Abstract
BACKGROUND AND PURPOSE: Coil herniation into the parent artery after detachment is an uncommon complication of embolization of the intracranial aneurysm. We report our experience with stent reconstruction of the lumen and flow of the internal carotid artery (ICA) after coil herniation during embolization for intracranial ICA aneurysms and the possible mechanisms of coil herniation.
MATERIALS AND METHODS: A series of 216 consecutive patients was treated by endovascular coil embolizations for intracranial aneurysms. Of these patients, there were 9 (4 men, 5 women; 32–68 years of age) complicating with coil herniation into the ICA and undergoing stent deployment to reconstruct the ICA lumen (n = 8) or both lumen and flow (n = 1). Wide-neck aneurysms were found in 8 and narrow-neck, in 1. Aneurysms were in the posterior communicating artery (n = 5) and the paraophthalmic (n = 3) and cavernous portions (n = 1) of the ICA. Self-expandable stents were deployed in the ICA in 6; balloon-mounted stents were selected in 3.
RESULTS: The causes of coil herniation appeared to be coil instability after detachment (n = 6), excessive embolization (n = 1), microcatheter-related problems (n = 1), or being pushed by subsequent coil embolization (n = 1). Endovascular stent placement to reconstruct the lumen and/or flow of the ICA was technically successful in all 9 patients; 1 needed a second stent due to further coil migration. No significant procedure-related complications were found. Clinical follow-up was 8–35 months.
CONCLUSION: Coil herniation occasionally occurs during endovascular embolization of ICA aneurysms because of coil instability after detachment, excessive embolization, microcatheter-related problems, or pushing by subsequent coil embolization. In this small series, stent placement was safe and effective in the reconstruction of the arterial lumen and/or restoration of flow past a herniated coil mass.
Endovascular detachable coil embolization of intracranial aneurysms has increasingly become an alternative treatment technique to neurosurgical aneurysm clipping.1 Despite increasing clinical experience and technologic improvements, endovascular treatment still has inherent risks of morbidity and mortality. The most common complication of endovascular embolization of the aneurysm is thromboembolic events, which may result from poor technique, endovascular devices, and/or poor flushing of the catheter systems. These complications may occur in 2.5%–28% of patients treated.2-4 Stent-assisted aneurysm embolization is a well-known tool in the management of intracranial wide-neck aneurysms to prevent coil protrusion into the parent vessel and may allow safer and denser packing of the aneurysm sac.5-8 However, to our knowledge, stent as a salvage procedure to reconstruct the lumen and/or blood flow of the parent artery during the procedure has not been well evaluated.
The purpose of our study was to report our experience using stents to reconstruct the lumen and/or blood flow of the parent artery after coil herniation in the internal carotid artery (ICA) during embolization of intracranial ICA aneurysms and to report possible mechanisms of coil herniation.
Materials and Methods
From January 2000 to August 2007, 216 consecutive patients underwent 238 endovascular coil embolizations for intracranial aneurysms in our institution. Of these, 9 underwent stent deployment to reconstruct the lumen (n = 8) or lumen and blood flow (n = 1) of the parent artery, while we attempted endosaccular embolization of the intracranial ICA aneurysms. Of the 9, there were 4 men and 5 women, 32–68 years of age (mean, 51 years). Patients presented with subarachnoid hemorrhages (n = 6), headache (n = 1), ptosis (n = 1), or were asymptomatic (n = 1). The aneurysms in the ICA were located in the posterior communicating artery (n = 5) or the paraophthalmic (n = 3) or cavernous (n = 1) portions of the ICA. The sizes of the aneurysms varied from 4.5 to 10 mm in their maximal dimension. Wide-neck aneurysms, with a neck larger than 4 mm (n = 1) and/or neck-to-dome ratio larger than 0.5 (n = 7), were found in 8 patients. However, stent- or balloon-assisted coil embolization was not attempted initially in these 8 wide-neck aneurysms. Information, including the patient's age and sex, clinical manifestations, and aneurysm location and size, is summarized in the Table.
