Abstract
BACKGROUND AND PURPOSE: Chronic middle cerebral artery (MCA) occlusion is more common than generally thought. It is important to assess the cerebral hemodynamic status in patients with this chronic condition. We investigated the cerebral hemodynamic and metabolic disturbances in these patients in relation to the development of the collateral vasculature.
MATERIALS AND METHODS: We studied 13 patients with chronic unilateral MCA occlusion who had a minor or no stroke by using positron-emission tomography (PET). PET was performed by the oxygen 15 (15O) gas steady-state inhalation method. The intracranial arteries were evaluated by digital subtraction angiography. We divided the patients into 2 subgroups according to whether they had a normal or increased oxygen extraction fraction (OEF) in the occluded MCA territory and compared the 2 groups.
RESULTS: Of the 13 patients, 9 were classified into the normal OEF and 4 were classified into the increased OEF group. In the increased OEF group, the mean OEF values were also increased in the territories of the ipsilateral anterior cerebral artery, ipsilateral posterior cerebral artery, and contralateral MCA. The patients in the increased OEF group had more than 1 steno-occlusive lesion in the major intracranial arteries (P = .008). Three of the 4 patients in the increased OEF group also had vascular lesions in the collateral pathways to the MCA territory.
CONCLUSION: Most patients with chronic MCA occlusion did not show severe hemodynamic impairment. Those with increased OEF tended to have other areas of severe hemodynamic impairment and other vascular lesions, especially in the collateral pathways.
Chronic middle cerebral artery (MCA) occlusion as a cause of hemodynamic stroke has not been a prominent clinical issue in the Western world. The most common cause of MCA occlusion is embolism, and sudden occlusion of the proximal MCA by an embolus is one of the most frequent causes of major stroke.1 In addition, emboli, especially those from the heart, frequently undergo lysis, and no residual vascular obstruction is found in more than half of the patients.2,3 Atherosclerotic thrombosis, on the other hand, is reported to be a rare cause of MCA occlusion.4,5 However, atherosclerosis of the intracranial arteries is more common in Asians.6–9 There are a number of patients with permanent proximal MCA occlusion who have a minor or no stroke. MCA occlusion is sometimes identified incidentally by MR angiography in asymptomatic patients. It is important to assess the cerebral hemodynamic status in these patients because hemodynamic compromise markedly increases the risk of a subsequent stroke in patients with occlusive cerebral artery disease.10–12
The cerebral hemodynamic studies in terms of chronic MCA occlusion are much fewer than those of carotid occlusion. Two positron-emission tomography (PET) studies and several studies using single-photon emission CT (SPECT) demonstrated that hemodynamic compromise does not always exist in patients with MCA occlusion.13–16 However, it is unclear which factor is related to the hemodynamic status. In the present study, we investigated the hemodynamic status in patients with unilateral MCA occlusion in a chronic state by using PET and evaluated whether angiographic findings and the other factors were associated with the hemodynamic status.
Materials and Methods
We selected 13 consecutive patients with unilateral MCA occlusion, 10 men and 3 women, 8 symptomatic and 5 asymptomatic, 39–72 years of age (mean age, 57.6 ± 10.3 years), who had undergone a PET study between October 2000 and August 2006 at our institution. None of the patients had large infarcts of more than 30 mm in diameter in the internal carotid artery territory. Patients who had undergone a PET study after extracranial-intracranial bypass surgery were excluded. Symptomatic patients had a history of transient ischemic attacks (n = 3) or a minor disabling ischemic stroke (n = 5, modified Rankin Scale grade ≤317). In the asymptomatic patients, including those who had nonfocal symptoms such as dizziness or headache, MCA occlusion was detected incidentally by MR angiography. We performed the PET study at least 1 month after symptom onset in the symptomatic patients. Clinical information on these patients, including their histories, neurologic deficits, medication, and atherosclerotic risk factors, was obtained from the medical records at the time of the PET study (Table 1). PET studies were also performed in 6 healthy volunteers (2 men and 4 women; 26 to 43 years of age; mean age, 33.0 ± 6.6 years) to determine the cutoff point for the normal oxygen extraction fraction (OEF) value. None of the volunteers had a history of neurologic or medical disease, including hypertension or diabetes mellitus. Informed written consent was obtained from each volunteer before the PET study. All protocols were approved by the Ethics Committee for Clinical Research of our institution.
