Abstract
BACKGROUND AND PURPOSE: CT and MR angiographies have been reported to visualize the artery of Adamkiewicz (AKA) noninvasively to prevent spinal cord ischemia in surgery of thoracic descending aortic aneurysms. The purpose of this work was to compare the usefulness of CT angiography (CTA) with intra-arterial contrast injection (IACTA) with that of conventional CTA with intravenous contrast injection (IVCTA).
MATERIALS AND METHODS: We enrolled 32 consecutive patients with thoracic or thoracoabdominal aortic aneurysms who were scheduled for surgical repair or endovascular stent-graft treatment. All of the CTA images were obtained using a 16-detector row CT scanner and 100 mL of contrast material (370 mg/mL) injected at a rate of 5 mL/s. Contrast was injected via the antecubital veins of 15 patients and via a pig-tail catheter placed at the proximal portion of the descending aorta in 17 patients who underwent IVCTA and IACTA, respectively. Two datasets were reconstructed from 2 consecutive scans. The AKA was identified as a characteristic hairpin curved vessel in the anterior midsagittal surface of the spine and by the absence of further enhancement in the second rather than in the first phase. Continuity between the AKA and aorta was confirmed when the vessel could be traced continuously by paging the oblique coronal multiplanar reconstruction or original axial images.
RESULTS: Intra-arterial contrast injection was significantly more sensitive in identifying the AKA than IVCTA: 16 (94.1%) of 17 versus 9 (60.0%) of 15 (P = .033). Continuity between the AKA and aorta through intercostal or lumbar artery was confirmed in 14 (87.5%) of 16 and 5 (55.6%) of 9 of the IACTA and IVCTA groups, respectively.
CONCLUSION: Intra-arterial contrast injection detected the AKA at a high rate and verified continuity from the aorta to the AKA.
Paraplegia and paraplesis secondary to spinal cord ischemia remain serious complications of surgical repair or of endovascular treatment for descending thoracic aortic aneurysm (TAA) or thoracoabdominal aortic aneurysm (TAAA). The incidence ranges between 5% and 11% of thoracoabdominal surgeries.1–4 The great anterior radiculomedullary artery (the artery of Adamkiewicz [AKA]) is the dominant feeder of the spinal cord. One possible cause of spinal cord ischemia during surgery is failure to reestablish the spinal cord blood supply, and many reports have stressed the importance of reattaching the intercostal or lumbar arteries related to the AKA.5,6 Preoperative AKA identification and display of intercostal and lumbar arteries help surgeons to determine the appropriate range of aortic lesions that require graft replacement and intercostal or lumbar arteries requiring reconstruction.7
The most reliable way to visualize the AKA is selective intercostal arterial angiography, the detection rate of which is 43%–86%.8–12 However, selective angiography is time consuming, and complications including spinal cord injury can develop.10,11 Recently, MR angiography (MRA)13–18 and CT angiography (CTA)17–21 have been used to visualize the AKA less invasively with reported detection rates of 67%–93% and 68%–90%, respectively. However, these rates could be further improved.
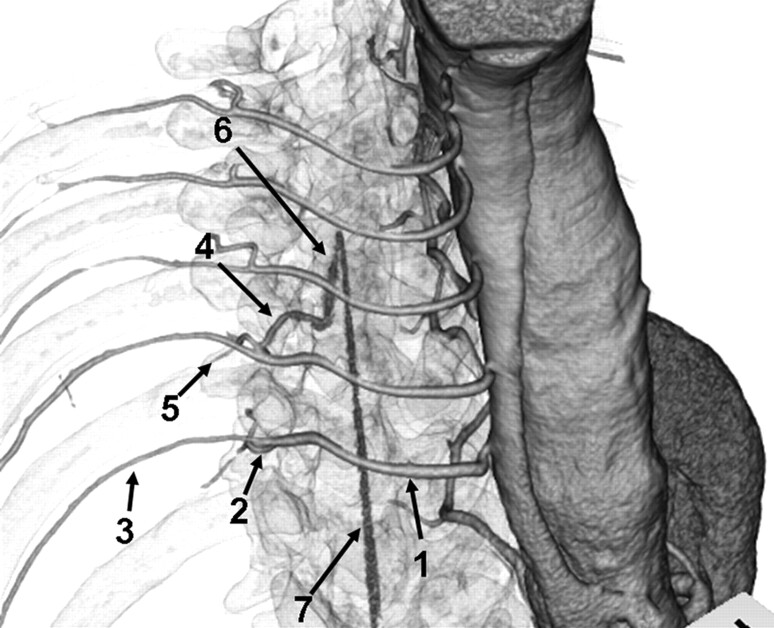
The AKA is a small vessel with a diameter of 0.5–1.5 mm22,23 that is surrounded by osseous structures. In addition, intercostal or lumbar arteries and dorsal branches run very close to the osseous structures (Fig 1). Due to these anatomic features, the contrast-to-noise ratio (CNR) in the spinal canal is decreased, and the AKA and its continuity with the aorta can be obscured. Robust aortic contrast enhancement is necessary to detect small vessels, but CTA with intravenous contrast injection (IVCTA) has limitations with respect to elevating aortic enhancement, because contrast material is diluted in the circulation of the right side of the heart. Nojiri et al24 showed that CTA with intra-arterial contrast injection (IACTA) could track the AKA to the aorta because of high contrast. The present study compares the abilities of IACTA and IVCTA to detect the AKA.