Demography and outcomes of 9 patients with ICA aneurysms after stent management of coil herniation to the parent artery
| Case No. | Sex/Age (years) | Clinical Manifestation | Aneurysm Location (ICA) | Aneurysm Size (mm) | Aneurysm Neck (mm) | Cause of Coil Herniation | Stent | Complication | Follow-Up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/48 | SAH | PcomA | 7 × 5 | 4 | Coil instability | BMS | Nil | 32 |
| 2 | F/52 | SAH | Paraophthalmic | 5 × 4 | 3 | Coil instability | SES | Nil | 23 |
| 3 | F/62 | SAH | Paraophthalmic | 6 × 6 | 3 | Coil instability | SES | Nil | 19 |
| 4 | F/46 | SAH | PcomA | 7 × 6 | 4 | Coil instability | SES | Nil | 20 |
| 5 | F/63 | Ptosis, DCCF after embolization | Cavernous | 10 × 10 | 6 | Excessive embolization | BMS | TIA | 35 |
| 6 | M/45 | SAH | PcomA | 6 × 3 | 3 | Coil push | SES | TIA | 12 |
| 7 | F/68 | SAH | PcomA | 4.5 × 4 | 3.5 | Microcatheter-related | BMS | Nil | 25 |
| 8 | M/40 | Headache | PcomA | 6 × 3 | 3 | Coil instability | SES | Nil | 10 |
| 9 | M/32 | Asymptomatic | Paraophthalmic | 5 × 4 | 2.5 | Coil instability | SES | Nil | 8 |
Note:—SAH indicates subarachnoid hemorrhage; PcomA, posterior communicating aneurysm; BMS, balloon-mounted stent; SES, self-expandable stent; TIA, transient ischemic attack; DCCF, direct carotid cavernous fistula.
With the patients under general anesthesia, the femoral arteries were catheterized by means of a percutaneous technique. A bolus of intravenous heparin was routinely administered after the first coil was detached into the aneurysm sac, and we maintained an activated clotting time (ACT) from 1.5 to 2 times baseline throughout the whole procedure. Through the 6- or 7F femoral sheath and guiding catheter, a microcatheter was advanced into the aneurysm sac. A total of 3–8 detachable coils (Matrix; Boston Scientific, Fremont, Calif) were placed into the aneurysm sac under direct fluoroscopic and digital road-mapping guidance. Before detaching each coil, we confirmed adequate and accurate placement of the coil into the aneurysm sac by subtraction road-mapping or angiography. Unfortunately, coil loop or mass (Figs 1 and 2) herniation into the parent artery occurred in all 9 patients. Compromise of the ICA lumen was detected in 8 patients, whereas both lumen and blood flow compromise was found in 1 patient (Fig 2). At that moment, the decision was made to isolate the herniated coil mass and to reconstruct the ICA lumen or flow by placing an intracranial stent. A 300-cm supporting microguidewire (Transcend, Boston Scientific) was navigated into the branches of the middle cerebral artery (MCA). Then a balloon-mounted coronary stent (n = 3; Bx stent; Cordis, Miami Lakes, Fla) (Fig 2) was selected when a softer and more flexible self-expandable stent was not available. Since the launch of the self-expandable intracranial stent in Taiwan (Neuroform; Boston Scientific), the Neuroform stent has been used in 6 patients (Fig 1).
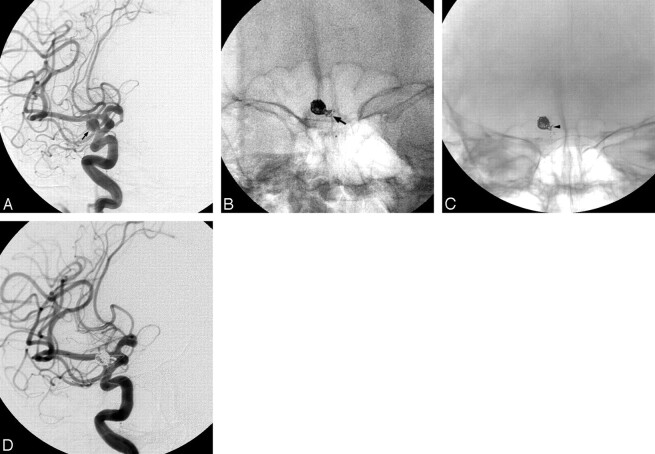
Fig 1.
Images of a 62-year-old woman who presented with subarachnoid hemorrhage. A, Right carotid angiogram shows a right ICA paraophthalmic narrow-neck aneurysm (arrow). B, Coil herniation into the ICA is found at the end of the embolization because of instability of the detached coil (arrow). C, A self-expandable stent was deployed into the ICA to push the herniated coil into the aneurysm sac and fix it to the vascular wall (arrowhead). D, Postembolization angiogram reveals subtotal occlusion of the aneurysm sac with patency of the ICA lumen. Note a supporting guidewire in the ICA and MCA.