Table 1:
Clinical backgrounds of the 13 patients
| Patient No. | Age | Sex | Presentation | Onset PET (months) | Occlusion Site | Cause of Occlusion | Site of Infarct | Infarct Size (mm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 46 | M | No symptom | R M1 p | Unknown | L corona radiata | 29 | |
| 2 | 62 | M | No symptom | L M1 p | Atherosclerotic | None | ||
| 3 | 66 | M | No symptom | L M1 p | Atherosclerotic | None | ||
| 4 | 62 | M | No symptom | L M1 p | Atherosclerotic | R corona radiata | 6 | |
| 5 | 48 | M | Minor stroke | 182 | L M1 p | Atherosclerotic | L putamen | 25 |
| 6 | 44 | M | Minor stroke | 38 | L M1 p | Atherosclerotic | L putamen, corona radiata | 29 |
| 7 | 72 | M | Minor stroke | 1081 | L M1 p | Atherosclerotic | L frontal cortex | 23 |
| 8 | 63 | M | TIA | 27 | R M1 d | Atherosclerotic | R temporo-occipital cortex | 22 |
| 9 | 39 | M | TIA | 238 | R M1 p | Atherosclerotic | None | |
| 10 | 58 | M | No symptom | R M1 p | Atherosclerotic | L putamen | 4 | |
| 11 | 64 | F | Minor stroke | 129 | R M1 p | Atherosclerotic | R internal capsule, corona radiata | 5 |
| 12 | 56 | F | Minor stroke | 273 | R M1 p | Atherosclerotic | R corona radiata, frontal subcortex | 30 |
| 13 | 69 | F | TIA | 36 | R M1 p | Atherosclerotic | R frontal cortex | 10 |
Note:—TIA indicates transient ischemic attack; L, left; R, right; p, proximal; d, distal.
PET Measurements
All subjects were scanned with a Headtome V/SET 2400W system (Shimadzu, Kyoto, Japan), which acquires 63 sections with an intersection distance of 3.1 mm. All scans were obtained at a resolution of 3.7-mm full width at half maximum in the transaxial direction and at 5 mm in the axial direction. Before the PET study, a germanium 68 (68Ge)/gallium 68 (68Ga) transmission scanning was performed for 10 minutes for attenuation correction. Images were reconstructed with an ordered-subset expectation maximization algorithm (12 iterations with 4 ordered subsets). For the oxygen 15 (15O)-labeled gas steady-state method, 15O–labeled carbon dioxide (550 MBq/min) and 15O2 (1300 MBq/min) were administered by inhalation through a mask.18 The scanning time was 9 minutes, and arterial blood was manually sampled from the radial artery 4 times during each scanning. The concentration of the radiotracer activity in the whole blood and plasma was measured with a well counter; the arterial hemoglobin concentration, pH, saturation level of oxygen in hemoglobin, partial pressure of oxygen in the blood, and partial pressure of carbon dioxide in the arterial blood (PaCO2) were also measured. Inhalation of 2000-MBq C15O and a 9-minute scanning period were used to measure the cerebral blood volume (CBV). Arterial sampling was manually performed 3 times during the scanning, and the radiotracer activity in whole blood was measured. Cerebral blood flow (CBF), the cerebral metabolic rate of oxygen (CMRO2), and the OEF were calculated by the steady-state method, and CMRO2 and OEF were corrected according to the CBV.19
Angiographic Assessment
All patients underwent digital subtraction angiography for clinical purposes within 4 months before or after the PET study. Digital subtraction angiography was performed with an Integris V3000 scanner (Philips Medical Systems, Best, the Netherlands). The results were reviewed independently by a neuroradiologist and one of the investigators (M.T.), who were blinded to the PET results. No patient had either occlusion or stenosis of >50% in the internal cerebral artery.
