Abstract
BACKGROUND AND PURPOSE: Pilomyxoid astrocytoma (PMA) is a recently described variant of pilocytic astrocytoma (PA) with unique clinical and histopathologic characteristics. Because the histopathology of PMA is distinct from that of PA, we hypothesized that PMAs would display distinctive imaging characteristics. We retrospectively reviewed the imaging findings in a large number of patients with PMA to identify these characteristics.
MATERIALS AND METHODS: CT and MR images, pathology reports, and clinical information from 21 patients with pathology-confirmed PMA from 7 institutions were retrospectively reviewed. CT and MR imaging findings, including location, size, signal intensity, hemorrhage, and enhancement pattern, were tabulated.
RESULTS: Patients ranged in age from 9 months to 46 years at initial diagnosis. Sex ratio was 12:9 (M/F). Twelve of 21 (57%) tumors were located in the hypothalamic/chiasmatic/third ventricular region. Nine (43%) occurred in other locations, including the parietal lobe (2/21), temporal lobe (2/21), cerebellum (2/21), basal ganglia (2/21), and fourth ventricle (1/21). Ten (48%) tumors showed heterogeneous rim enhancement, 9 (43%) showed uniform enhancement, and 2 (9%) showed no enhancement. Five (24%) masses demonstrated intratumoral hemorrhage.
CONCLUSION: This series expands the clinical and imaging spectrum of PMA and identifies characteristics that should suggest consideration of this uncommon diagnosis. One third of patients were older children and adults. Almost half of all tumors were located outside the typical hypothalamic/chiasmatic region. Intratumoral hemorrhage occurred in one quarter of patients. PMA remains a histologic diagnosis without definitive imaging findings that distinguish it from PA.
Pilomyxoid astrocytoma (PMA) is a rare primary central nervous system (CNS) tumor recently described as a histologic variant of pilocytic astrocytoma (PA).1,2 PMA differs from PA both clinically and histopathologically. PMA typically occurs at an earlier age and is associated with a significantly worse prognosis.3 Identifying an astrocytoma as PMA is important for patient management because its more malignant biologic behavior and shorter recurrence-free survival may justify more aggressive treatment.4
To date, there are only 3 small case series of PMA in the imaging literature.5-7 Unique imaging characteristics that distinguish PMA from PA have not yet been identified. Because the histopathology of PMA is quite distinct from that of PA, we hypothesized that PMAs would display imaging characteristics that might suggest the correct preoperative diagnosis. We retrospectively reviewed the imaging findings in 21 patients with pathologically proved PMA to identify any unique imaging characteristics. This article expands the clinical manifestations and imaging spectrum of these rare tumors. We report some features that—when present—may suggest PMA.
Materials and Methods
A retrospective review of images, pathology reports, and clinical information of 21 patients with pathology-confirmed PMA was collected from 7 different institutions. Five cases were previously published as case reports.7-9 Contributors searched their respective institutional teaching files, PACS archives, and pathology department data base archives. Pathology reports were available in all cases and documented the diagnosis of PMA. Original specimens were available in 5 cases and were reviewed by our neuropathologist. Patient demographics and clinical presentation were recorded. Age and sex were available in all cases. At least 1 presenting symptom was noted in all patients.
Noncontrast-Enhanced CT (NCCT) scans were obtained in 12/21 patients. Three patients had contrast-enhanced CT (CECT) studies. Tumor location, size (maximal diameter), hemorrhage, calcification, and attenuation characteristics, and contrast enhancement on CT were tabulated.
MR imaging was performed in all patients. Signal intensity relative to gray matter on multiple MR images, surrounding edema, presence or absence of diffusion restriction, MR spectroscopy signature, and presence and pattern of enhancement following contrast administration were noted. Standard precontrast T1-weighted spin-echo sequences (TR/TE = 400–700/8–20 ms) were available for review in 17/21 patients; T2-weighted fast spin-echo sequences, in 20/21 patients (TR/TE = 3000–7000/100–120 ms); and fluid-attenuated inversion recovery (FLAIR) fast spin-echo scans, in 15/21 (TR/TE = 8000–9000/110–150 ms). T2* (gradient recalled-echo [GRE]) sequences were performed in 2/21 cases (TR/TE = 500/26 ms; flip angle, 20°). Contrast-enhanced T1-weighted spin-echo sequences were performed in all patients. Echo-planar diffusion-weighted imaging (DWI) was performed in 11/21, and MR spectroscopy (TE = 135 and 30 ms), in 4/21 patients.