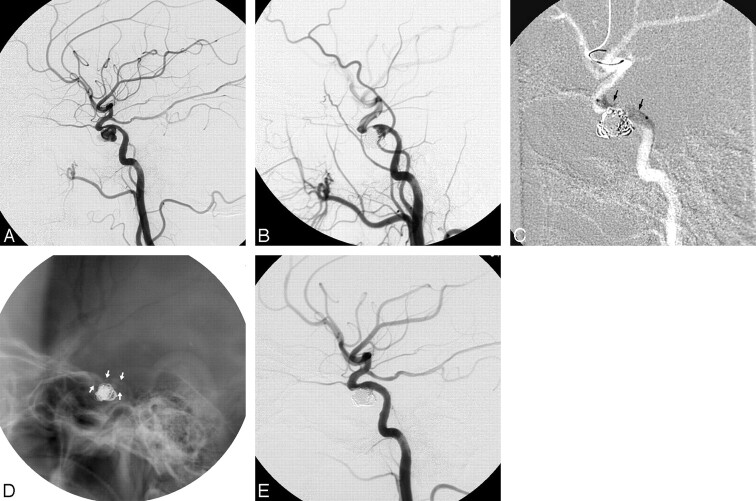
Fig 2.
Images of a 63-year-old woman who had ptosis after balloon embolization of a traumatic carotid cavernous fistula. A, A left carotid angiogram reveals a wide-neck aneurysm at the stenotic cavernous portion of the ICA. B, Excessive embolization with coil herniation into the ICA leading to compromise of the ICA lumen and flow is found. C–E, A balloon-mounted stent (black and white arrows) is deployed into the ICA to reconstruct the lumen and blood flow of the ICA.
The stent was navigated and deployed into the ICA to bridge the aneurysm neck, and it pushed the herniated coil back into the aneurysm sac or fixed the coil loop between the vascular wall and the stent mesh. In 1 patient, herniation of the first coil basket was found when the second coil was advanced into the aneurysm sac. Further movement of the coil loop into the distal ICA and MCA during the advancement of the Neuroform stent was depicted. To complete reconstruction of the ICA lumen and trap the free coil into the vascular wall, we deployed a second Neuroform stent into the ICA and proximal MCA. Subsequent coil embolization of the residual aneurysm sac was performed uneventfully.
Postembolization conventional and rotational angiograms were routinely obtained to assess the effect of aneurysm occlusion and patency of the ICA and its intracranial branches. After the procedure, patients were given intravenous heparinization to keep the ACT approximately 2 times baseline for 24 hours. We immediately gave oral 300-mg clopidogrel and 200-mg aspirin via a nasogastric tube after the procedure, followed by oral 75-mg clopidogrel and 100-mg aspirin daily for 6 months.
Results
A summary of the data on aneurysms treated, stent selection, results, and follow-up are presented in the Table. The presumed causes of coil herniation were instability of the detached coil in the aneurysm sac (n = 6, Fig 1), excessive embolization (n = 1, Fig 2), microcatheter removal (n = 1), or being pushed by subsequent coil embolization (n = 1). Coil herniation leading to compromise of ICA flow was found in only 1 patient. Stent deployment as an end procedure was successful in 8 patients. One patient underwent subsequent coil embolization to create more attenuated packing. Initial complete angiographic occlusion was achieved in 5 aneurysms; a neck remnant and/or residual aneurysm sacs with partial contrast filling were achieved in 4. Angiographic follow-up examinations of these 9 aneurysms after 6–24 months demonstrated 5 complete occlusions, 2 neck remnants, and 2 residual aneurysms. Further endovascular treatment was not given in 4 patients with neck remnants or residual aneurysms because of the relatively stable coil mass in the aneurysm sac. Two patients had transient ischemic attacks related to the stented ICA because of flow compromise or further distal coil migration. They presented with temporary hemiparesis compared with baseline neurologic status of pre-embolization and recovery to baseline within 24 hours. No other periprocedual neurologic deficit was found. Clinical follow-up for these patients was 8–35 months (mean, 20 months).
Discussion
Coil-related thromboembolic complications in embolization of the intracranial aneurysms included stretching, breaking, and migration of coils and herniation into the parent artery.9-11 Detached coil herniation into the parent artery usually occurs at the end stage of the procedure. It may result from instability of the coil in the aneurysm sac, excessive embolization, microcatheter removal, or the coil being pushed out by subsequent coil embolization. In general, in the late stage of embolization, advancement of the coil into the aneurysm sac may be difficult and risky because of the limited volume of the aneurysm sac associated with increased friction and resistance while the coil is being advanced. If the whole coil is forced into the aneurysm sac and temporarily supported by the attached pusher, the coil may prolapse into the parent artery after detachment because of sudden loss of the supporting force of the pusher. Coil instability associated with coil herniation was the most common cause of complications in our series and occurred in 6 patients. Shorter and softer coils should be selected in the later stages of endosaccular embolization to avoid this problem.
