planar gradient-echo sequencing was not part of the imaging protocol. Descriptive statistics were used to document results. The overall frequency of brain lesions in all patients with CSVT was calculated. The frequency of brain lesions was also calculated for each group (neonates, infants, and older children) of patients separately.
MR imaging examinations were performed on a 1.5T scanner (LX; GE Healthcare, Milwaukee, Wis) with the patient in the supine position. MR imaging included diffusion-weighted images, axial T1, axial fast spin-echo (FSE) T2, coronal FSE T2, and sagittal T1. Other sequences were obtained on the basis of clinical indication and MR findings. Coronal 2D time-of-flight MRV was performed with an inferior saturated band to eliminate signal intensity from the arterial structures. The parameters include TE, 5 to 6 ms; TR, 50 ms; flip angle, 60°; section thickness, 1.5 mm; matrix, 256 × 160; and FOV, 20 cm. Maximum intensity projections were reconstructed from the source images, and multiple oblique and orthogonal projections were reviewed on PACS workstations (GE Healthcare). CT/CTV was performed on 8-row multidetector CT (LightSpeed Ultra; GE Healthcare) with the patients in the supine position. An unenhanced brain CT examination was performed before the contrast injection. A bolus of 2.5 mL/kg of iohexol USP 65% (Omnipaque 300; GE Healthcare, Oakville, Canada) was given intravenously by hand injection in the neonates and infants. A power injector was used to inject contrast material only in older children (>1 year) to decrease the possibility of rupture of veins. When the power injector was used, a bolus of 2.5 mL/kg of iohexol USP 65% was injected with a rate of 2 mL/s. Total injection time varied depending on the body weight of the children. The imaging was performed immediately after completion of the injection of contrast. The axial unenhanced and enhanced images were acquired parallel to the orbitomeatal line, and the same coverage and same imaging parameters were used. The imaging parameters include section thickness of 5 mm, x-ray tube voltage of 120 kV, x-ray current of 80 mA, 2 images per rotation, and a FOV of 22 cm. The axial enhanced images were then reconstructed at 1.25 mm, and sagittal and coronal reformatted images were generated. The axial reconstructed images and the sagittal and coronal reformatted images were reviewed.
Results
Seventy-one children (34 neonates, 10 infants, 27 older children) with CSVT were included in the study. Nineteen of 34 neonates and 7 of 27 older children had only CT/CTV. Three neonates and 3 older children had both CT/CTV and MR imaging/MRV. In 2 of those 3 older children who had both CT/CTV and MR imaging/MRV, the results of both CT/CTV and MR imaging/MRV were negative for brain lesions. In 1 of 3 older children and in all 3 neonates who had both CT/CTV and MR imaging/MRV, results of both imaging techniques were positive for both CSVT and brain lesions. Most neonates in the previous years of the study had CT/CTV from neonatal transport issues and MR accessibility logistics. As the age of the patient has increased (neonate to older children), there were more MR imaging/MRV examinations (On-line Table 1).
The most common locations for CSVT were the transverse sinuses, the superior sagittal sinus (SSS), and the straight sinus in the neonates and infants. The most common locations in the older children were the SSS, the transverse sinus, and the sigmoid sinus (On-line Table 2). Overall, 37 (52%) of 71 children with CSVT had brain parenchymal lesions. Twenty-one (62%) of 34 neonates, 4 (40%) of 10 infants, and 12 (44%) of 27 older children had brain lesions. Mean age of the older children with brain lesions was 10 years. The most common location of brain lesions in neonates were the frontal and parietal lobes, whereas the most common location of brain lesions in the older children were the frontal lobe and the thalamus (Fig 1). The parenchymal lesions in infants were mostly seen in the frontal lobe and the cerebellar hemisphere (On-line Table 3). The lesions were smaller in the neonates compared with the lesions in the infants and older children (On-line Table 4). The location of brain lesions correlated with the corresponding venous drainage territory in 16 (76%) of 21 neonates, all infants, and all older children with brain lesions.7 The location of the brain lesions did not correlate with the corresponding venous drainage territory in 5 of 21 neonates with brain lesions. In the first patient, the location of the CSVT was the right transverse sinus, whereas the location of the brain lesions was the right temporal and left frontal lobes. In the second patient, the CSVT was in the right transverse sinus, whereas the brain lesions were in the left parietal lobe (Fig 2). In the third patient, the CSVT was in the bilateral transverse and sigmoid sinuses; however, the brain lesion was in the right frontal lobe. In the fourth patient, the location of the CSVT was the right transverse sinus and torcular, the brain lesions were located in the left frontal deep white matter and bilateral parietal lobes. In the last patient, the CSVT was in the bilateral transverse sinuses, and the brain lesions were in the right thalamus, left temporal lobe, and right frontal deep white matter. In the patients with transverse sinus thrombosis, images were viewed carefully to exclude congenital narrowing of the transverse sinus or artifactual flow gaps in the transverse sinus. When contrast-enhanced images were not available, spin-echo images were viewed along with time-of-flight MR venography to definitively diagnose the thrombosis. In 16 (76%) of 21 neonates, the brain lesions were hemorrhagic. In the infants, 3 (75%) of 4 parenchymal lesions were hemorrhagic, whereas 4 (33%) of 12 older children had hemorrhagic parenchymal lesions.