Results
Patient demographics, clinical presentation, and imaging findings in all 21 patients are summarized in on-line Table 1. Patient age ranged from 9 months to 46 years, with a mean of 7 years (median 5 years) at time of initial diagnosis. There were 12 males and 9 females. Presenting symptoms in order of frequency included headache, vomiting, visual changes, seizure, failure to thrive, and hemiplegia. Two patients had neurofibromatosis type 1 (NF-1).
Clinical, pathologic, and imaging characteristics of PMA and PA*
| PMA | PA | |
|---|---|---|
| Pathology | Monomorphous piloid cells with myxoid background angiocentric pattern | Heterogeneous/mixed piloid and protoplasmic cells |
| Rosenthal fibers, eosinophilic granular bodies, and microcalcifications rare1,2 | Rosenthal fibers, eosinophilic granular bodies, and microcalcifications common1,2 | |
| Clinical | ||
| Infants, children, and adults3 | Young children and, less commonly, adults3 | |
| More aggressive | Less aggressive | |
| Less progression-free and overall survival | Greater progression-free and overall survival | |
| More recurrences3 | Fewer recurrences3 | |
| Most common location: hypothalamus/optic chiasm17 | Most common location: posterior fossa17 | |
| May be associated with NF-19,10 | May be associated with NF-110 | |
| Imaging | ||
| Hypointense T1 | Hypointense T1 | |
| Hyperintense T2/FLAIR5 | Hyperintense T2/FLAIR5 | |
| Variable contrast enhancement5 | Variable contrast enhancement5 | |
| Solid with central necrosis5 | Often cystic5 | |
| Calcification (<10%)5 | Calcification (10%)5 | |
| Intratumoral hemorrhage common (12%–25%)8,16,32,33 | Intratumoral hemorrhage less common (∼8%)29 |
Although PMA is most commonly found in the hypothalamic/chiasmatic region, because of its rarity, an undiagnosed hypothalamic/chiasmatic tumor is most likely to be the more common PA.
Four patients with original pathology specimens initially diagnosed as PMA were confirmed by second-opinion review of our neuropathologist. A fifth patient (case 20) was originally diagnosed with PA in 1997, before the identification of PMA as a distinct tumor entity. Because imaging findings were so atypical for PA, the teaching file case had been noted as inconsistent with the pathologic diagnosis. The original slides were re-reviewed by our neuropathologist and found to be consistent with PMA.
Twelve of 21 (57%) tumors were located in the optic pathway/hypothalamus/third ventricle region, including those of both patients with NF-1. Nine (43%) occurred in atypical locations, including the parietal lobe (2/21), temporal lobe (2/21), cerebellum (2/21), basal ganglia (2/21), and fourth ventricle (1/21). Median tumor maximal diameter was 4 cm (range, 2–11 cm). One patient had a large bitemporal tumor (Fig 1).
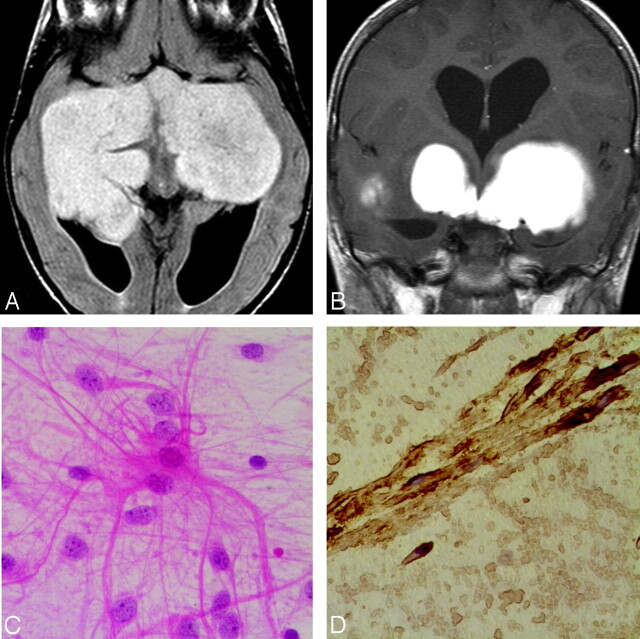
Fig 1.
Case 9. A, Axial FLAIR sequence in a 3-year-old girl with progressive blindness shows a large solid lobulated suprasellar and bitemporal PMA, with uniform hyperintensity. B, Coronal contrast-enhanced T1-weighted image shows a homogeneously enhancing suprasellar and bitemporal PMA. C, Photomicrograph shows classic hairlike (“piloid”) astrocytes in a myxoid background (hematoxylin-eosin, original magnification ×300). D, Photomicrograph shows that the tumor is strongly positive for glial fibrillary acidic protein, confirming its astrocytic origin.
