Abstract
BACKGROUND AND PURPOSE: Hypotension and bradycardia are common in carotid artery stenting (CAS) and are particularly worrisome in the high risk patient who is typically referred for CAS. The purpose of this work was to assess the incidence and predictors of hypotension and bradycardia and the risk of their delayed occurrence after CAS.
MATERIALS AND METHODS: A total of 53 men and 40 women (median age, 71 years) with symptomatic (57%) or asymptomatic (42%) carotid artery stenosis had CAS performed in our institution between December 2002 and January 2007. Patient vital sign records for the 12 hours post-CAS were analyzed. The relative decrease of blood pressure and pulse rate were used as primary end points, and the requirement of pressor or anticholinergic drugs was used as a surrogate end point. Significant predictors of hypotension and bradycardia were analyzed with a logistic regression model. Cumulative freedom from hypotension and bradycardia was calculated by using the Kaplan-Meier method. Negative predictive value (NPV) of screening for early hypotension and bradycardia was determined.
RESULTS: The incidence of hypotension, bradycardia, and both was 14%, 23%, and 15%, respectively. Drug intervention was required in 45 patients (48%). Asymptomatic stenosis was an independent predictor of hypotension and bradycardia. Stenosis proximity to the bifurcation and dilation percentage were independent predictors of the drug intervention requirement. Seven patients (8%) had new onset of hypotension or bradycardia later than 6 hours post-CAS. The NPV of early hypotension and bradycardia was 97% and 93%, respectively.
CONCLUSION: In this retrospective study, the risk of hypotension or bradycardia after CAS is significantly influenced by the degree of dilation performed, and the risk of their delayed occurrence may justify a minimum of 12 hours postprocedural vital sign monitoring.
Hypotension and bradycardia can occur during or after carotid artery stent placement (CAS) due to the stretching of the carotid sinus baroreceptors by the balloon and the stent. The role of this phenomenon as a predictor of adverse outcomes is still debated.1-5 Because CAS represents a valuable alternative to carotid endarterectomy (CEA) among the high-risk patient population,6 patients with severe carotid artery stenosis also having cardiac or cerebrovascular comorbidities are increasingly referred for CAS. Those patients may be more vulnerable to hypotension or bradycardia. In fact, patients with coronary disease undergoing cardiac or noncardiac surgery are at higher risk of myocardial infarction (MI) if prolonged hypotension occurs during the procedure.7-10 It has also been suggested that hypotension associated with CAS increases the risk of cerebrovascular events in impairing the distal washout of residual emboli not intercepted by the protection device.2,11
The concern about the potential harmfulness of hypotension and bradycardia during or after CAS has enhanced the scientific interest about its risk factors. So far, the reported predictors vary from one study to another.1-3,12-18 The study end points, as well as the time point and the duration of the vital sign (VS) monitoring, are also highly variable. Absolute blood pressure (BP) and pulse rate (PR) values2,3,13,14,16-18 or absolute decrease in their initial values1,3,16 are used in most reports, and a standardized follow-up time for VS monitoring is rarely defined.3,14,18
This study aimed to assess the predictors of hypotension and bradycardia occurring in the 12 hours after CAS by using the relative decrease of BP and PR as primary end points and the requirement of pressor or anticholinergic drugs as a surrogate end point. Because the current discussion about the safety of performing CAS in an outpatient setting has also increased the concern about the risk of hypotension and bradycardia after CAS, a secondary objective of the study was to evaluate the risk of their delayed appearance and the negative predictive value (NPV) of early (0–6 hours post-CAS) screening.
Materials and Methods
The study was approved by the institutional review board of the Weill Cornell Medical College, and the procedures followed for this study were in accordance with institutional guidelines.