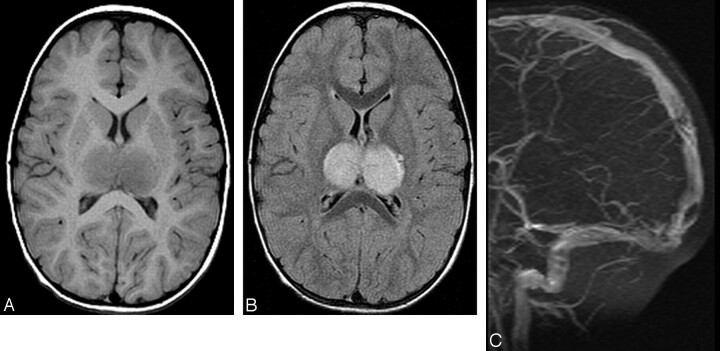
Fig 1.
A 3½-year-old boy is presented with a headache after severe gastroenteritis. A, Axial T1-weighted and (B) axial FLAIR images demonstrate edematous swollen thalami bilaterally. (C) Maximum intensity projection 2D time of flight MRV image reveals nonvisualization of the internal cerebral veins and straight sinus. Filling defects consistent with clots are also noted in the posterior superior sagittal sinus.
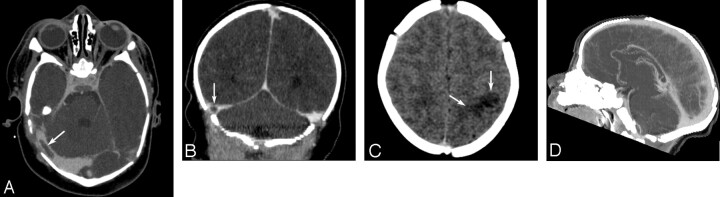
Fig 2.
An 8-day-old term neonate presented with a seizure. (A) Axial and (B) coronal CT venography MPR images demonstrate a thrombus in the right transverse sinus (arrows). (C) Unenhanced axial CT image shows focal hypoattenuated lesion consistent with edema in the left parietal lobe (arrows). D, Sagittal CT venography maximum intensity projection image reveals patency of superior sagittal sinus without thrombosis.
Discussion
CSVT is not an uncommon condition in children and can have serious clinical consequences. The diverse clinical presentation has made CSVT diagnosis difficult and often delayed. The risk factors for CSVT are age dependent, frequently multiple, and different from those reported in adults.1 In the pediatric age group, neonates comprise the largest group, though the true incidence in neonates is still unknown because reliable epidemiologic data are lacking.1 That 48% of children with CSVT were neonates in our study supports the general belief that neonates are more prone to this condition than older children. The SSS and transverse sinus were more frequently involved in our patient population, which is similar to other studies in the literature.1,8,9
Anatomic studies with use of postmortem vascular injections have made extensive contributions to our understanding of the cerebral venous drainage system.10-12 The venous drainage of the cerebral hemispheres is divided into the superficial and the deep systems. The superficial system consists of the SSS, the transverse sinuses, torcular, sigmoid sinuses, and the internal jugular veins. The deep system consists of the deep basal veins draining into the paired internal cerebral veins that unite with the inferior sagittal sinus and then drain into the vein of Galen, the straight sinus, and the torcular7 (Fig 3).