NCCT scans showed uniform hypoattenuation in 7 of 12 patients. Hemorrhage was identified in 5 patients. Four tumors contained mixed hyperattenuations suggestive of intratumoral hemorrhage, and an additional 1 displayed gross fluidfluid levels. Only 1 case demonstrated calcification, with a partial rim and central punctuate pattern. Contrast was administered to 3 patients. All 3 tumors showed rim enhancement.
MR signal intensity varied widely. Seventeen patients had T1-weighted scans available for review. Relative to gray matter, tumors were uniformly hypointense in 12/17 patients (71%). Two of 17 (12%) showed uniform isointensity, 1/17 (6%) demonstrated mixed hypointensity-hyperintensity, 1/17 (6%) showed hypointensity-isointensity, and 1/17 (6%) showed intratumoral fluid-fluid levels suggesting hemorrhage.
T2 sequences were available for review in all except 1 patient. Fourteen of 20 (70%) showed uniform hyperintensity; 2 of 15 showed mixed iso- and hyperintense signal intensity. Three of 20 (15%) showed small areas of adjacent edema surrounding the tumor. None demonstrated extensive edema. Four patients had intratumoral hemorrhages, ranging from 1 to 3 cm in maximal diameter. One patient (case 20) also had subarachnoid hemorrhage adjacent to the tumor. All hemorrhages were hypointense on T2-weighted images (T2WI); 1 demonstrated fluid-fluid level (case 11). T2* (GRE) sequences were performed in 2 patients with hemorrhage. Both showed heterogeneous hyperintensity within the tumor and uniform hypointensity in the areas of hemorrhage.
FLAIR sequences were performed in 15 patients. Seven of 15 (47%) showed uniform hyperintensity, 5/15 (33%) showed heterogeneous hyperintensity, 2/15 (13%) showed an isointense center with a rim of hyperintensity, and 1/15 (7%) showed fluid-fluid levels. Three of 15 (20%) showed small areas of adjacent edema.
Contrast-enhanced T1 sequences were available for review in all patients and showed the following patterns: 8 of 21 (38%) showed solid uniform enhancement, 6/21 (29%) showed heterogeneous enhancement with rim enhancement, 5/21 (24%) showed rim enhancement alone, and 2/21 (9%) patients showed no enhancement. MR angiography performed in 2/5 patients with intratumoral hemorrhage showed no evidence of abnormal vascularity. No patients in whom DWI was performed (11/21) showed evidence of diffusion restriction. Proton MR spectroscopy was performed in 6 patients. All spectra revealed elevated choline (Cho) and lipids, with decreased creatine (Cr) and N-acetylaspartate (NAA). Quantitative data were available in 2 patients (mean Cho/Cr = 2.94 ± 1.71 at TE = 135 ms and 6.24 ± 0.86 at 30 ms).
Discussion
The first reports of PMA described this lesion as a strictly pediatric tumor, occurring at a mean age of 18 months versus 58 months for PA.3 Early investigators thus suggested that PMA was a “juvenile” variant of PA.2 However, review of all PMA cases subsequently reported in the literature reveals a larger age range (on-line Table 2). The mean age at presentation of patients in our series was 7 years (median, 5 years), with fully one third of tumors occurring in adolescents and young adults. The oldest patient in our series was 46 years old. Therefore, PMA is certainly not an exclusively pediatric tumor.
Before our series, the association of PMA with NF-1 had been reported 3 times.9,10 One of these previously reported cases, a 9-year-old girl, is included in our series. A second patient in our series, a 9-month-old girl, also has NF-1. These 4 cases of PMA in the setting of NF-1 suggest that PMA may be another NF-1-associated tumor.