Coil herniation resulting from excessive embolization commonly occurs in wide-neck aneurysms; in this situation, the coil mass may easily prolapse through the neck into the parent artery. Flow compromise may occur while the parent artery is small or stenosis is present, such as occurred in patient 5 of our series. A stent- or balloon-assisted technique should be initiated before coil embolization to prevent coil herniation in wide-neck aneurysms. Microcatheter removal associated with coil herniation was seen in 1 patient in our series. This occurred because an already-detached coil was brought out by the shaped and curved microcatheter. Therefore, meticulous removal and straightening of the microcatheter by the coil pusher or the microguidewire may be of great help in reducing this complication. Coil herniation resulting from displacement of the first coil by subsequent coil embolization may occasionally occur; particularly if the first coil was short, soft, or without a good basket. Longer and stiffer coils, such as a standard coil, should be selected as a first coil to form a solid and stable basket to avoid this problem. In general, coil herniation will more likely occur in treating wide-neck aneurysms. Occasionally, it may occur in narrow-neck aneurysms as shown in 1 patient of our series.
Some alternative endovascular procedures may be applicable to this complication. Retrieving the fractured migrated coil in the parent artery by snaring has been reported, with promising results.12,13 Nevertheless, we do not recommend the use of a microsnare to retrieve the herniated coil with whole-loop partial herniation into the parent artery because it carries too many risks, including dislodging the whole basket leading to undesirable mobilization of other coils already stably placed in the aneurysm, which may prolapse out of the aneurysm sac into the parent artery, further exacerbating the situation.
The balloon-remodelling technique may be useful in the endovascular management of coil herniation or coil-related complications,14 by navigation of a proper balloon into the parent artery across the aneurysm neck, whereby the herniated coil/mass may be pushed back into the aneurysm sac after balloon inflation. Nevertheless, this maneuver may be ineffective in most cases because of coil recoil back into the parent artery while the balloon is deflated. In addition, this technique was considered only when the diameter of the herniated coil was no more than 50% of the lumen of the parent artery or the coil loop was less than the width of the neck of aneurysm. Otherwise, there is a risk of increasing the instability of the herniated coil mass. Once the balloon is inflated, the coil mass may be further forced into the wall of the parent artery.
Recently, stents have been selected for managing coil-related thromboembolic events such as proximal stent fixation of fractured coils or migrated coils.9,11 There are 2 major advantages to using stents to manage this complication in comparison with the balloon-remodeling technique. First, unlike the balloon technique, stents can be used in coil herniation regardless of the volume or diameter of the herniated coils/mass in relation to the lumen of the parent artery or aneurysm neck. After the stent is successfully deployed, the herniated mass can be pushed back into the aneurysm sac or can be fixed between the wall of the parent artery and the stent, with reconstruction of the lumen of the parent artery. Second, the stent itself bridging across the aneurysm neck may interfere with the blood flow patterns within the aneurysm, promote thrombus formation, and reduce the effect of further coil compaction and aneurysm recurrence as well as decrease the aneurysm bleeding rate.15
During the stent- or balloon-assisted technique, a supporting guidewire has to be navigated into the intracranial branch before advancement of the balloon or stent. A major concern with this technique is to avoid the supporting wire passing through the coil loop/mass en route to the intracranial area, because subsequently introducing the stent may cause further migration of the herniated coil mass/loop into its distal parent artery or its intracranial branches. In this situation, a second stent is needed to fix the coil to the wall of the parent artery. This occurred in case 9 of our series. If a larger coil loop or mass prolapses into the most cross-sectional area of the lumen of the parent artery, passing the supporting wire through the coil loop/mass into the distal intracranial branch is usually unavoidable. In this circumstance, gentle advancement of the supporting wire and stent is necessary to avoid vigorous migration of the herniated coil loop or mass. In this series, a balloon-mounted coronary stent was selected for 3 patients before the advent of the self-expandable stent in our institution. However, navigation of a coronary stent may be difficult in a tortuous parent artery and carries a risk of injury to the parent artery.