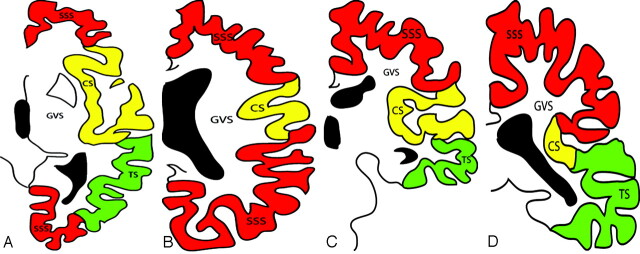
Fig 3.
Diagrammatic representation of the cerebral venous territories.7 Transverse diagrams at the level of the basal ganglia (A) and (B) at the level of the corona radiata, coronal diagrams at the level of the basal ganglia (C) and (D) at the level of thalami. SSS: Territory of the superior sagittal sinus; CS: Territory of the cavernous sinus; TS: Territory of the transverse sinus; GVS: Territory of the Galen vein system.
On the lateral surface of the frontal lobe, the territory of the SSS drains the anterior part of the superior, middle, and inferior frontal gyri anteriorly; the superior part of the inferior frontal gyrus inferiorly, and the portions adjacent to the central sulcus of the ascending frontal and parietal gyri posteriorly. On the medial surface of the frontal lobe, the territory of the SSS includes the medial surface of the superior frontal gyrus, the adjacent part of the cingulate gyrus, and the paracentral lobule. On the lateral surface of the parietal lobe, the SSS drains the posterior part of the postcentral gyrus, the upper part of the supramarginal and angular gyri, and the anterior part of the cuneus. On the medial surface of the parietal lobe, the venous territory of the SSS includes the posterior part of the paracentral lobule, the upper part of the cingulate gyrus, the precuneus, and the adjacent part of the occipital lobe.13 The transverse sinus drains the lateral surface of the temporal lobe, except for the superior temporal gyrus and the inferior surface of the occipital lobe. Occasionally, the veins of the lateral surface of the occipital lobe join the transverse sinus.12 The deep (Galen) venous system includes the basal vein of Rosenthal and the internal cerebral veins14,15 (Fig 3). It has been reported that there is no correlation between the location and the size of the brain lesions and the site and extent of the sinus thrombosis in adult patients, suggesting that adults might better tolerate CSVT.6 However, the results of our study demonstrated good correlation between the site and extent of the sinus thrombosis and the location of the brain lesions in children except in 5 neonate patients. In these neonates, the site of the thrombosis was the transverse sinus, the sigmoid sinus, or the torcular, or all 3, whereas the site of the brain lesions also included the frontal or parietal lobes in addition to the territory of the transverse and sigmoid sinuses. There was no definite thrombosis of the SSS in these patients. We suspect that these neonates may have had thrombosis of the medullary or cortical veins that may not have been detected on imaging.
CT/CTV is a rapid, readily available, and accurate technique for the detection of CSVT. The clinical status of neonates may change very rapidly, and they may not be clinically stable for a long period. For this reason, CT/CTV was the imaging of choice most of the time for scanning neonates because of its advantages of a very short scanning period and its availability. MR imaging/MRV was the imaging of choice in older children because of their relatively clinically stable condition and tolerance for a longer imaging (compared with CT) time compared with neonates. In addition, MR imaging/MRV has the advantages of providing detailed soft tissue information especially for the parenchymal lesions without ionizing radiation. One potential pitfall of MRV in neonates is that there is high proportion of flow gaps in the venous sinuses, particularly the posterior aspect of the SSS, which could be attributed to the age-related smaller caliber of the sinus, smaller venous flow, and skull molding.16 There were 4 of 5 neonates who did not demonstrate correlation between the site of the brain lesions and the venous territory of the thrombosed sinus and had only CT/CTV instead of MR imaging/MRV. In our experience, it is more difficult to detect the thrombosed cortical/medullary veins on CT/CTV as opposed to MR imaging/MRV. These patients actually may have had thrombosed cortical/medullary veins that could have perhaps correlated with the brain lesions.