PMA has a strong geographic predilection for the hypothalamic/chiasmatic region.2,11 The first case series reporting imaging findings in 4 patients with PMA described a solid hypothalamic tumor with homogeneous contrast enhancement and T2 hyperintensity extending into the adjacent deep white and gray matter.5 A large solid hypothalamic tumor in a very young patient (Fig 1) has been considered the classic pattern, with few tumors before 2004 being reported outside this region. Nearly half the PMAs in our series occurred in atypical locations (Figs 2 and 3), suggesting that this tumor may occur anywhere along the central neuraxis. This observation is supported by a number of recently reported cases found in atypical locations,2,3,8,11-13 including recent reports of 4 spinal PMA tumors (on-line Table 2).6,14
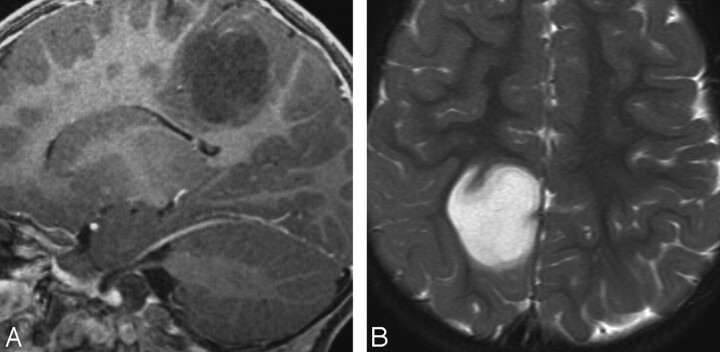
Fig 2.
Case 6. A, Sagittal contrast-enhanced T1-weighted image in a 2-year-old boy with seizures shows a nonenhancing tumor in the posterior parietal cortex. B, Axial T2WI in the same patient shows homogeneous hyperintensity without surrounding edema. PMA was found at surgery.
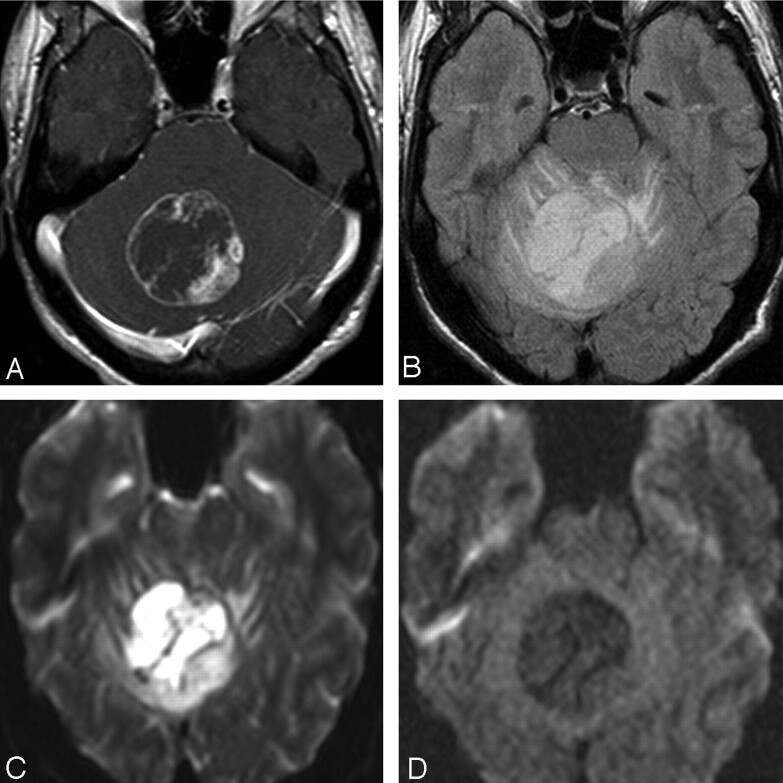
Fig 3.
Case 13. A, Axial contrast-enhanced T1-weighted image in a 17-year-old boy with intractable headaches shows a rim-enhancing PMA of the cerebellar vermis. B, FLAIR image shows heterogeneous hypertintensity with extension into adjacent white matter of the cerebellar folia. C and D, DWI (C) and apparent diffusion coefficient map (D) show no restricted diffusion. This case demonstrates atypical age, location, and enhancement pattern.
Both our data and these most recent reports also suggest a relationship between atypical tumor location and age. Two of 3 hemispheric tumors recently reported were in adults,8,13 and 3 of the 4 spinal cord tumors occurred at 6, 8, and 29 years of age.6,14 In our series, 2 cortical tumors were found in 13- and 24-year-old patients. Cerebellar tumors (n = 2) and basal ganglia tumors (n = 2) were also found in patients older than 13 years. This finding confirms that atypical tumor locations are more common in older patients. Some investigators have commented on the recent increase in adult PMA diagnoses.15,16 One possible explanation is an increased awareness of the entity by pathologists and radiologists alike (eg, our case 20).
Size and morphology of PMA varies widely.17 The tumor size in our series was variable, with 5 tumors measuring ≥6 cm in maximal diameter.