Patient Population
Patients were identified from a registry maintained by the Weill Cornell Medical College Division of Interventional Neuroradiology. We included all of the patients admitted with a diagnosis of carotid atherosclerotic disease and stenosis who were treated with CAS in our department between December 2002 and January 2007 (n = 93). All of the patients had a minimum follow-up of 30 days poststenting. Most patients were enrolled in a US Food and Drug Administration–approved research protocol (Capture 2 or Carotid Revascularization Endarterectomy versus Stenting Trial).19,20 Patients who had CAS to treat a nonatherosclerotic lesion, such as a carotid artery dissection or a pseudoaneurysm, were excluded.
Standardized Treatment and Follow-up
All of the CASs were performed as an inpatient procedure. Patients were admitted the morning of the procedure. They were premedicated with 325 mg of acetyl salicylic acid and 75 mg of clopidogrel (Plavix) daily for 3 days and were told to hold antihypertensive medications on the morning of the procedure. The procedures were performed under mild sedation. A baseline angiogram was performed. The stenosis length, the lumen diameter of the stenotic segment, and the lumen diameter of the parent artery segment were measured. An intravenous heparin bolus of 70 IU/kg of weight was administered. An anticholinergic drug (glycopyrrolate or atropine) was administered before angioplasty. The CAS procedure was performed in a standard fashion, including the following: placement of a protection device, prestent angioplasty, stent placement with a self-expandable stent, and poststent angioplasty if necessary. Prestent and poststent angioplasty were performed by using a 4.5–5.5 × 20-mm Viatrac angioplasty balloon (Abbott Vascular, Santa Clara, Calif). In case of very tight stenosis, a preangioplasty was performed by using a 2–4 × 20-mm Viatrac angioplasty balloon. Primary stent placement (without prestent angioplasty) was used only twice. After CAS, the patient's VSs and neurologic signs were monitored for a minimum of 12 hours. The BP and PR were monitored with an arterial line and were recorded every 15 minutes during the first hour, every 30 minutes during the second hour, and every hour thereafter. If the patient had a sustained low systolic BP (BP <90 mm Hg, lasting >30 minutes, despite fluid intravenous bolus administration), pressors were started; if the patient had a sustained low PR (PR <50, lasting >30 minutes), anticholinergics (glycopyrrolate or atropine) were administered. Sustained hypertension was treated with a nicardipine drip. The patient's home antihypertensors were held during the first 12 hours after the procedure. A follow-up examination was performed at 1 month. Any new neurologic or cardiac events that had occurred in the first 30 days poststenting were recorded.
Clinical and Radiologic Data
Table 1 displays the baseline variables collected. First VSs taken on patient arrival in the angiography suite (before the administration of any medication), poststenting VSs recorded during the first 12 hours, administration of pressors or anticholinergics, and any clinical manifestation of hypotension or bradycardia were collected. Hospitalization lasting more than 1 night due to hypotension or bradycardia was noted. All of the medical progress notes during the hospitalization and at follow-up were examined to search for neurologic or cardiac events reported within 30 days of the procedure. Neurologic events were categorized as transient ischemic attack (TIA; neurologic deficit lasting <24 hours), minor stroke (neurologic deficit lasting >24 hours, with a National Institutes of Health Stroke Scale score [NIHSS] <4), and major stroke (neurologic deficit lasting >24 hours, with a NIHSS >4). MI was defined as a creatine kinase level higher than 2 times the upper limit of normal with a positive myocardial band isoenzyme fraction.
Table 1.