The anatomic information evaluated included the following: 1) the side and portion of the MCA occluded, 2) occlusion or stenosis in the ipsilateral anterior cerebral artery (ACA) or posterior cerebral artery (PCA), 3) presence of the anterior and posterior communicating arteries, 4) occlusion or stenosis in the contralateral major cerebral arteries, 5) occlusion or stenosis in the basilar artery or the intracranial vertebral arteries, and 6) the segment of the MCA that showed retrograde contrast filling from the ACA or PCA. The degree of stenosis was expressed as the percentage stenosis of the narrowest luminal diameter relative to the diameter of the normal distal lumen, and a stenosis percentage of >50% was considered significant.
Data Analysis
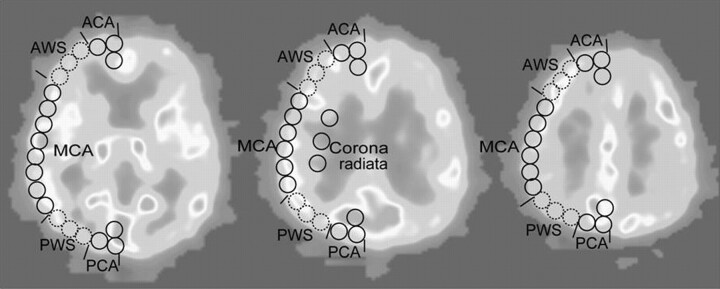
All PET measurements were analyzed with the Dr. View Linux 1.1 image analysis software system (AJS, Tokyo, Japan). We analyzed the images in 3 tomographic planes: the levels of the basal ganglia and thalamus, body of the lateral ventricle, and the centrum semiovale. Each image was examined by placing circular regions of interest, 10 mm in diameter, over the gray matter of the cortex (Fig 1). According to the atlas of Kretschmann and Weinrich,20 the region of interest included the cortical territories of the ACA, MCA, PCA, and the corona radiata. The anatomic watershed areas between the ACA and MCA and between the MCA and PCA were excluded in the analysis to examine each territory independently. Areas of prior infarction identified on the CMRO2 images and MR images were also excluded from the analysis. The mean values of the CBF, CBV, CMRO2, OEF, and the CBF/CBV ratio were then calculated. These values were compared between the symptomatic and asymptomatic patients. Next the patients were divided into 2 groups, the increased OEF group and the normal OEF group in terms of the absolute OEF values in the occluded MCA territory. Because the mean OEF value in the MCA territory in the 12 hemispheres from the 6 healthy volunteers was 42.0 ± 2.3%, we defined pathologically increased OEF as an OEF of >49.3% (absolute hemispheric values of the upper 99% confidence limits in healthy volunteers).10,12
Fig 1.
Locations of regions of interest: cortical territories of the ACA, MCA, PCA, and the corona radiata. The watershed areas between the ACA and MCA (AWS) and the watershed areas between the MCA and PCA (PWS) were excluded from the analysis.
Differences in the mean hemispheric values of the CBF, CBV, CMRO2, and the CBF/CBV ratio between the symptomatic and asymptomatic groups or between the normal and increased OEF groups were tested with a 2-tailed t test. Differences in the proportions of the clinical variables between the normal and increased OEF groups were tested by the Fisher exact test (2-sided). Differences in the means of the background clinical parameters between the normal and increased OEF groups were tested by the 2-tailed t test. A value of P < .05 was considered to be statistically significant.
Results
All except 1 of the patients had unilateral occlusion at the proximal site of the M1 portion. The remaining 1 patient had occlusion at the distal site of the M1 portion (Table 1). The average regional values in the occluded MCA territory were as follows: CBF, 42.1 ± 7.4 mL · 100 g−1 · min−1; OEF, 46.3 ± 4.9%; CMRO2, 3.3 ± 0.52 mL · 100 g−1 · min−1; CBV, 4.9 ± 1.4 mL · 100 g−1; CBF/CBV, 9.3 ± 2.9 minutes−1. The average regional values in the contralateral MCA territory were as follows: CBF, 45.5 ± 7.3 mL · 100 g−1 · min−1; OEF, 44.2 ± 5.2%; CMRO2, 3.4 ± 0.54 mL · 100 g−1 · min−1; CBV, 4.6 ± 1.3 mL · 100 g−1; CBF/CBV, 10.6 ± 3.5 minutes−1. There were no differences in the values between the symptomatic and asymptomatic patients. (Table 2)
Table 2:
Cerebral blood flow and metabolism in the symptomatic and asymptomatic patients
| Asymptomatic Patients | Symptomatic Patients | P | |
|---|---|---|---|
| CBF (mL · 100 g−1 · min−1) | 44.7 ± 6.0 | 40.5 ± 8.7 | .36 |
| OEF (%) | 42.9 ± 5.7 | 48.5 ± 5.7 | .11 |
| CBV (mL · 100 g−1) | 3.3 ± 0.4 | 3.3 ± 0.7 | .92 |
| CMRO2 (mL · 100 g−1 · min−1) | 4.9 ± 1.4 | 4.9 ± 1.8 | .97 |
| CBF/CBV (min−1) | 9.7 ± 2.8 | 9.1 ± 3.4 | .77 |
Note:—CBF indicates cerebral bloodflow; OEF, oxygen extraction fraction; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate of oxygen.