The T1, T2, and FLAIR signal-intensity characteristics of our tumors are consistent with prior descriptions. However, enhancement patterns in our patients were highly variable. Only 8 (38%) tumors showed uniform enhancement. Eleven (52%) tumors displayed some rim enhancement (Fig 3), suggesting central necrosis or cystic degeneration, both of which have been described in some PMAs.5 Two tumors (9%) in our series showed no enhancement. Thus, we found a much broader spectrum of enhancement patterns.
Of particular interest are the 5 PMAs in our series that demonstrated spontaneous intratumoral hemorrhage. Nearly 25% of patients had evidence of hemorrhage (Fig 4). Our literature review of PMAs showed a 12% incidence of hemorrhage (on-line Table 2). In 1997, before the first description of PMA, 1 of our patients (case 20) was initially imaged for symptoms suggestive of subarachnoid hemorrhage (“worst headache of my life”). Emergency NCCT scanning at an outside hospital confirmed subarachnoid hemorrhage. An angiogram was obtained and was negative for aneurysm. When the patient was referred for further evaluation, MR imaging disclosed a hemorrhagic hypothalamic-optic chiasm tumor, which was subsequently diagnosed as a pilocytic astrocytoma. This tumor was re-examined in 2007 because of the unusual finding of hemorrhage on the initial MR image (Fig 5). On re-evaluation, it was found to have histologic features consistent with the diagnosis of PMA.
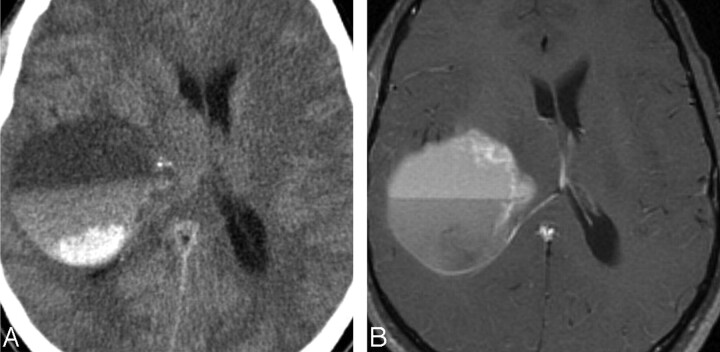
Fig 4.
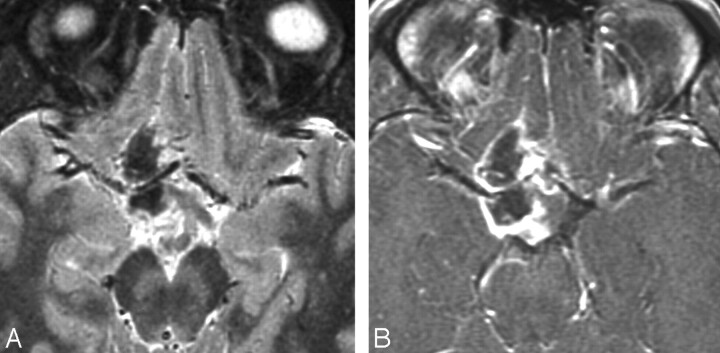
Case 11. A, Hemorrhage in PMA. Axial NCCT scan in a 24-year-old man with headache, confusion, and gait instability shows a right temporal lobe PMA with fluid-fluid levels and hyperattenuation consistent with hemorrhage. B, Axial contrast-enhanced T1-weighted image shows anteromedial rim enhancement and fluid-fluid levels.
Fig 5.
Case 20. A, Axial T2WI in a 21-year-old man with headache shows a heterogeneous supra- and juxtasellar mass. The hypointense core suggests hemorrhage, which was confirmed at surgery. B, Axial contrast-enhanced T1-weighted image shows a rim-enhancing tumor surrounding a nonenhancing hemorrhagic core. Hemorrhagic exophytic hypothalamic/chiasmatic tumor was partially resected and originally diagnosed as PA in 1997. Re-examination of the specimen in 2007 showed features consistent with PMA.
In contrast to PMA, hemorrhage is relatively rare in PAs, with mostly isolated case reports in the current literature.18-28 Until recently the rate of hemorrhage in PA was estimated at <1%. However, a recent retrospective series of 134 patients with a tissue diagnosis of PA found an 8% spontaneous hemorrhage rate, much higher than that previously reported.29 Hemorrhagic PAs were found primarily in older patients, the average being 20 years of age (range, 5–58 years). Notably, none of the tumors with spontaneous hemorrhages occurred in the cerebellum, and only 1 was found in the hypothalamus, the typical locations for PA.29 Could some of these tumors have been PMAs? The article is unclear, because some PAs included in this study had a tissue diagnosis established before the identification of PMA as a unique tumor entity. We hypothesize that at least some previously reported cases of PA in older patients with spontaneous intratumoral hemorrhage may indeed have been PMAs, which might be documented if re-evaluation of pathology specimens were possible, as occurred in our case 20. When present, intratumoral hemorrhage may be an important feature suggestive of PMA.