Intracranial venous hypertension undoubtedly plays an important role in the pathophysiology of brain parenchymal changes. It is assumed that increased intraluminal venous pressure causes decrease in cerebral blood flow and cerebral perfusion pressure. This might induce an energy failure and a disruption of the blood-brain barrier that results in vasogenic edema and hemorrhagic transformation from increased venous pressure.17-19 The protective mechanisms against the abrupt increase of intravenous pressure have been described in the literature. One of these mechanisms is the opening of reserve capillaries that causes an increase in cerebral blood volume during the early phase of sinus occlusion.20 This mechanism serves as a safety valve for excess pressure. Another protective mechanism is the opening of the end-to-end anastomosis of the meningeal veins to other venous channels that preserves a functioning vascular drainage system.21 A higher percentage of brain lesions in neonates (62%) compared with infants (40%) and older children (44%) in our study suggested that CSVT is not well tolerated in children, particularly in neonates. We postulate that the reason for this result may be the deficiency of the protective mechanisms because of immaturity, particularly in the neonates. One potential supportive piece of evidence is that sinus thrombosis alone may be well tolerated in many cases, and the brain lesions resulting from cerebral venous thrombosis do not correlate with sinus involvement in adult patients.6 If the venous collateral pathways are not readily open after sinus thrombosis, intravenous pressure may easily increase rapidly, which may cause injury to the blood-brain barrier very quickly. In addition, if the capillary reserve capacity does not function efficiently, a sufficient amount of cerebral blood volume may not be sustained, which may result in ischemic injury to the brain in the early stage of sinus thrombosis. Venous hemorrhage is considered the final consequence in the pathologic process and may develop, depending on the degree of intrasinus venous pressure distal to the occlusion site and on the time interval from venous occlusion.20 There was a high incidence of hemorrhage in neonates (76%) and infants (75%) with CSVT in our study. In general, the reason for this tendency to hemorrhage in patients with CSVT is the raised venous pressure that gets transmitted in a retrograde fashion proximal to the obstruction. This increases the venular pressure and the capillary hydrostatic pressure that results in leakage of the capillary fluid into the interstitial space, causing edema. The fluid leakage is almost always accompanied by “diapedesis” of red blood cells that usually is the cause of a high incidence of hemorrhagic venous infarcts in CSVT. Most of the edema is vasogenic and hence reversible, but if the process continues unabated, the capillary hydrostatic pressure and interstitial pressure can exceed the arteriolar pressure. This can sometimes cause true “ischemic infarction” also, because of impairment to the arterial inflow.4,22
The brain lesions in neonates with CSVT tended to be smaller compared with the lesions in older children with CSVT in our study. It is difficult to explain the exact mechanism of the smaller size of the lesions in neonates compared with the size of the lesions in older children. One possible reason for this result in our study is the inclusion of tiny deep white-matter abnormalities as parenchymal lesions. This pattern of injury has been detected only in neonates, and inclusion of these lesions may have caused the predominance of smaller size of the lesions in neonates. Medullary vein thrombosis is one of the suggested mechanisms for these tiny deep white-matter lesions. Another possible mechanism for these lesions is subclinical hypoxic-ischemic encephalopathy, which could have also contributed to the deep white-matter lesions.
Conclusion
CSVT is not an uncommon disorder in children, and it occurs most commonly in neonates. Neonates are more vulnerable to brain lesions, and the lesions tend to be more hemorrhagic in neonates and infants with CSVT. The location of the brain lesions associated with CSVT is within the territory of the dural venous sinus. This finding suggests that CSVT is not well tolerated in children compared with adults and warrants early diagnosis. Prompt diagnosis and treatment of CSVT may prevent potential serious damage to the brain parenchyma.
Supplementary Material
Footnotes
indicates article with supplemental on-line tables.