There were 9 patients with normal OEF values in the occluded MCA territory (normal OEF group, patients 1–9) and 4 patients with increased OEF values (increased OEF group, patients 10–13) in the occluded MCA territory. The clinical backgrounds were not significantly different between the 2 groups, except for a higher proportion of women in the increased OEF group (Table 3). There were no significant differences in the hemodynamic parameters, such as the hemoglobin concentration, blood pressure, or O2 content, which could influence the OEF values between the 2 groups. The PaCO2 levels remained stable throughout the PET examination (Table 3).
Table 3:
Background hemodynamic parameters and clinical characteristics in the normal and increased OEF groups
| Normal OEF (n = 9) | Increased OEF (n = 4) | |
|---|---|---|
| Hemoglobin (g/dL) | 13.5 ± 1.6 | 11.8 ± 0.5 |
| O2 content (mL/dL) | 17.8 ± 2.5 | 15.6 ± 0.9 |
| Mean blood pressure (mm Hg) | 93.7 ± 10.1 | 89.2 ± 5.7 |
| PaCO2 during C15O2 inhalation (mm Hg) | 39.6 ± 2.3 | 40.0 ± 1.8 |
| PaCO2 during 15O2 inhalation (mm Hg) | 39.9 ± 2.8 | 40.9 ± 2.2 |
| Age (mean ± SD) (yr) | 55.8 ± 11.6 | 61.8 ± 5.9 |
| Sex (male/female) (No.)* | 9/0 | 1/3 |
| Symptomatic (No.) | 5 | 3 |
| Hypertension (No.) | 6 | 4 |
| Diabetes mellitus (No.) | 2 | 0 |
Note:—OEF indicates oxygen extraction traction; PaCO2, partial pressure of carbon dioxide.
P < .05.
Compared with the normal OEF group, the increased OEF group showed higher CBV values and lower CBF/CBV ratios in the occluded MCA territory (Table 4). The increased OEF group also showed significantly higher OEF values in the other cortical territories (Table 4).
Table 4:
Cerebral blood flow and metabolism in the normal and increased OEF groups
| Normal OEF (n = 9) | Increased OEF (n = 4) | |
|---|---|---|
| Ipsilateral MCA | ||
| CBF (mL · 100 g−1 · min−1) | 42.6 ± 8.1 | 40.9 ± 8.1 |
| OEF (%) | 43.1 ± 3.9 | 53.7 ± 3.1 |
| CBV (mL · 100 g−1)* | 4.3 ± 1.3 | 6.3 ± 1.4 |
| CMRO2 (mL · 100 g−1 · min−1) | 3.3 ± 0.6 | 3.4 ± 0.5 |
| CBF/CBV (min−1)* | 10.5 ± 2.9 | 6.6 ± 1.2 |
| Ipsilateral corona radiata | ||
| CBF | 30.6 ± 9.2 | 28.9 ± 4.9 |