Baseline characteristics of the patient population (N = 93)
| Characteristic | n (%) |
|---|---|
| Age, median (interquartile range, range), y | 71 (59–77, 45–90) |
| Male | 53 (57) |
| Symptomatic stenosis | 53 (57) |
| Asymptomatic stenosis | 39 (42) |
| Diabetes | 28 (30) |
| Coronary atherosclerotic disease | 52 (56) |
| Hypertension | 70 (75) |
| Neck radiotherapy | 3 (3) |
| Ipsilateral endarterectomy | 4 (4) |
| Current smokers | 27 (29) |
| Taking antihypertensors at home | 68 (73) |
A different investigator reviewed each angiogram. The following assessments were done: stenosis percentage (North American Symptomatic Carotid Endarterectomy Trial),21 stenosis proximity to the bifurcation (indicated as less than or greater than 10 mm from the bifurcation), residual stenosis percentage poststenting, and presence of calcifications. The dilation percentage was calculated as follows:
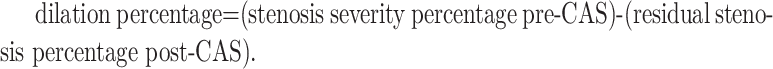 |
End Points
Hemodynamic depression is considered to be an occurrence of hypotension or bradycardia after CAS. Hypotension was defined as any decrease in systolic BP of more than 40% of the prestent value. Bradycardia was defined as any decrease in the PR of more than 20% of the prestent value. Active treatments of hypotension or bradycardia with either pressor or anticholinergics were separately used as a surrogate end point to overcome the possible omission of some hypotension or bradycardia record in the charts and to separate the clinically significant episodes that were severe enough to require an intervention.
Statistical Analysis
Univariate analyses were performed to determine whether associations exist between the patient characteristics of interest (Tables 1 and 2) and the outcomes of hypotension, bradycardia, and drug intervention for hemodynamic depression. χ2 tests and Fisher exact tests were used for categoric variables, and t tests were used for continuous variables. A liberal P value of .15 was used as a cutoff for deciding whether a patient characteristic had a potentially significant relationship with the 3 outcomes. Any characteristic with a univariate P value less than or equal to .15 was retained in a multivariate logistic regression model. A characteristic with a P value less than .05 in the multivariate model was considered statistically significant. A McNemar test of association was also performed to determine whether a relationship exists between a patient's BP and PR status within 0–6 hours and their status within 6–12 hours after surgery. Cumulative freedom from hypotension and bradycardia was calculated at different time points by using the Kaplan-Meier method. In several cases, several CASs were performed at different times on the same patient to treat either a restenosis or a contralateral stenosis. For such cases, we used only the data from the first procedure so that all of the subjects entered into the analyses were fully independent.
Table 2.
Distributions of anatomic and procedural data
| Variable | n (%) |
|---|---|
| Stenosis severity | |
| <50% | 4 (4) |
| 50%–70% | 38 (41) |
| >70% | 51 (55) |
| Stenosis <10 mm to bifurcation | 58 (62) |
| Presence of coarse calcification | 36 (39) |
| Premedication | |
| Glycopyrrolate | 65 (70) |
| Atropine | 8 (9) |
| Both | 3 (3) |
| Unknown | 17 (18) |
| Number of dilations | |
| 0–1 | 40 (43) |
| 2 | 35 (38) |
| 3–5 | 13 (14) |
| Unknown | 5 (5) |
| Residual stenosis after stenting | |
| 0%–10% | 53 (57) |
| 11%–20% | 19 (20) |
| 21%–30% | 15 (16) |
| 31%–40% | 6 (7) |
| Dilation percentage obtained* | |
| 10%–39% | 12 (13) |
| 40%–69% | 39 (42) |
| 70%–99% | 42 (45) |
The formula is (stenosis severity percentage before carotid artery stent placement) − (residual stenosis percentage after carotid artery stent placement).
Results
Population Characteristics and Anatomic and Procedural Data
Between December 2002 and January 2007, 100 CASs were performed on 93 patients for treatment of extracranial carotid artery stenosis. Five patients had an additional CAS performed later, either for a restenosis (n = 2) or a contralateral stenosis (n = 3); 1 patient had 2 additional CASs, 1 for a restenosis and 1 for a contralateral stenosis. One patient had more than 1 CAS performed within 1 month. All of the patients were admitted for at least 12 hours after the CAS. Table 1 gives the baseline characteristics, and Table 2 gives the anatomic and procedural data of the cohort. Intraprocedural asystole occurred in 3 patients and was rapidly reversed with an anticholinergic. No patients needed transcutaneous pacing.