References
- 1.deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med 2001;345:417–23 [DOI] [PubMed] [Google Scholar]
- 2.Tsutsumi K, Shiokawa Y, Sakai T, et al. Venous infarction following the interhemispheric approach in patients with acute subarachnoid hemorrhage. J Neurosurg 1991;74:715–19 [DOI] [PubMed] [Google Scholar]
- 3.Yuh WT, Simonson TM, Wang AM, et al. Venous sinus occlusive disease: MR findings. AJNR Am J Neuroradiol 1994;15:309–16 [PMC free article] [PubMed] [Google Scholar]
- 4.Dormont D, Anxionnat R, Evrard S, et al. MRI in cerebral venous thrombosis. J Neuroradiol 1994;21:81–99 [PubMed] [Google Scholar]
- 5.Fries G, Wallenfang T, Hennen J, et al. Occlusion of the pig superior sagittal sinus, bridging and cortical veins: multistep evolution of sinus-vein thrombosis. J Neurosurg 1992;77:127–33 [DOI] [PubMed] [Google Scholar]
- 6.Bergui M, Bradac GB, Daniele D. Brain lesions due to cerebral venous thrombosis do not correlate with sinus involvement. Neuroradiology 1999;41:419–24 [DOI] [PubMed] [Google Scholar]
- 7.Meder JF, Chiras J, Roland J, et al. Venous territories of the brain. J Neuroradiol 1994;21:118–33 [PubMed] [Google Scholar]
- 8.Sebire G, Tabarki B, Saunders DE, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain 2005;128:477–89 [DOI] [PubMed] [Google Scholar]
- 9.Heller C, Heinecke A, Junker R, et al. Cerebral venous thrombosis in children. A multifactorial origin. Circulation 2003;108:1362–67 [DOI] [PubMed] [Google Scholar]
- 10.Hassler O. Deep cerebral venous system in man. A microangiographic study on its areas of drainage and its anastomoses with the superficial cerebral veins. Neurology 1966;16:505–11 [DOI] [PubMed] [Google Scholar]
- 11.Oka K, Rhoton AL Jr., Barry M, et al. Microsurgical anatomy of the superficial veins of the cerebrum. Neurosurgery 1985;17:711–48 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan HA. The transcerebral venous system. An anatomical study. Arch Neurol 1959;1:148–52 [DOI] [PubMed] [Google Scholar]
- 13.McCord GM, Goree JA, Jimenez JP. Venous drainage to the inferior sagittal sinus. Radiology 1972;105:583–89 [DOI] [PubMed] [Google Scholar]
- 14.Ono M, Rhoton AL Jr., Peace D, et al. Microsurgical anatomy of the deep venous system of the brain. Neurosurgery 1984;15:621–57 [DOI] [PubMed] [Google Scholar]
- 15.Wolfram-Gabel R, Maillot C, Koritke JG. The vascular pattern in the tela choroidea of the prosencephalon in man. J Neuroradiol 1987;14:10–26 [PubMed] [Google Scholar]
- 16.Widjaja E, Shroff M, Blaser S, et al. 2D time-of-flight MR venography in neonates: anatomy and pitfalls. AJNR Am J Neuroradiol 2006;27:1913–18 [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai FY, Wang AM, Matovich VB, et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. AJNR Am J Neuroradiol 1995;16:1021–29 [PMC free article] [PubMed] [Google Scholar]
- 18.Doege CA, Tavakolian R, Kerskens CM, et al. Perfusion and diffusion magnetic resonance imaging in human cerebral venous thrombosis. J Neurol 2001;248:564–71 [DOI] [PubMed] [Google Scholar]
- 19.Peeters E, Stadnik T, Bissay F, et al. Diffusion-weighted MR imaging of an acute venous stroke: case report. AJNR Am J Neuroradiol 2001;22:1949–52 [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaiwa H, Kuchiwaki H, Inao S, et al. Changes in the cerebrocortical capillary network following venous sinus occlusion in cats. Surg Neurol 1995;44:172–79, discussion 179–80 [DOI] [PubMed] [Google Scholar]
- 21.Schaller B, Graf R, Sanada Y, et al. Hemodynamic changes after occlusion of the posterior superior sagittal sinus: an experimental PET study in cats. AJNR Am J Neuroradiol 2003;24:1876–80 [PMC free article] [PubMed] [Google Scholar]
- 22.Bousser MG. Why cerebral infarction is an emergency. Bull Acad Natl Med 2002;186:1159–74; discussion 1174–77 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.