Fig 1.
Anatomic course of the AKA. Right anterosuperior view of a 3D volume-rendered CT image of IACTA with semitransparent skeletal system. Intercostal and lumbar arteries (1) originate from the aorta, and divide into posterior (2) and anterior (3) branches. Anterior branches run through the intercostal groove. Posterior branches subdivide into the radiculomedullary artery (4) and muscular branch (5). Radiculomedullary artery courses to the spine and enters the vertebral foramen. The AKA (6) is the largest anterior radiculomedullary artery and joins the anterior spinal artery (7) in a characteristic hairpin curve.
Methods
Patients
Between March 2005 and September 2006, 32 consecutive patients with TAA or TAAA who were scheduled for surgical repair or an endovascular stent-graft underwent CTA to visualize the AKA. The patient group was composed of 22 men and 10 women aged 22–87 years (mean, 68.1 years). Twenty patients had TAAA and 12 had TAA. Of these, 11 had dissecting aneurysms. One patient had a background of Takayasu disease, and another had Malfan syndrome.
The patients were assigned to 2 groups according to whether the injection method was IVCTA (n = 15) or IACTA (n = 17; Table 1). Thirteen patients underwent IVCTA before July 25, 2005, when IACTA was initiated. From then until September 2006, 17 patients underwent IACTA. After July 25, 2005, 2 patients with insufficient renal function (serum creatinine >1.5 mg/dL) underwent IVCTA 3 or more days after coronary angiography to avoid a large amount of contrast medium in a single day. Thus, 15 patients underwent IVCTA.
Table 1:
Patient data and results of CT angiography
| Variable | IVCTA | IACTA | P |
|---|---|---|---|
| No. of patients | 15 | 17 | |
| Age (range), years | 70.6 (54–87) | 65.9 (22–83) | .168 |
| Male:female | 9:6 | 13:4 | .450 |
| TAA:TAAA | 3:12 | 9:8 | .125 |
| Dissecting:true | 7:8 | 4:13 | .266 |
| AKA detection, n/N (%) | 9/15 (60.0) | 16/17 (94.1) | .033 |
| Confirmation of continuity, n/N (%) | 5/9 (55.6) | 14/16 (87.5) | .142 |
| CT value of aorta, mean ± SD, HU | 352.2 ± 46.0 | 965.4 ± 634.1 | .002 |
Note:—IVCTA indicates intravenous CT angiography; IACTA, intra-arterial CT angiography; TAA, thoracic aortic aneurysm; TAAA, thoracoabdominal aortic aneurysm; HU, Hounsfield unit; AKA, artery of Adamkiewicz.
This study was concordant with our institutional ethics guidelines, and all of the patients provided written informed consent to participate in the study.
Scanning Protocol
We obtained CT images from the levels of the seventh thoracic and the third lumbar vertebrae in the craniocaudal direction using a 16-detector row multisection CT (Brilliance16; Philips Medical Systems, Best, the Netherlands). Scanning was consecutively repeated to obtain early and late-phase images. Scanning variables were composed of 0.75 seconds per rotation, 0.75-mm collimation, 0.666–0.875 pitch, 120 kV, and 300–400 mA (according to individual physique).
Injection Protocol and Scan Timing
IVCTA.