| OEF | 39.2 ± 7.7 | 46.8 ± 5.5 |
| CBV* | 3.1 ± 1.3 | 5.0 ± 0.7 |
| CMRO2 | 2.1 ± 0.5 | 2.1 ± 0.3 |
| CBF/CBV* | 11.5 ± 6.0 | 6.0 ± 1.5 |
| Contralateral MCA | ||
| CBF | 46.8 ± 5.7 | 42.7 ± 11.4 |
| OEF† | 41.8 ± 3.9 | 50.0 ± 4.3 |
| CBV | 4.3 ± 1.2 | 5.5 ± 1.6 |
| CMRO2 | 3.5 ± 0.5 | 3.3 ± 0.7 |
| CBF/CBV | 11.6 ± 3.3 | 8.4 ± 3.9 |
| Ipsilateral ACA | ||
| CBF | 41.3 ± 6.2 | 36.0 ± 8.9 |
| OEF† | 40.8 ± 3.8 | 50.4 ± 5.7 |
| CBV | 4.6 ± 1.4 | 5.5 ± 1.2 |
| CMRO2 | 3.0 ± 0.3 | 2.8 ± 0.6 |
| CBF/CBV | 10.0 ± 4.1 | 6.7 ± 2.0 |
| Ipsilateral PCA | ||
| CBF | 43.8 ± 8.3 | 41.0 ± 7.2 |
| OEF† | 44.3 ± 4.2 | 53.5 ± 4.4 |
| CBV | 4.4 ± 1.5 | 6.3 ± 1.7 |
| CMRO2 | 3.2 ± 0.5 | 3.4 ± 0.6 |
| CBF/CBV | 10.0 ± 3.4 | 6.7 ± 1.2 |
Note:—CBF indicates cerebral bloodflow; OEF, oxygen extraction fraction; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate of oxygen; MCA, middle cerebral artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery.
P < .05.
P < .01.
None of the patients showed vascular lesions in the basilar artery or either vertebral artery. Two patients had unilateral intracranial vertebral arterial stenosis (patients 9 and 10). The remaining vascular findings are shown in Fig 2. Significantly, all patients in the increased OEF group had >1 intracranial steno-occlusive lesion (P = .008), especially in the ACA. All except 1 patient had collaterals either from the ACA or PCA, which reconstituted the M1 or M2 segment. One patient in the increased OEF group showed no retrograde contrast filling of the occluded MCA territory from either the ACA or the PCA.
Fig 2.
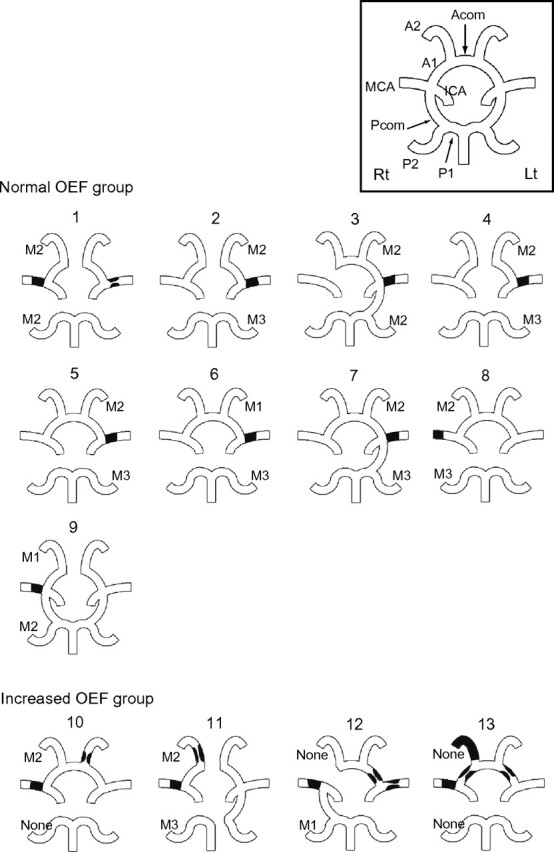
Angiographic assessment of the patients. This diagram shows schematic representations of the circle of Willis and the major intracranial vessels in each patient. Black lesions indicate the location of the stenosis or occlusion. Acom indicates the anterior communicating artery; Pcom, the posterior communicating artery; Rt; right, Lt; left; M1-M3, collaterals reconstituting the M1, M2, or M3 segments; None, little or no significant reconstitution of the territory of the occluded vessel.