When a coil loop extends into the parent artery without compromising the ICA flow, the patient can be placed on antiplatelet treatment. However, we preferred to solve this complication of a coil loop or coil mass herniation to the parent artery, particularly when the herniated coil was pulsatile in the parent artery, because a herniated coil loop may have a risk of further migration to the distal parent artery and its intracranial branches by blood flow of the parent artery and lead to a thromboembolic event. After stent placement, stents have the advantage of pushing the coil back into the aneurysm sac and/or permanently fixing the coil between the arterial wall and stent. Regarding thrombogenesis of the stent and herniated coil loop, the stent itself may be not less thrombogenic than a coil loop initially, but the stent can show complete endothelialization in several months after deployment in the parent artery16; therefore, antiplatelet medication can be shortened to 3–6 months. Nevertheless, a lifetime antiplatelet regime may be necessary in patients with a herniated coil loop in the parent artery without stent placement.
Generally, 1 stent is enough to reconstruct the lumen of the parent artery by pushing the coil mass back into the aneurysm sac and/or fixing the coil loop to the vascular wall. However, a second stent may be needed in those patients with longer free coil in the parent artery and cannot be covered by 1 stent only. After stent placement, a loading dose of oral clopidogrel and aspirin is given, followed by a postprocedural dual antiplatelet regime for 3–6 months. We did not encounter any major clinical complications related to this technique, though 2 patients experienced transient minor symptoms. However, dual antiplatelet medication in those patients with stent placement with ruptured aneurysms still has a risk of rebleeding.
Conclusions
Herniation of a coil mass into the parent artery may occasionally occur in aneurysms after coil detachment for several reasons, particularly wide-neck aneurysms. These include coil instability in the aneurysm sac, excessive embolization, microcatheter-related factors, and being pushed by subsequent coil embolization. Stent placement is a simple, safe, and effective method to reconstruct the lumen/flow of the parent artery with lower periprocedural risk.
Footnotes
This work was supported in part by a grant from the Taipei Veterans General Hospital (V96C1–073, V97C1–139).
References
- 1.Molyneux A, Kerr R, Stratton I, et al, for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;26 ;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 2.Park H, Horowitz M, Jungreis C, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2005;26:506–14 [PMC free article] [PubMed] [Google Scholar]
- 3.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1541–47 [PMC free article] [PubMed] [Google Scholar]
- 4.Vinuela F, Duckwiler G, Maward M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 5.Higashida RT, Halbach VV, Down CF, et al. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol 2005;26:1751–56 [PMC free article] [PubMed] [Google Scholar]
- 6.Luo CB, Wei CJ, Chang FC, et al. Stent-assisted embolization of internal carotid artery aneurysms. J Chin Med Assoc 2003;66:460–66 [PubMed] [Google Scholar]
- 7.Weber W, Bendszus M, Kis B, et al. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: initial clinical and angiographic results in 31 aneurysms. Neuroradiology 2007;49:555–61 [DOI] [PubMed] [Google Scholar]
- 8.Biondi A, Janardhan V, Katz JM, et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery 2007;61:460–69 [DOI] [PubMed] [Google Scholar]
- 9.Fressler RD, Ringer AJ, Qureshi AI, et al. Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: technical note. Neurosurgery 2000;46:248–53 [PubMed] [Google Scholar]
- 10.Phatouros CC, McConachie NS, Jaspan T. Post-procedure migration of Guglielmi detachable coils and mechanical detachable spirals. Neuroradiology 1999;41:324–27 [DOI] [PubMed] [Google Scholar]
- 11.Schutz A, Solymosi L, Vince GH, et al. Proximal stent fixation of fractured coils: technical note. Neuroradiology 2005;47:874–78 [DOI] [PubMed] [Google Scholar]
- 12.Dinc H, Kuzeyli K, Kosucu P, et al. Retrieval of prolapsed coils during endovascular treatment of cerebral aneurysms. Neuroradiology 2006;48:269–72 [DOI] [PubMed] [Google Scholar]
- 13.Standard SC, Chavis TD, Wakhloo AK, et al. Retrieval of a Guglielmi detachable coil after unraveling and fracture: case report and experimental results. Neurosurgery 1994;35:994–99 [DOI] [PubMed] [Google Scholar]
- 14.Sugiu K, Martin J, Jean B, et al. Rescue balloon procedure for an emergency situation during coil embolization for cerebral aneurysms. J Neurosurg 2002;96:373–76 [DOI] [PubMed] [Google Scholar]
- 15.Canton G, Levy DI, Lasheras JC, et al. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg 2005;103:891–902 [DOI] [PubMed] [Google Scholar]
- 16.Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery 2005;56:E416. [DOI] [PubMed] [Google Scholar]