The contrast material, iopamidol (Iopamiron; Schering, Berlin, Germany), was injected (100 mL; 370 mg/mL; 5 mL/s) via a 20-gauge plastic intravenous catheter placed in the antecubital vein and followed by a 30-mL saline flush. The start time of the scan was determined by using conventional real-time monitoring (Bolus Tracking, Phillips Medical Systems). Regions of interest were placed on the descending aorta at the level of the seventh thoracic vertebra. Low-dose dynamic monitoring scanning (120 kV; 100 mA) started 15 seconds after the intravenous contrast material injection started, and 1 image per second was obtained. The trigger threshold was set at 100 Hounsfield units (HU) above the baseline (absolute value, 140–160 HU). Scanning automatically started 4 seconds after reaching the threshold.
IACTA.
Patients underwent IACTA after coronary angiography as a routine preoperative evaluation of coronary stenosis. After coronary angiography (radial or brachial arterial approach), a 5F Pigtail Catheter (Terumo, Tokyo, Japan) was advanced to the proximal portion of the descending aorta under fluoroscopy, and visualization of intercostal arteries was confirmed by manual injection of contrast material. Patients were then transferred to the CT room, and 100 mL of the same contrast material was injected via the catheter at a rate of 5 mL/s. The CT scan started 4 seconds after starting the injection.
Image Procession and Diagnosis
Two datasets were reconstructed from the 2 consecutive scans with a section width of 0.8 mm, a reconstruction interval of 0.4 mm, and 200-mm FOV. Image datasets were transferred to a workstation (Zio M900; Ziosoft, Tokyo, Japan). From the first phase image, an oblique coronal multiplanar reconstruction (MPR; 0.8-mm thickness, 0.8-mm intervals) fitting the curvature of the AKA was obtained. Exactly the same MPR sections were obtained from the second phase to distinguish the AKA from the radiculomedullary vein, which can be similarly shaped. Curved planar reformation (CPR) and 3D volume rendering were applied to display continuity between the AKA and the aorta.
The AKA was identified by the consensus of 2 experienced radiologists (K.U. and A.K.) who specialize in cardiac and vascular imaging. The analytical criteria of the AKA comprised detection of the AKA and confirmation of continuity between the AKA and aorta.13,18 The detection criteria of the AKA were the presence of a characteristic hairpin curved vessel ascending from the intercostal foramen to the anterior midsagittal surface of the spine and the absence of further enhancement in the second phase compared with the first phase (Figs 2 and 3). Continuity was confirmed when the vessel could be continuously traced via the intercostal or lumbar artery by paging oblique coronal MPR or original axial images. To compare the contrast bolus between the 2 groups, the CT value of the aorta was measured in sections of the midlevel of the scan range (approximately the 10th or 11th thoracic vertebra). A region of interest was placed in the dorsal portion of the aorta from which the intercostal or lumbar artery arose. To assess differences in the features of the contrast bolus between the 2 groups, we visually evaluated sagittal partial maximum intensity projection (MIP) images to determine whether contrast material was dorsally concentrated or homogeneously distributed.
Fig 2.
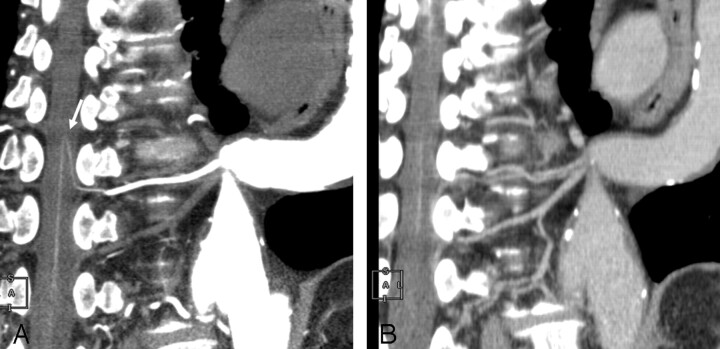
IACTA image obtained from a 68-year-old man with TAA. A, CPR of the first phase shows a hairpin curved vessel continuously to the aorta through the intercostal artery (arrow). B, On a CPR image obtained from the second phase in the same profile as that of the first phase, enhancement of this vessel is decreased. This vessel is identified as the AKA.
Fig 3.