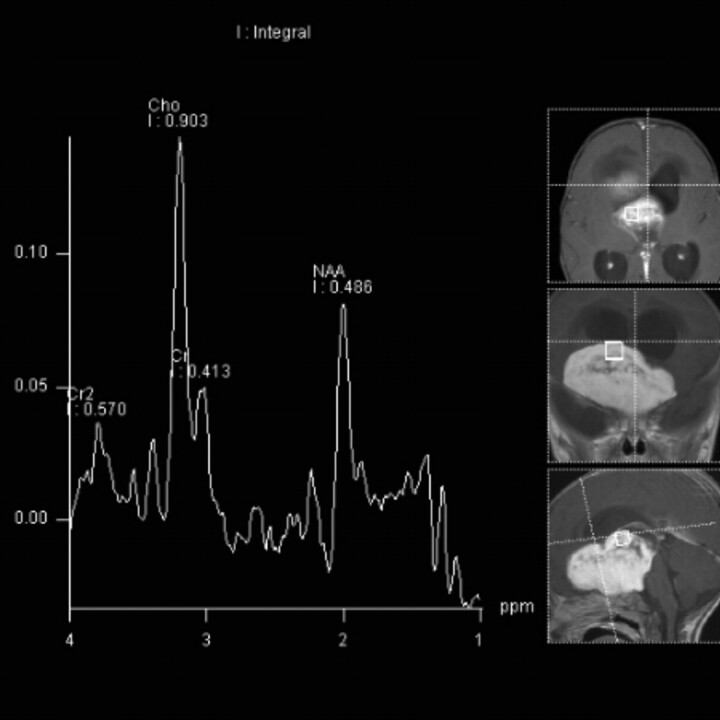
MR spectroscopy data in PMAs are similar to those described for PA. All 6 of our patients demonstrated elevated Cho and lipids and decreased Cr and NAA (Fig 6). These profiles are characteristic of aggressive tumors and are considered to be paradoxically elevated in low-grade astrocytomas (PA and PMA).7,30,31 Two recent articles evaluating MR spectroscopy in PMA also found that the Cho/Cr ratio was not appreciably different in PMA compared with PA. They did, however, find increased Cho/Cr in the peritumoral region, a finding consistent with the more infiltrative and aggressive nature of PMA.7
Fig 6.
Case 7. MR spectroscopy (TE = 135 ms) in a 9-month-old boy with failure to thrive shows increased Cho and lipids with decreased Cr and NAA within the tumor. Multivoxel MR spectroscopy map showed Cho/Cr = 6.1.
PA and PMA are difficult to distinguish on the basis of imaging findings alone (Table). Whereas PA is more commonly found in the posterior fossa,11 its second most common intracranial location is the hypothalamus/optic chiasm, the most common location of PMA. The cerebral hemispheres are rare sites for PA, but 4 of our patients had hemispheric or cortical PMAs. The most prominent imaging characteristic that suggests PMA versus PA is the presence of intratumoral hemorrhage. Although hemorrhage can certainly occur in PAs, it is less common (≤8%)29 compared with PMA (nearly 25% in our series and 12% in previously reported cases).8,16,32,33
When reported clinical outcomes of PA and PMA of the hypothalamic/chiasmatic region are compared, PMA is associated with shorter progression-free survival (26 versus 147 months), shorter overall survival (60 versus 233 months), and higher frequency of recurrence (76% versus 50%), often with prominent CSF dissemination.3,11 Hypothalamic/chiasmatic PAs are often treated conservatively without histologic confirmation.34 We suggest that a neoplasm in this location, when it occurs either with intratumoral hemorrhage1 or in patients outside the typical age for PA, 2 may be a PMA, not a PA. Some authors suggest a confirmed diagnosis of PMA versus PA might warrant more aggressive treatment.4,32
Conclusion
We found a broader clinical and imaging spectrum of PMA than previously reported. One third of our patients were adolescents and young adults. Whereas PMA predominantly affects the hypothalamic/chiasmatic region, we demonstrate that it may be found anywhere along the central neuraxis, with nearly half of our patients having neoplasms outside this region. Intratumoral hemorrhage, if present, is a feature more frequently encountered in PMA. Although we suggest that an older patient with a hemorrhagic neoplasm in a location atypical for PA should be considered as having a possible PMA and thus warranting histologic confirmation for treatment planning, PMA remains a histologic diagnosis. There are no definitive pathognomonic imaging findings to distinguish it from PA.