Incidence of Hypotension and Bradycardia after CAS
Baseline VS values were available for 88 patients, and post-CAS values were available for all of the patients (Table 3). The maximal percentage decrease in systolic BP and PR was calculated in all 88 of the patients for whom the baseline VSs were found. Twenty-five patients developed at least 1 episode of hypotension, and 33 patients developed at least 1 episode of bradycardia in the 12 hours after the procedure. Forty-five patients (51%) developed either hypotension or bradycardia, 12 (14%) developed only hypotension, 20 (23%) developed only bradycardia, and 13 (15%) developed both. No clinical manifestation of hypotension or bradycardia was found in the charts of any of those patients.
Table 3.
Baseline pre-CAS and lowest post-CAS systolic blood pressure and pulse rate
| Variable | Baseline Pre-CAS | Lowest Post-CAS Values |
||
|---|---|---|---|---|
| 0–3 h | 3–6 h | 6–12 h | ||
| SBP | 159 (144–175, 102–221) | 117 (98–130, 70–163) | 115 (102–130, 63–167) | 109 (97–129, 75–176) |
| PR | 66 (60–76, 40–108) | 60 (53–68, 40–98) | 60 (54–69, 42–99) | 60 (51–69, 34–102) |
Note:—Data show the median (intraquartile range, range). CAS indicates carotid artery stent placement; SBP, systolic blood pressure; PR, pulse rate.
Active Treatment of Hypotension and Bradycardia
Forty-five (48%) of the 93 patients received either vasopressors or anticholinergics drugs (glycopyrrolate or atropine) during the 12 hours after CAS. No patient required transcutaneous or transvenous pacing. Eight patients (9%) were hospitalized for more than 1 night due to hypotension and bradycardia. No clinical manifestation of hypotension or bradycardia was found in the charts of any of those patients.
Factors Associated with Hemodynamic Depression after CAS (Univariate Analysis)
Stenosis proximities (<10 mm) to bifurcation (P = .03) and older age (P = .047) were significantly associated with hypotension; asymptomatic stenosis (P = .004) and being on antihypertensive medication (P = .03) were significantly associated with bradycardia; and the stenosis proximity to bifurcation (P = .04) and the dilation percentage [(stenosis severity percentage pre-CAS) − (residual stenosis percentage post-CAS)] (P = .04) were significantly associated with the use of a drug intervention for the treatment of hypotension or bradycardia after CAS. The association of asymptomatic stenosis with hypotension (P = .07); chronic hypertension (P = .11) with bradycardia; and the stenosis severity with the need for a drug intervention (P = .13) after CAS were also considered noteworthy to deserve further analysis with a logistic regression model. The presence of calcification, the number of dilations performed, the stent placement side, the taking of antihypertensive medication, chronic hypertension, diabetes, coronary artery disease (CAD), smoking, and sex and age of the patient were not associated with any of the study end points.
Independent Predictors of Hemodynamic Depression
The stenosis clinical presentation (asymptomatic stenosis), the stenosis proximity to the bifurcation, and the dilation percentage were all independently associated with the study end points. The stenosis severity was marginally associated with the drug intervention requirement (P = .06; 95% confidence interval [CI], 1.00–1.34). Table 4 summarizes the result of the logistic regressions for those factors.
Table 4.
Independent predictors of hypotension and bradycardia after CAS
| Variable | End Point | P | OR | 95% CI |
|---|---|---|---|---|
| Asymptomatic stenosis | Hypotension | .03 | 3.4 | 1.24–2.30 |
| Bradycardia | .03 | 3.1 | 1.1–1.8 | |
| <10 mm to bifurcation | Hypotension | .06 | 3.8 | 0.95–14.90 |
| Drug intervention | .02 | 2.9 | 1.2–7.2 | |
| Stenosis severity | Drug intervention | .06 | 1.15* | 1.00–1.34 |
| Dilation percentage | Drug intervention | .02 | 1.17* | 1.03–1.33 |
Note:—OR indicates odds ratio; CI, confidence interval; CAS, carotid artery stent placement.