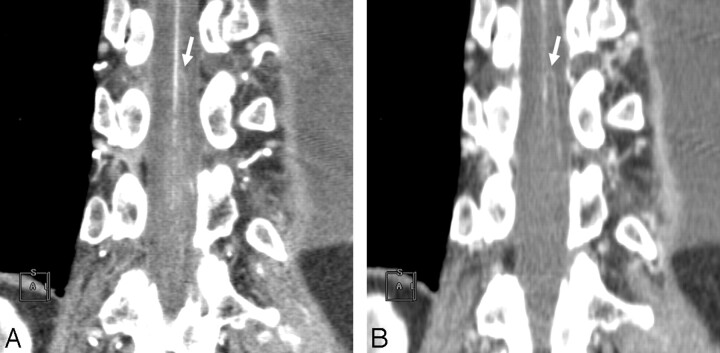
IACTA image obtained from a 77-year-old man with TAA. Oblique coronal MPR of the first (A) and second (B) phases show a hairpin curved vessel (arrows). The vessel is more enhanced in the second than in the first phase and is not connected to arteries. This vessel is identified as the radiculomedullary vein.
Statistical Analysis
Data were processed using StatMate (ATMS, Tokyo, Japan). Variables were compared between the IVCTA and IACTA groups using the 2-sided Fisher exact test for proportions and a 2-tailed t test for the means of CT values and patient ages.
Results
No complications were associated with the angiographic procedure or CT imaging. Table 1 summarizes the results. The AKA was detected more sensitively by IACTA than IVCTA: 16 (94.1%) of 17 versus 9 (60.0%) of 15 (P = .033). Continuity between the AKA and aorta through the intercostal or lumbar artery was confirmed in 14 (87.5%) of 16 and in 5 (55.6%) of 9 of the IACTA and IVCTA groups, respectively. The CT value of the aorta was significantly higher in IACTA than in IVCTA (965.4 ± 634.1 versus 352.2 ± 46.0 HU; P = .002). Contrast material concentrated in the dorsal portion of the aorta in IACTA but homogeneously enhanced the aorta in IVCTA. The intercostal and lumbar arteries were displayed more clearly by IACTA than by IVCTA (Fig 4). The AKA originated from a left intercostal or lumbar artery in 72% of the patients and at the level of the ninth to 12th intercostal arteries in 84% (Fig 5). We found hairpin curved vessels that were visible in the first phase images but were enhanced stronger in the second phase images in 10 patients of the IACTA group and 9 of the IVCTA group. They were identified as radiculomedullary veins that may mimic the AKA in the first phase image.
Fig 4.
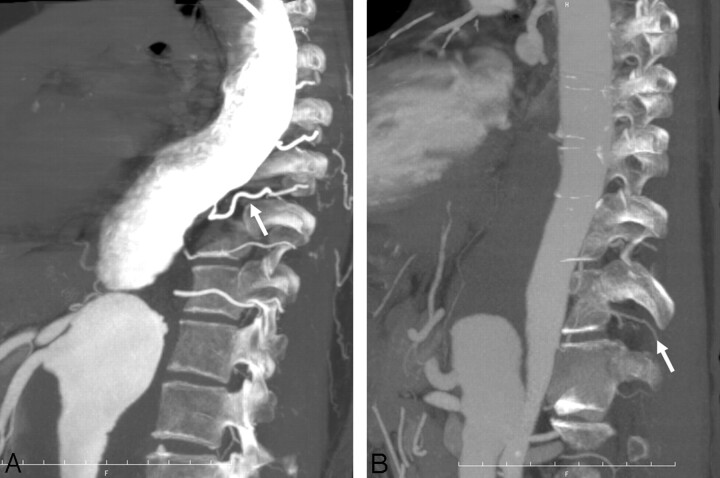
A, Oblique sagittal partial MIP image of IACTA obtained from a 73-year-old man with TAAA. Contrast material is not mixed throughout the aorta, and dorsal portion of the aorta and the intercostal or lumbar arteries (arrow) are enhanced over 1000 HU. B, Oblique sagittal partial MIP image of IVCTA obtained from a 58-year-old man with TAAA. The aorta is homogeneously enhanced, but intercostal or lumbar arteries (arrow) are poorly visualized in comparison with IACTA.
Fig 5.
Laterality and levels of the AKA in all of the patients.
