Abstract
SUMMARY: We present a patient with progressive dural calcifications, thickening, and enhancement presumably related to the development of nephrogenic systemic fibrosis (NSF). Head CT demonstrated progressive dural calcifications, whereas MR imaging demonstrated progressive dural thickening and enhancement during a 3-year period in which the patient received several gadolinium-enhanced MR imaging studies. To the best of our knowledge, dural calcifications are the only described intracranial finding of NSF.
Nephrogenic systemic fibrosis (NSF), previously referred to as nephrogenic fibrosing dermopathy, is a relatively new, rare systemic condition first described in the literature in 2000.1 NSF occurs only in people with renal disease, with more than 215 documented cases in the NSF Registry at Yale University.2 All but a few of the known cases have been associated with intravenous administration of gadolinium-based contrast material.3 Manifestations of the disease are primarily cutaneous, but multiorgan system involvement has been described.4–7 Dural involvement has been described at autopsy; however, the depiction of the progressive imaging changes has not been documented.4,6,8 We report a patient with progressive dural calcification, thickening, and enhancement as a manifestation of NSF.
Case Report
A 52-year-old woman with a medical history of end stage renal disease secondary to adult polycystic kidney disease required hemodialysis starting in 2004. She had 8 gadolinium-enhanced MR imaging studies of the brain and abdomen between September 2004 and November 2006, all performed with gadodiamide.
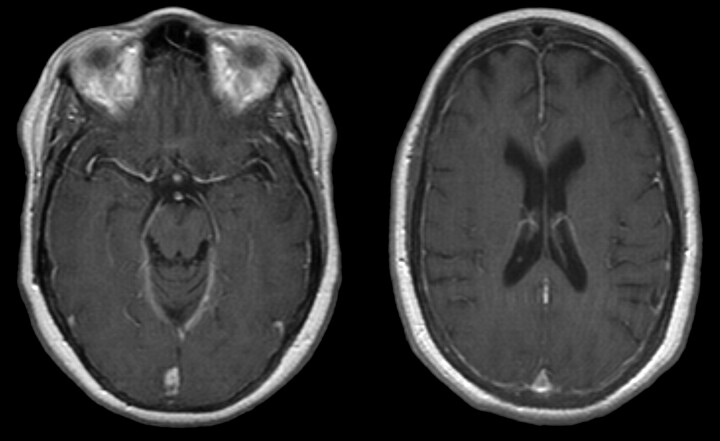
The initial brain MR imaging examination in September 2004 was performed for elevated serum prolactin levels. A pituitary microadenoma was seen, but results of the study were otherwise normal. A follow-up study in October 2005 demonstrated an interval decrease in adenoma size as well as new dural thickening and enhancement (Fig 1). The dural thickening and enhancement progressed on the gadolinium-enhanced MR imaging study obtained in March 2006.
Fig 1.
October 2005. Axial T1-weighted postgadolinium MR imaging examination demonstrates diffuse dural enhancement. Changes in bone marrow are likely secondary to anemia.
Because of the uncommon nature of the meningeal abnormalities, the patient had a right frontal craniotomy and dural biopsy in May 2006. The dura mater contained multiple areas of fibrosis and calcification with rare, multinucleated giant cells adjacent to the foci of calcification (Fig 2).
Fig 2.
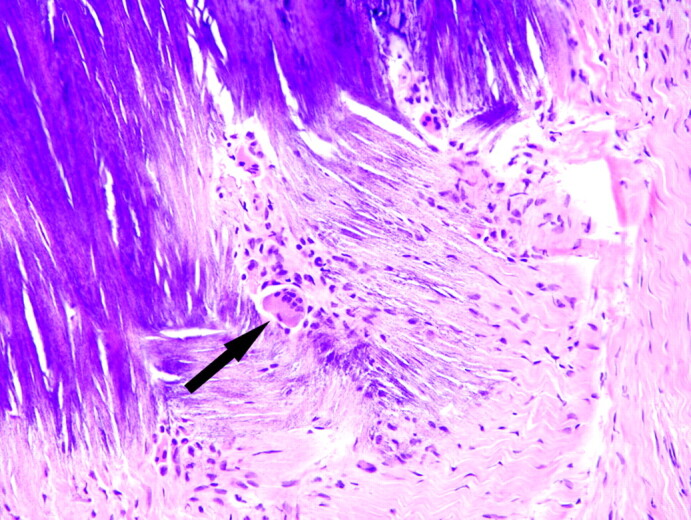
May 2006. Section of dura mater from the right frontal lobe shows fibrosis and blue-staining area of calcification. A multinucleated giant cell (arrow) is in the area of fibrosis next to the focus of calcification (hematoxylin-eosin stain).