Discussion
We confirmed the findings in previous studies of cerebral hemodynamic and metabolic disturbances in patients with chronic unilateral MCA occlusion. The hemodynamic status in the occluded MCA territory varied among the patients. The mean regional cortical CBF was only 5.7% lower than that in the contralateral MCA territory. Nine of the 13 patients with MCA occlusion showed no hemodynamic compromise in the chronic state. Of the 5 patients reported by Derdeyn et al,15 2 had an increased OEF and the rest had a normal OEF and reduced CBF. In a previous SPECT study, 9 of 29 patients showed reduced cerebrovascular reactivity in the occluded MCA territory.13
One of the new findings of our study was that an increase of the OEF was associated with multiple intracranial steno-occlusive arterial lesions. In particular, 3 of the 4 patients in the increased OEF group had steno-occlusive lesions in the collateral pathways. Blood supply to the occluded MCA territory is maintained mainly via pial anastomoses and their upstream vessels of both the ipsilateral ACA and PCA. One patient had stenosis of the ipsilateral ACA, and another had stenosis of both the ipsilateral ACA and PCA. In Patient 12, the ipsilateral ACA was supplied from the contralateral side through the anterior communicating artery because the ipsilateral A1 was congenitally absent. However, stenosis was observed in the contralateral A1. In contrast, none of the subjects in the normal OEF group showed vascular lesions in the collateral pathways. Steno-occlusive lesions in the collateral pathways upstream of the pial anastomosis reduce the pial arterial pressure (ie, the perfusion pressure in the territory of the occluded MCA) and may thus cause misery perfusion. In the increased OEF group, the OEF values were also increased in the territories of the ipsilateral ACA, ipsilateral PCA, and contralateral MCA. The steno-occlusive lesions may directly induce misery perfusion in their original territory. A similar pathologic process was reported in patients with Moyamoya disease. Mugikura et al21 indicated that progressive steno-occlusive changes in the anterior and posterior circulations were associated with the frequency and extent of cerebral infarctions in patients with Moyamoya disease.
Poor collateral development is considered a risk factor for stroke in patients with occlusive cerebral artery disease. Some studies have indicated an association between collateral vasculature and the hemodynamic status.22–24 However, this association was limited to patients with carotid disease, and the results obtained remain controversial to date.25,26 One of the reasons for these conflicting results is that collateral pathways can be varied and complicated in patients with carotid occlusion and it is difficult to assess all potential collateral routes. Compared with that in patients with carotid artery occlusion, the collateral vasculature is simpler in patients with MCA occlusion. In the report by Derdeyn et al,15 the MCA territory was supplied solely by pial collaterals in all 5 patients with MCA occlusion, regardless of the OEF.15 They concluded that pial collateralization is not a specific sign of increased OEF. Our subjects did not demonstrate significant association between the OEF values and pial collateral development either. Pial collaterals from the ACA and PCA play a complementary role, and most patients showed retrograde contrast filling of the occluded MCA segment or at least 1 segment distal to the occluded site. Our results indicate that a major arterial lesion upstream of the pial arteries may be a more important factor causing hemodynamic compromise.
The treatment for patients with MCA occlusion associated with hemodynamic impairment has not been established. It is not clear whether surgical treatment for providing additional blood supply to the brain in these patients can reduce the incidence of stroke. For the case of symptomatic carotid artery occlusion, there is an ongoing trial designed to determine whether extracranial-to-intracranial (ECIC) arterial bypass surgery can reduce the incidence of stroke in patients with increased OEF.27 The patients with increased OEF in the present study had steno-occlusive lesions in the collateral pathways and other areas showing hemodynamic impairment. Progression of collateral stenosis could induce extensive infarction, and ECIC bypass surgery may be expected to be effective in these patients. One patient with increased OEF had undergone ECIC bypass surgery aimed at reducing the risk of stroke before coronary bypass surgery, and the cerebral blood flow had improved. However, few patients with MCA occlusion show hemodynamic compromise; therefore, the usefulness of bypass surgery should be evaluated in a multicenter trial.