Discussion
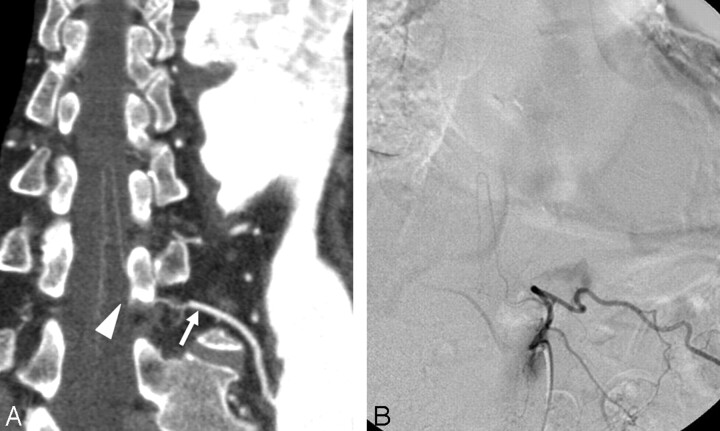
The detection rates of the AKA and the confirmation rates of continuity were higher with IACTA compared with those of previous reports using IVCTA (Table 2). The AKA and its anatomic course from the intercostal or lumbar arteries to the anterior spinal artery are very thin, and the artery runs close to osseous structures, which frequently reduce the CNR of the spinal vasculature and complicate vessel tracking.18 We applied a slower rotation speed and smaller helical pitch than usual to obtain higher mAs and, thus, elevate the CNR. Furthermore, contrast material is diluted in aortic aneurysms, which reduces the CNR. To obtain sufficient arterial opacification, rapid injection (4.0–4.5 mL/s) and a relatively high dose (130–150 mL) of contrast material are considered necessary to visualize the AKA by using IVCTA.20 Aortic visualization is enhanced by IACTA because contrast material is directly injected without dilution in the circulation of the right heart. Similar to the report of Nojiri et al,24 we injected 100 mL of contrast material at a rate of 5.0 mL/s through a Pigtail catheter placed in the proximal portion of the descending aorta and started CT scanning with a 4-second delay after the injection start. The dorsal portion of the aorta and intercostal or lumbar arteries tended to have extremely high attenuation values in IACTA (Fig 4) because of the high specific gravity of the contrast material (1.405 at 370 mg/mL of Iopamidol; 37°C). High z-axis (craniocaudal) resolution is also important to visualize thin arteries. Collimation of 1 mm improved the spatial resolution and arterial tracking compared with 2-mm collimation.20 When IACTA was applied using 0.75-mm collimation and intra-arterial contrast injection, the detection rate of the AKA was 94%, and continuity was confirmed at a rate of 88%. We could not detect the AKA by using IACTA in 1 patient despite sufficient enhancement of the aorta and intercostal arteries. The large anterior radiculomedullary artery might not have been located within the scan range of this patient. One of 2 patients in whom the AKA was detected but continuity was not confirmed underwent selective intercostal angiography in the course of stent-graft deployment, and the AKA originating from the 11th intercostal artery visualized by using IACTA was confirmed by selective spinal arterial angiography (Fig 6).
Table 2:
Detection rates of the artery of Adamkiewicz
| Method (Reference) | Detection Rate, % (n/N) | Confirmation Rate, % (n/N) | Detectors Row | Phase | Scan Duration Injection Rate |
|
|---|---|---|---|---|---|---|
| Seconds | mL/s | |||||
| IVCTA (19) | 90 (63/70) | 32 (20/63) | 4 | 1 | 40 | 3.5 |
| IVCTA (20) | 90 (9/10) | 89 (8/9) | 8 | 1 | 40 | 4.0–4.5 |
| IVCTA (18) | 83 (25/30) | 72 (18/25) | 16 | 1 | NA | 3.5 |
| IACTA (24) | 100 (39/39) | 90 (35/39) | 4 or 6 | 1 | NA | 4.0–7.0 |
| IVCTA (present study) | 60 (9/15) | 56 (5/9) | 16 | 2 | 25 | 5.0 |
| IACTA (present study) | 94 (16/17) | 88 (14/16) | 16 | 2 | 25 | 5.0 |
Note:—IVCTA indicates intravenous CT angiography; IACTA, intra-arterial CT angiography; NA, not assessed.
Fig 6.
A, CPR image of the AKA obtained from IACTA. A hairpin curved vessel ascends to the midsagittal surface of the spinal cord, but continuity with the left 11th intercostal artery (arrow) is disturbed by a vertebral pedicle (arrowhead). B, Selective angiography of the left 11th intercostal artery demonstrates the AKA originating from this location.
A few points should be considered when performing IACTA. The catheter tip should be advanced to a suitable position to visualize the intercostal and lumbar arteries when a dissecting ascending aortic aneurysm is associated with a patent false lumen. We placed the catheter tip immediately proximal to the entry to enhance both true and false lumens in patients with dissection and confirmed visualization of intercostal and lumber arteries by test injections of contrast material. None of the patients studied here had dissection of the ascending aorta or aortic arch. Patients with Stanford type A dissection had undergone total arch replacement before IACTA. Collateral circulation from branches of the subclavian artery, such as the internal thoracic artery, might not have been visualized when contrast material was injected from the proximal descending aorta.