Data are for every 5% increase in stenosis severity or dilation percentage.
Late Occurrence of Hypotension and Bradycardia
Table 5 gives the distribution of the first and last occurrence of hypotension and bradycardia according to different time point used (0–3, 3–6, and 6–12 hours). The incidence of hypotension and bradycardia 6 hours after CAS was 16 (17%) and 24 (26%), respectively. For patients who did have an early episode of hypotension and bradycardia (0–6 hours after CAS), the risk of having a recurrent episode 6 hours after the procedure was 61% for hypotension and 68% for bradycardia. McNemar test showed that the presence of early hypotension was significantly associated with its late occurrence (P = .03), whereas the presence of early bradycardia was not (P = .17). Seven patients (8%) had either bradycardia (5 patients) or hypotension (2 patients) occurring, for the first time, later than 6 hours after the procedure. The NPV of early hypotension and early bradycardia in predicting their later occurrence was 97% (95% CI, 93%–100%) and 93% (95% CI, 88%–98%), respectively. Cumulative freedom from any episode of hypotension was 80% in the first 3 hours, 92% at 3–6 hours, and 97% at 6–12 hours after the procedure. Cumulative freedom from any episode of bradycardia was 73% in the first 3 hours, 94% at 3–6 hours, and 91% at 6–12 hours after the procedure.
Table 5.
Distribution of the first and last occurrence of hypotension and bradycardia according to time after CAS
| Variable | Hypotension (n = 25 Patients) |
Bradycardia (n = 33 Patients) |
||
|---|---|---|---|---|
| First Episode, n (%) | Last Episode, n (%) | First Episode, n (%) | Last Episode, n (%) | |
| 0–3 h | 17 (68) | 6 (24) | 24 (73) | 8 (24) |
| 3–6 h | 6 (24) | 3 (12) | 4 (12) | 1 (3) |
| 6–12 h | 2 (8) | 16 (64) | 5 (15) | 24 (73) |
Note:—CAS indicates carotid artery stent placement.
Complications
No patient had a major stroke, 2 patients (2%) had a TIA, and 5 patients (5%) had a minor stroke. Three of the 5 recovered within 72 hours, 1 within 1 month, and 1 had a visual blind spot that persisted at 1 month. This patient had a Rankin Score (RS) of 1 at 1 month. For all of the other patients, the 1-month RS was 0. One patient had a seizure due to a reperfusion injury (mild subarachnoid hemorrhage). No patients had an MI. All of the patients who had a TIA or a minor stroke had either hypotension or bradycardia in the 12 hours after the CAS. Three of them required vasopressors or anticholinergics. The patient who had a reperfusion injury had received vasopressors to treat hypotension and bradycardia.
Discussion
In the published literature, the rate of hypotension after CAS varies from 10% to 42% and the rate of bradycardia from 27% to 37%.1-3,12,13,15-18 To date, the risk factors that have been found to be independently associated with a higher risk of hypotension and bradycardia during or after CAS are as follows: older age,14,18 female,18 previous MI,13,18 history of CAD,14 intraprocedural hypotension or bradycardia,13 stenosis localization (on the carotid bulb or within 10 mm of the carotid bulb),2,3,16 stenosis length,3 presence of calcification,2,16 fibrous plaque,16 eccentric plaque,16 high balloon-to-artery diameter ratio,3 and presence of a contralateral stenosis,3; whereas a history of a previous CEA was found to be associated with a lower risk.2
In the present study, because the baseline values of systolic BP and PR are variable from one patient to another, we preferred to use their relative percentage change rather than their absolute value. Using this end point, the incidences of hypotension and bradycardia during the first 12 hours after CAS in our study were 28% and 38%, respectively. We fixed a standard time for detection of hypotension and bradycardia at 12 hours, because all of the patients were kept in the intensive care unit for hemodynamic monitoring for at least this much time. We are aware that some hypotension or bradycardia can happen after 12 hours so that the estimated risk of late occurrence of hypotension and bradycardia in our series could have caused us to underestimate the real risk. This limitation is related to the retrospective nature of the study, in which the time to follow-up was established for a clinical purpose and not for research reasons. We also considered the need for drug intervention (vasopressors or anticholinergics) as a surrogate end point to overcome the possible omission of some hypotension or bradycardia record in the charts and to separate the clinically significant hypotension and bradycardia episodes that were considered severe enough to require an intervention. We found that 48% of patients in our cohort received either a vasopressor or an anticholinergic drug in the 12 hours after the procedure.