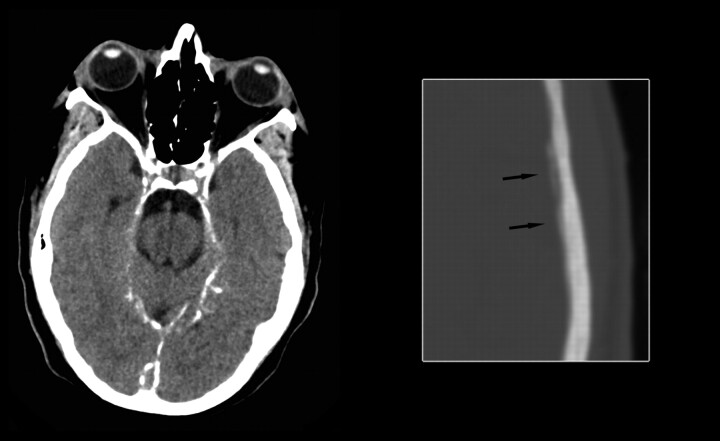
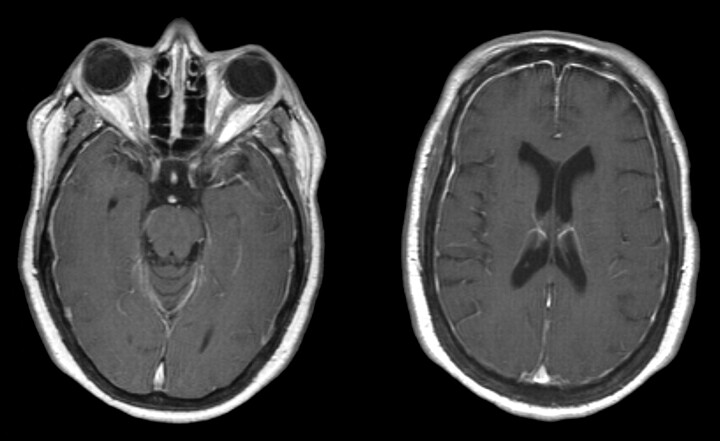
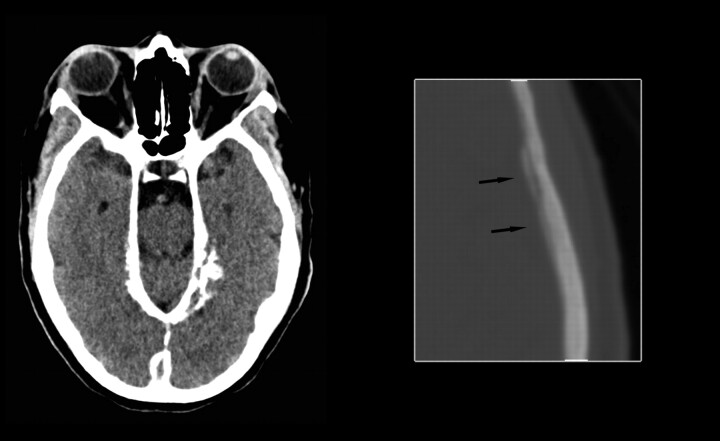
Head CT scan obtained in June 2006 demonstrated diffuse dural calcification (Fig 3). Follow-up gadolinium-enhanced MR imaging examination in November 2006 showed further progression of dural thickening and enhancement (Fig 4). Head CT scan in March 2008 demonstrated progressive calcification (Fig 5).
Fig 3.
June 2006. Axial noncontrast CT image at the level of the tentorium in brain windows and magnified image at the level of the lateral ventricles in bone windows demonstrate diffuse dural calcification (arrows on magnified image).
Fig 4.
November 2006. Axial T1-weighted postgadolinium MR imaging examination demonstrates progressive thickening and enhancement of the dura.
Fig 5.
March 2008. Axial noncontrast CT image at the level of the tentorium in brain windows and magnified image at the level of the lateral ventricles in bone windows demonstrate progressive dural calcification (arrows on magnified image).
In April 2007, the patient presented with progressive skin thickening and decreased range of motion of her hands during a 4-month period. Results of skin biopsy from the right palm revealed fibrocellular thickening of dermal collagen, patchy calcification of blood vessel walls and dermal collagen, and increased Alcian-blue–positive mucin staining consistent with NSF.