PET has been the standard perfusion imaging technique used to select patients who would benefit from ECIC bypass surgery; however, it is not easily available for daily clinical practice. Surrogate perfusion imaging techniques that are more easily accessible are needed for the management of each patient. Perfusion-weighted MR imaging (PWI) and quantitative CBF imaging, such as CT perfusion or SPECT with an acetazolamide challenge test, are reasonable choices for this purpose. Unfortunately, their quantification is less reliable than that possible with PET, and they are not recommended for the evaluation of patients with chronic ischemia by the guidelines of the American Heart Association.28 These techniques should be compared with PET in the same settings to determine their roles. More recent studies comparing PWI with PET in chronic occlusive carotid disease and Moyamoya disease reported that patients with abnormally increased OEF as measured by PET could be discovered on the basis of the time-to-peak delay of more than the threshold as measured by PWI.29,30
There are 2 limitations of this study. First, the controls were much younger than the study patients. We used the OEF values in the control group to determine the cutoff point of the normal OEF values for the patients. However, several studies have reported that OEF values are not significantly influenced by age.31–33 Leenders et al34 reported the correlations between the PET data and age in each part of the brain. A slight increase of the OEF with age was seen in some regions but not in the MCA cortical ribbon. According to these reports, the assumption of mean OEF value in the control group would not be much different from the original one if their ages were matched with those of the patients. We determined the upper 99% confidence limits of the OEF values instead of the upper 95% confidence limits as the cutoff, to make the cutoff applicable to older patients. Second, this study was performed in a retrospective manner, and the association between the status of the collaterals and cerebral hemodynamics was not assessed with time. The conditions of the collaterals may be affected with time from the onset. Also, PET was performed after varying times from onset in the symptomatic patients. There were no differences in the OEF, CBV, or the mean transit time between the symptomatic and asymptomatic patients with MCA occlusion in the chronic state; however, the results may differ in the acute state. Prospective studies are needed to confirm the findings in these patients.
Conclusions
Most of the patients with chronic MCA occlusion with no symptoms or only a minor stroke did not show any severe hemodynamic impairment. Those with increased OEF tended to have other areas of severe hemodynamic impairment and other vascular lesions in the collateral pathways.
Acknowledgments
We thank Y. Nakamura, M. Sasagaki, and K. Fujino for their assistance during this clinical research.
Footnotes
This work was supported by the Molecular Imaging Program on “Research Base for Exploring New Drugs” from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and the Research Promotion Program on Health from the National Institute of Biomedical Innovation, Japan.
References
- 1.Olsen TS, Skriver EB, Herning M. Cause of cerebral infarction in the carotid territory: its relation to the size and the location of the infarct and to the underlying vascular lesion. Stroke 1985;16:459–66 [DOI] [PubMed] [Google Scholar]
- 2.Zanette EM, Roberti C, Mancini G, et al. Spontaneous middle cerebral artery reperfusion in ischemic stroke: a follow-up study with transcranial Doppler. Stroke 1995;26:430–33 [DOI] [PubMed] [Google Scholar]
- 3.Fieschi C, Bozzao L. Transient embolic occlusion of the middle cerebral and internal carotid arteries in cerebral apoplexy. J Neurol Neurosurg Psychiatry 1969;32:236–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lhermitte F, Gautier JC, Derouesne C. Nature of occlusions of the middle cerebral artery. Neurology 1970;20:82–88 [DOI] [PubMed] [Google Scholar]
- 5.Fisher CM. Cerebral ischemia: less familiar types. Clin Neurosurg 1971;18:267–336 [PubMed] [Google Scholar]
- 6.Kieffer SA, Takeya Y, Resch JA, et al. Racial differences in cerebrovascular disease: angiographic evaluation of Japanese and American populations. Am J Roentgenol Radium Ther Nucl Med 1967;101:94–99 [PubMed] [Google Scholar]
- 7.Liu HM, Tu YK, Yip PK, et al. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke 1996;27:650–53 [DOI] [PubMed] [Google Scholar]
- 8.Leung SY, Ng TH, Yuen ST, et al. Pattern of cerebral atherosclerosis in Hong Kong Chinese: severity in intracranial and extracranial vessels. Stroke 1993;24:779–86 [DOI] [PubMed] [Google Scholar]