Considering that stretching of the carotid sinus baroreceptors by the balloon and the stent is incriminated in hypotension and bradycardia, the proximity of the stenosis to the carotid bulb and the magnitude of the dilation performed are expected to influence its occurrence. Indeed, stenosis localization on the carotid bulb was found as an independent risk factor in our series, as well as in 3 previous series.2,3,16 This factor was significantly associated with the need for drug intervention. The risk of developing hypotension and bradycardia significant enough to require drug intervention was also independently influenced by the magnitude of the dilation performed: a larger difference in stenosis severity before and after CAS in our series was associated with a higher risk of developing hypotension or bradycardia that required drug intervention. This effect of what we can call “aggressiveness of dilation” on hemodynamic status has been tested in several studies by using different measures, such as the absolute difference between the diameter of the stenosis before and after angioplasty,2,17 the absolute values of the stent and balloon diameter,13 and the balloon-to-artery diameter ratio.3 Only Leisch et al3 found an association between the balloon-to-artery diameter ratio and the occurrence of carotid sinus reaction during the procedure. On 51 patients analyzed, Pappada et al17 found the difference between the diameter of the artery and the stenosis to be associated with hypotension after CAS, but this association was not confirmed in their multivariate analysis. Because balloon dilation has also been incriminated for embolisms occurring during CAS, some authors have suggested the performance of carotid stent placement without deliberate use of angioplasty.22,23
In our series, the stenosis severity was marginally associated with hypotension and bradycardia requiring drug intervention. This association was independent of the aggressiveness of the dilation performed during the procedure. None of the series published thus far found this factor to be an independent predictor of hypotension and bradycardia.
Asymptomatic stenosis was significantly associated with occurrence of hypotension and bradycardia in our study, but no significant association was found between this factor and the need for drug intervention. There was no significant difference in the stenosis percentage between the symptomatic and asymptomatic groups, and asymptomatic stenosis remained a significant risk factor even after entering stenosis severity in the model. None of the series published so far have found asymptomatic presentation to be associated with hypotension and bradycardia. Qureshi et al13 found an association between stent placement of a symptomatic stenosis and the occurrence of hypotension. However, this association was not significant in the multivariate analysis (P = .06; 95% CI, 0.005–1.113). The meaning of the relationship between asymptomatic stenosis and occurrence of hypotension and bradycardia in our series remains to be elucidated.