Discussion
NSF is a rare, systemic disease linked to patients with renal failure who are exposed to intravenous gadolinium contrast. In 2006, Grobner9 suggested gadolinium as the cause of NSF. The disease was first described in the literature as skin thickening with “brawny hyperpigmentation” in 15 patients.1 Typical presenting symptoms are skin and subcutaneous tissue fibrosis in the extremities, then the trunk.7 Symptoms mimic scleroderma and other systemic fibrosing disorders.7 Serologic markers of scleroderma including antinuclear antibodies, anti-Scl 70 antibodies, and anticentromere antibodies are absent in NSF.10 Initially, NSF was believed to involve the skin and subcutaneous tissues, but subsequent reports documented involvement of the myocardium, muscle, lung, pericardium, pleura, kidney, bone, testis, and dura.4–8
The diagnosis of NSF is made by a combination of clinical, laboratory, and histopathologic findings.2,7 Prognosis is variable, but NSF may be fatal.7,11 Treatment leading to improved renal function may lead to relief of symptoms and may halt progression.7,11,12
Dural fibrosis from NSF was first reported in 2006.4,6 Both spinal and intracranial dural involvement have been described.4,6,8 Microscopic examination demonstrates fibrosis with a spindle-cell proliferation, areas of calcification, collections of CD68-positive mononuclear cells, and occasional multinucleated giant cells.4 The histologic changes in the dura mater of our patient were similar to those previously reported.4,6,8
The differential diagnosis for dural thickening and enhancement is extensive and includes sarcoidosis, tuberculosis, Wegener granulomatosis, intracranial hypotension, lymphoma, and metastatic disease. The differential diagnosis for dural calcifications includes physiologic calcifications, previous hemorrhage, previous infection, pseudoxanthoma elasticum, hyperparathyroidism, basal cell nevus syndrome, and idiopathic calcification.13
Virtually all patients with endstage renal disease have resultant hyperparathyroidism, and “metastatic calcification” may develop, with calcium deposition in the soft tissues and dura mater. However, dural thickening and enhancement are not typically seen in these patients. The patient in this case report had serum calcium values within normal limits and did not have other foci of soft tissue calcification. Her serum phosphorus levels ranged from low to elevated but were predominantly within normal limits. Although hyperparathyroidism cannot be excluded as the cause of the dural calcification because of the dural thickening and enhancement, the lack of other evidence of metastatic calcification, and her normal serum calcium levels, it is believed that the dural disease most likely represents a manifestation of NSF.
We suggest that dural calcification, thickening, and enhancement in patients with renal disease and a history of exposure to gadolinium may represent an early manifestation of NSF. Neuroradiologists must recognize these findings as a part of the spectrum of disease in NSF and may be the first to suggest the diagnosis if these radiologic findings precede cutaneous manifestations.
References
- 1.Cowper SE, Robin HS, Steinberg HM, et al. Scleromyxedema-like cutaneous disease in renal-dialysis patients. Lancet 2000;356:1000–01 [DOI] [PubMed] [Google Scholar]
- 2.Cowper SE. The International Center for Nephrogenic Fibrosing Dermopathy Research (ICNFDR). Available at http://www.icnfdr.org. Accessed March 13,2008
- 3.Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: A summary of the medical literature reporting. Eur J Radiol 2008;66:230–34 [DOI] [PubMed] [Google Scholar]
- 4.Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med 2006;130:209–12 [DOI] [PubMed] [Google Scholar]
- 5.Kucher C, Steere J, Elenitsas R, et al. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory distress. J Am Acad Dermatol 2006;54:S31–34 [DOI] [PubMed] [Google Scholar]
- 6.Saenz A, Mandal R, Kradin R, et al. Nephrogenic fibrosing dermopathy with involvement of the dura mater. Virchows Arch 2006;449:389–91 [DOI] [PubMed] [Google Scholar]
- 7.Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol 2008;66:191–99 [DOI] [PubMed] [Google Scholar]
- 8.Krous HF, Breisch E, Chadwick AE, et al. Nephrogenic systemic fibrosis with multiorgan involvement in a teenage male after lymphoma, Ewing's sarcoma, end-stage renal disease, and hemodialysis. Pediatr Dev Pathol 2007;10:395–402 [DOI] [PubMed] [Google Scholar]
- 9.Grobner T. Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis [published erratum appears in Nephrol Dial Transplant 2006; 21:1745]. Nephrol Dial Transplant 2006;21:1104–08 [DOI] [PubMed] [Google Scholar]
- 10.Broome DR, Girgus MS, Baron PW, et al. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007;188:586–92 [DOI] [PubMed] [Google Scholar]
- 11.Wiginton CD, Kelly B, Oto A, et al. Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue. AJR Am J Roentgenol 2008;190:1060–68 [DOI] [PubMed] [Google Scholar]
- 12.Knopp EA, Cowper SE. Nephrogenic systemic fibrosis: early recognition and treatment. Semin Dial 2008;21:123–28 [DOI] [PubMed] [Google Scholar]
- 13.Dorenbeck U, Leingärtner T, Bretschneider T, et al. Tentorial and dural calcification with tertiary hyperparathyroidism: a rare entity in chronic renal failure. Eur Radiol 2002;12:S11–13 [DOI] [PubMed] [Google Scholar]