- 9.Suh DC, Lee SH, Kim KR, et al. Pattern of atherosclerotic carotid stenosis in Korean patients with stroke: different involvement of intracranial versus extracranial vessels. AJNR Am J Neuroradiol 2003;24:239–44 [PMC free article] [PubMed] [Google Scholar]
- 10.Yamauchi H, Fukuyama H, Nagahama Y, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry 1996;61:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grubb RL Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–60 [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi H, Fukuyama H, Nagahama Y, et al. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med 1999;40:1992–98 [PubMed] [Google Scholar]
- 13.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002;33:1857–62 [DOI] [PubMed] [Google Scholar]
- 14.Kuroda S, Houkin K, Kamiyama H, et al. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 2001;32:2110–16 [DOI] [PubMed] [Google Scholar]
- 15.Derdeyn CP, Powers WJ, Grubb RL Jr. Hemodynamic effects of middle cerebral artery stenosis and occlusion. AJNR Am J Neuroradiol 1998;19:1463–69 [PMC free article] [PubMed] [Google Scholar]
- 16.Sgouropoulos P, Baron JC, Samson Y, et al. Severe stenoses and persistent occlusions of the middle cerebral artery: hemodynamic and metabolic consequences studied by positron tomography [in French]. Rev Neurol (Paris) 1985;141:698–705 [PubMed] [Google Scholar]
- 17.United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: Interim results—UK-TIA study group. Br Med J (Clin Res Ed) 1988;296:316–20 [PMC free article] [PubMed] [Google Scholar]
- 18.Frackowiak RS, Lenzi GL, Jones T, et al. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr 1980;4:727–36 [DOI] [PubMed] [Google Scholar]
- 19.Lammertsma AA, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab 1983;3:416–24 [DOI] [PubMed] [Google Scholar]
- 20.Kretschmann H-J, Weinrich W. Neuroanatomy and Cranial Computed Tomography. New York; Thieme-Stratton;1986. :60–74
- 21.Mugikura S, Takahashi S, Higano S, et al. The relationship between cerebral infarction and angiographic characteristics in childhood Moyamoya disease. AJNR Am J Neuroradiol 1999;20:336–43 [PMC free article] [PubMed] [Google Scholar]
- 22.Powers WJ, Press GA, Grubb RL Jr, et al. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med 1987;106:27–34 [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi H, Kudoh T, Sugimoto K, et al. Pattern of collaterals, type of infarcts, and haemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatry 2004;75:1697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebeskind DS. Collateral circulation. Stroke 2003;34:2279–84 [DOI] [PubMed] [Google Scholar]
- 25.Derdeyn CP, Shaibani A, Moran CJ, et al. Lack of correlation between pattern of collateralization and misery perfusion in patients with carotid occlusion. Stroke 1999;30:1025–32 [DOI] [PubMed] [Google Scholar]
- 26.Bisschops RH, Klijn CJ, Kappelle LJ, et al. Collateral flow and ischemic brain lesions in patients with unilateral carotid artery occlusion. Neurology 2003;60:1435–41 [DOI] [PubMed] [Google Scholar]
- 27.Grubb RL Jr, Powers WJ, Derdeyn CP, et al. The carotid occlusion surgery study. Neurosurg Focus 2003;14:e9. [DOI] [PubMed] [Google Scholar]
- 28.Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: a scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003;34:1084–104 [DOI] [PubMed] [Google Scholar]
- 29.Kajimoto K, Moriwaki H, Yamada N, et al. Cerebral hemodynamic evaluation using perfusion-weighted magnetic resonance imaging: comparison with positron emission tomography values in chronic occlusive carotid disease. Stroke 2003;34:1662–66 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, Nariai T, Nagaoka T, et al. Quantitative evaluation of cerebral hemodynamics in patients with Moyamoya disease by dynamic susceptibility contrast magnetic resonance imaging: comparison with positron emission tomography. J Cereb Blood Flow Metab 2006;26:291–300 [DOI] [PubMed] [Google Scholar]
- 31.Marchal G, Rioux P, Petit-Taboue MC, et al. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 1992;49:1013–20 [DOI] [PubMed] [Google Scholar]
- 32.Pantano P, Baron JC, Lebrun-Grandie P, et al. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 1984;15:635–41 [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Kanno I, Uemura K, et al. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 1986;17:1220–28 [DOI] [PubMed] [Google Scholar]
- 34.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization: normal values and effect of age. Brain 1990;113 (Pt 1):27–47 [DOI] [PubMed] [Google Scholar]