Our analyses failed to demonstrate any significant association of age, sex, history of CAD, and presence of calcification with hypotension and bradycardia. This may reflect a lack of power of our study compared with other larger series,2,14 rather than a real absence of association. However, some series entered into the logistic regression model only variables with a P value lower than .05, neglecting the possible confounding effect of other variables and allowing potentially biased associations to appear in their multivariate analyses.3,18
In our series, the incidences of hypotension and bradycardia more than 6 hours after CAS were 16 (17%) and 24 (26%), respectively. Most of those patients had a previous episode occurring in the first 3–6 hours after the procedure. The risk of having recurrent hypotension or bradycardia 6 hours after the procedure was high among patients with an earlier episode (61% for hypotension and 68% for bradycardia). This risk was significantly higher for patients with early hypotension. On the other hand, the cumulative freedom from any episode of hypotension or bradycardia tended to increase with time, going from 80% in the first 3 hours to 97% at 6–12 hours for hypotension and from 73% in the first 3 hours to 91% at 6–12 for bradycardia. According to our results, the NPV for early hypotension or bradycardia to predict late episodes was high enough (97% and 93%, respectively) to consider their absence reassuring but not sufficient to justify discharging a patient without hypotension or bradycardia less than 12 hours after the procedure. In fact, in the absence of hypotension or bradycardia within 0–6 hours after the procedure, late hypotension or bradycardia was rare but still possible: in our cohort, 8% of patients experienced a first episode of bradycardia or hypotension occurring later than 6 hours after CAS. Moreover, the fact that patients were asked to hold antihypertensive medications before the procedure and were routinely administered anticholinergic drugs during the procedure, in addition to the relatively short duration of hemodynamic monitoring, can probably reduce the estimated incidence of hemodynamic depression in this cohort. McKevitt et al24 demonstrated that, on average, hemodynamic perturbations persist for more than 20 hours.
Despite a high rate of hypotension and bradycardia, no major stroke, MI, or death occurred in our series. However, all of the patients who had a TIA or a minor stroke also had an episode of hypotension or bradycardia. The size of our cohort was not powered to find an association between hypotension and bradycardia and the risk of cerebrovascular events after CAS. In a series of 461 patients, Gupta et al2 found persistent hemodynamic depression (defined as an episode of hypotension and bradycardia requiring continuous vasopressor support) to be associated with an increased risk of periprocedural major adverse clinical event or stroke. Mlekusch et al,14 in a series of 471 patients, found no differences between the groups of patients with and without hypotension or bradycardia in terms of the neurologic complication rate. As in our series, their rate of neurologic complications was low (7%), so a larger cohort could have been required. So far, the evidence is insufficient for a conclusion regarding the real impact of hypotension and bradycardia on patient clinical outcome after CAS.
Conclusions
Until a doubt remains concerning the harmfulness of hypotension and bradycardia after CAS, its risk and predictors mandate a particular attention. In this retrospective study, the risk of hypotension or bradycardia after CAS is significantly influenced by the degree of dilation performed. Although the absence of early hypotension and bradycardia in the first hours after CAS is reassuring, it does not exclude with certainty the possibility of its later occurrence. Because new onset of hypotension and bradycardia, though rare, can happen up to 6–12 hours after CAS, a minimum of 12 hours postprocedural VS monitoring is justified.
Footnotes
Support for the contribution of Mr. Rutledge and Dr. Mazumdar to this project came from the following grants: Center for Education and Research on Therapeutics (Agency of Health Research and Quality RFA-HS-05-14), Weill Cornell Medical College Institute for Clinical Research (through Tolly Vinik Trust), and Clinical and Translational Science Award (UL1-RR024996).