Supplementary Material
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, June 9–15, 2007; Chicago, Ill.
indicates article with supplemental on-line tables.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. Epub 2007 Jul 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol 1999;58:1061–68 [DOI] [PubMed] [Google Scholar]
- 3.Komotar RJ, Burger PC, Carson BS, et al. Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery 2004;54:72–79, discussion 79–80 [DOI] [PubMed] [Google Scholar]
- 4.Komotar RJ, Mocco J, Jones JE, et al. Pilomyxoid astrocytoma: diagnosis, prognosis, and management. Neurosurg Focus 2005;18:E7. [PubMed] [Google Scholar]
- 5.Arslanoglu A, Cirak B, Horska A, et al. MR imaging characteristics of pilomyxoid astrocytomas. AJNR Am J Neuroradiol 2003;24:1906–08 [PMC free article] [PubMed] [Google Scholar]
- 6.Mendiratta-Lala M, Kader Ellika S, Gutierrez JA, et al. Spinal cord pilomyxoid astrocytoma: an unusual tumor. J Neuroimaging 2007;17:371–74 [DOI] [PubMed] [Google Scholar]
- 7.Morales H, Kwock L, Castillo M. Magnetic resonance imaging and spectroscopy of pilomyxoid astrocytomas: case reports and comparison with pilocytic astrocytomas. J Comput Assist Tomogr 2007;31:682–87 [DOI] [PubMed] [Google Scholar]
- 8.Gottfried ON, Fults DW, Townsend JJ, et al. Spontaneous hemorrhage associated with a pilomyxoid astrocytoma: case report. J Neurosurg 2003;99:416–20 [DOI] [PubMed] [Google Scholar]
- 9.Khanani MF, Hawkins C, Shroff M, et al. Pilomyxoid astrocytoma in a patient with neurofibromatosis. Pediatr Blood Cancer 2006;46:377–80 [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez FJ, Perry A, Gutmann DH, et al. Gliomas in neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol 2008;67:240–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery 2003;53:544–53, discussion 554–55 [DOI] [PubMed] [Google Scholar]
- 12.Burel-Vandenbos F, Jouvet A, Chanalet S, et al. An unusual and misleading form of pilocytic astrocytoma. Ann Pathol 2006;26:126–28 [DOI] [PubMed] [Google Scholar]
- 13.Komotar RJ, Mocco J, Zacharia BE, et al. Astrocytoma with pilomyxoid features presenting in an adult. Neuropathology 2006;26:89–93 [DOI] [PubMed] [Google Scholar]
- 14.Komotar RJ, Carson BS, Rao C, et al. Pilomyxoid astrocytoma of the spinal cord: report of three cases. Neurosurgery 2005;56:191. [DOI] [PubMed] [Google Scholar]
- 15.Ceppa EP, Bouffet E, Griebel R, et al. The pilomyxoid astrocytoma and its relationship to pilocytic astrocytoma: report of a case and a critical review of the entity. J Neurooncol 2007;81:191–96 [DOI] [PubMed] [Google Scholar]
- 16.Komakula ST, Fenton LZ, Kleinschmidt-DeMasters BK, et al. Pilomyxoid astrocytoma: neuroimaging with clinicopathologic correlates in 4 cases followed over time. J Pediatr Hematol Oncol 2007;29:465–70 [DOI] [PubMed] [Google Scholar]
- 17.Burger PC, Cohen KJ, Rosenblum MK, et al. Pathology of diencephalic astrocytomas. Pediatr Neurosurg 2000;32:214–19 [DOI] [PubMed] [Google Scholar]
- 18.Charles NC, Nelson L, Brookner AR, et al. Pilocytic astrocytoma of the optic nerve with hemorrhage and extreme cystic degeneration. Am J Ophthalmol 1981;92:691–95 [DOI] [PubMed] [Google Scholar]
- 19.Garg A, Chugh M, Gaikwad SB, et al. Juvenile pilocytic astrocytoma presenting with subarachnoid hemorrhage: case report and review of the literature. J Neurosurg 2004;100:525–29 [DOI] [PubMed] [Google Scholar]
- 20.Golash A, Thorne J, West CG. Low grade pilocytic astrocytoma presenting as a spontaneous intracerebral haemorrhage in a child. Br J Neurosurg 1998;12:59–62 [DOI] [PubMed] [Google Scholar]