References
- 1.Dangas G, Laird JR Jr, Satler LF, et al. Postprocedural hypotension after carotid artery stent placement: predictors and short- and long-term clinical outcomes. Radiology 2000;215:677–83 [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Abou-Chebl A, Bajzer CT, et al. Rate, predictors, and consequences of hemodynamic depression after carotid artery stenting. J Am Coll Cardiol 2006;1538–43 [DOI] [PubMed]
- 3.Leisch F, Kerschner K, Hofmann R, et al. Carotid sinus reactions during carotid artery stenting: predictors, incidence, and influence on clinical outcome. Catheter Cardiovasc Interv 2003;58:516–23 [DOI] [PubMed] [Google Scholar]
- 4.Wong JH, Findlay JM, Suarez-Almazor ME. Hemodynamic instability after carotid endarterectomy: risk factors and associations with operative complications. Neurosurgery 1997;41:35–41 discussion 41–33 [DOI] [PubMed] [Google Scholar]
- 5.Howell M, Krajcer Z, Dougherty K, et al. Correlation of periprocedural systolic blood pressure changes with neurological events in high-risk carotid stent patients. J Endovasc Ther 2002;9:810–16 [DOI] [PubMed] [Google Scholar]
- 6.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501 [DOI] [PubMed] [Google Scholar]
- 7.Mangano DT. Dynamic predictors of perioperative risk. Study of Perioperative Ischemia (SPI) Research Group. J Card Surg 1990;5 (3 suppl):231–36 [DOI] [PubMed] [Google Scholar]
- 8.Eerola M, Eerola R, Kaukinen S, Kaukinen L. Risk factors in surgical patients with verified preoperative myocardial infarction. Acta Anaesthesiol Scand 1980;24:219–23 [DOI] [PubMed] [Google Scholar]
- 9.Munzer T, Stimming G, Brucker B, et al. Perioperative myocardial infarction and cardiac complications after noncardiac surgery in patients with prior myocardial infarction. I. Clinical data and diagnosis, incidence. Anaesthesist 1996;45:213–20 [DOI] [PubMed] [Google Scholar]
- 10.Jain U, Laflamme CJ, Aggarwal A, et al. Electrocardiographic and hemodynamic changes and their association with myocardial infarction during coronary artery bypass surgery. A multicenter study. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology 1997;86:576–91 [DOI] [PubMed] [Google Scholar]
- 11.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–82 [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn FO, Weissman NJ, Lederman RJ, et al. Acute hemodynamic changes during carotid artery stenting. Am J Cardiol 1998;82:1077–81 [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AI, Luft AR, Sharma M, et al. Frequency and determinants of postprocedural hemodynamic instability after carotid angioplasty and stenting. Stroke 1999;30:2086–93 [DOI] [PubMed] [Google Scholar]
- 14.Mlekusch W, Schillinger M, Sabeti S, et al. Hypotension and bradycardia after elective carotid stenting: frequency and risk factors. J Endovasc Ther 2003;10:851–59 discussion 860–51 [DOI] [PubMed] [Google Scholar]
- 15.Cayne NS, Faries PL, Trocciola SM, et al. Carotid angioplasty and stent-induced bradycardia and hypotension: Impact of prophylactic atropine administration and prior carotid endarterectomy. J Vasc Surg 2005;41:956–61 [DOI] [PubMed] [Google Scholar]
- 16.Nonaka T, Oka S, Miyata K, et al. Prediction of prolonged postprocedural hypotension after carotid artery stenting. Neurosurgery 2005;57:472–77 discussion 472–77 [DOI] [PubMed] [Google Scholar]
- 17.Pappada G, Beghi E, Marina R, et al. Hemodynamic instability after extracranial carotid stenting. Acta Neurochir (Wien) 2006;148:639–45 [DOI] [PubMed] [Google Scholar]
- 18.Trocciola SM, Chaer RA, Lin SC, et al. Analysis of parameters associated with hypotension requiring vasopressor support after carotid angioplasty and stenting. J Vasc Surg 2006;43:714–20 [DOI] [PubMed] [Google Scholar]
- 19.Hobson RW 2nd, Howard VJ, Brott TG, et al. Organizing the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): National Institutes of Health, Health Care Financing Administration, and industry funding. Curr Control Trials Cardiovasc Med 2001;2 (4):160–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv 2007;69:341–48 [DOI] [PubMed] [Google Scholar]
- 21.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 22.Maynar M, Baldi S, Rostagno R, et al. Carotid stenting without use of balloon angioplasty and distal protection devices: preliminary experience in 100 cases. AJNR Am J Neuroradiol 2007;28:1378–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Men S, Lownie SP, Pelz DM. Carotid stenting without angioplasty. Can J Neurol Sci 2002;29:175–79 [PubMed] [Google Scholar]
- 24.McKevitt FM, Sivaguru A, Venables GS, et al. Effect of treatment of carotid artery stenosis on blood pressure: a comparison of hemodynamic disturbances after carotid endarterectomy and endovascular treatment. Stroke 2003;34:2576–81 [DOI] [PubMed] [Google Scholar]