- 21.Hwang SL, Huang TY, Chai CY, et al. Hypothalamic juvenile pilocytic astrocytoma presenting with intracerebral hemorrhage. J Formos Med Assoc 1998;97:784–87 [PubMed] [Google Scholar]
- 22.Lones MA, Verity MA. Fatal hemorrhage in a cerebral pilocytic astrocytoma-adult type. Acta Neuropathol 1991;81:688–90 [DOI] [PubMed] [Google Scholar]
- 23.Lyons MK. Pilocytic astrocytoma with spontaneous intracranial hemorrhages in an elderly adult. Clin Neurol Neurosurg 2007;109:76–80 [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Akagi K, Abekura M, et al. Hypothalamic pilocytic astrocytoma presenting with intratumoral and subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 1997;37:849–51 [DOI] [PubMed] [Google Scholar]
- 25.Mesiwala AH, Avellino AM, Roberts TS, et al. Spontaneous cerebellar hemorrhage due to a juvenile pilocytic astrocytoma: case report and review of the literature. Pediatr Neurosurg 2001;34:235–38 [DOI] [PubMed] [Google Scholar]
- 26.Oka F, Yamashita Y, Kumabe T, et al. Total resection of a hemorrhagic tectal pilocytic astrocytoma: case report. Neurol Med Chir (Tokyo) 2007;47:219–21 [DOI] [PubMed] [Google Scholar]
- 27.Sorenson EJ, Silbert PL, Benarroch EE, et al. Transient amnestic syndrome after spontaneous haemorrhage into a hypothalamic pilocytic astrocytoma. J Neurol Neurosurg Psychiatry 1995;58:761–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ouwerkerk WJ, Dirven CM. Hematoma in a low-grade medullary astrocytoma: report of an unusual case and literature review. Childs Nerv Syst 1998;14:742–46 [DOI] [PubMed] [Google Scholar]
- 29.White JB, Piepgras DG, Scheithauer BW, et al. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J Neurosurg 2008;108:223–26 [DOI] [PubMed] [Google Scholar]
- 30.Cirak B, Horska A, Barker PB, et al. Proton magnetic resonance spectroscopic imaging in pediatric pilomyxoid astrocytoma. Childs Nerv Syst 2005;21:404–09 [DOI] [PubMed] [Google Scholar]
- 31.Hwang JH, Egnaczyk GF, Ballard E, et al. Proton MR spectroscopic characteristics of pediatric pilocytic astrocytomas. AJNR Am J Neuroradiol 1998;19:535–40 [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller CE, Frankel B, Smith M, et al. Suprasellar monomorphous pilomyxoid neoplasm: an ultrastructural analysis. Clin Neuropathol 2001;20:256–62 [PubMed] [Google Scholar]
- 33.Darwish B, Koleda C, Lau H, et al. Juvenile pilocytic astrocytoma ‘pilomyxoid variant' with spinal metastases. J Clin Neurosci 2004;11:640–42 [DOI] [PubMed] [Google Scholar]
- 34.Allen JC. Initial management of children with hypothalamic and thalamic tumors and the modifying role of neurofibromatosis-1. Pediatr Neurosurg 2000;32:154–62 [DOI] [PubMed] [Google Scholar]
- 35.Enting RH, van der Graaf WT, Kros JM, et al. Radiotherapy plus concomitant and adjuvant temozolomide for leptomeningeal pilomyxoid astrocytoma: a case study. J Neurooncol 2006;80:107–08. Epub 2006 Apr 28 [DOI] [PubMed] [Google Scholar]
- 36.Chadarevian JP, Halligan GE, Reddy G, et al. Glioneural phenotype in a diencephalic pilomyxoid astrocytoma. Pediatr Dev Pathol 2006;9:480–87 [DOI] [PubMed] [Google Scholar]
- 37.Melendez B, Fiano C, Ruano Y, et al. BCR gene disruption in a pilomyxoid astrocytoma. Neuropathology 2006;26:442–46 [DOI] [PubMed] [Google Scholar]
- 38.Hamada H, Kurimoto M, Hayashi N, et al. Pilomyxoid astrocytoma in a patient presenting with fatal hemorrhage: case report. J Neurosurg Pediatrics 2008;1:244–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.