Radiculomedullary veins can have a similar shape to the AKA, and they can run close to the radiculomedullary arteries at the level of the vertebral foramen. Thus, they can be enhanced in the first phase image because of a scan duration of 20–25 seconds, whereas the estimated interval between the arterial and venous phases is 10–15 seconds.13 Some vessels with a hairpin curve configuration in the first phase image that were more enhanced in the second were regarded as radiculomedullary veins (Fig 3). Without multiphase dynamic scanning, such vessels might be misdiagnosed as arteries if continuity with intercostal or lumbar arteries is not confirmed. Thus, confirmation of continuity between the aorta and the AKA by multiphase scanning is critical to avoid misdiagnosis.
Selective spinal arterial angiography is the “gold standard” for diagnosing the AKA. However, previous diagnoses of the AKA by using CTA and MRA did not have a “gold standard” probably because of the risk of selective angiography. Only 1 report has proved that MRA can be diagnostic in a subgroup of 3 patients who underwent selective spinal angiography.13 Thus, the true detection rates of the AKA by MRA and CTA are unknown. The detection rate of the AKA by using IVCTA in the present study was not as high as in previous reports (Table 2). One possible cause of this variance is that diagnostic criteria were not identical among the studies. Multiphase dynamic imaging was performed by using MR imaging,13–16 whereas single-phase imaging was performed by using CT.19–21,24 We believe that multiphase imaging can improve the precision of AKA diagnosis. The present study showed that the detection rates of IACTA were better than those of IVCTA when using the same scan variables, contrast dose, injection rate, and diagnostic criteria.
Arterial catheterization causes IACTA to be more invasive than IVCTA and MRA but is nevertheless safer and less time consuming than selective spinal angiography, which is associated with complications such as retroperitoneal bleeding, cerebral ischemia, renal failure, and spinal cord ischemia in 1.2%–4.6% of patients.10,11 We have not yet experienced any complications related to IACTA, which proceeded in addition to routine preoperative coronary angiography, with an elevated total dose of contrast material. For patients with insufficient renal function, we performed IVCTA on a different day from coronary angiography.
Although the contribution of information about the AKA to the outcome of thoracoabdominal aortic surgery remains controversial,9,11,12 some reports have suggested that preoperative AKA localization is useful in reducing the risk of postoperative ischemic spinal complications14,15 and in shortening surgical duration.16 Preoperative AKA localization aids in planning surgical strategies, and protection for the spinal cord can be more reliable.7 We believe that IACTA will improve diagnostic accuracy and would help to reduce postoperative spinal complications. In this study, 14 and 15 patients from the IVCTA and IACTA groups, respectively, underwent surgical repair or stent grafting, and 1 patient who underwent IVCTA developed hemiaplasia despite preoperative AKA demonstration. However, the study cohort was small, and further study is needed to confirm the efficacy of IACTA in the clinical setting.
Conclusions
The rate of detecting the AKA and the confirmation rate of continuity from the aorta to the AKA were high using IACTA. A high concentration of contrast material delivered via intra-arterial injection can improve the accuracy of visualizing the AKA. The AKA should be correctly identified so that radiologists can provide surgeons with the critical information needed for reconstruction of the intercostal and lumbar arteries. We believe that IACTA is a suitable preoperative means of investigating patients with TAA and TAAA.
References
- 1.Cambria RP, Clouse WD, Davison JK, et al. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg 2002;236:471–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estrera AL, Miller CC 3rd, Huynh TTT, et al. Neurologic outcome after thoracic and thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2001;72:1225–31 [DOI] [PubMed] [Google Scholar]
- 3.Hollier L, Money SR, Naslund TC, et al. Risk of spinal cord dysfunction in patients undergoing thoracoabdominal aortic replacement. Am J Surg 1992;164:210–13 [DOI] [PubMed] [Google Scholar]
- 4.Coselli JS, LeMaire SA, Miller CC III, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thoracic Surg 2000;69:409–14 [DOI] [PubMed] [Google Scholar]
- 5.Safi HJ, Miller CC III, Carr C, et al. Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg 1998;27:58–68 [DOI] [PubMed] [Google Scholar]
- 6.Wan IY, Angelini GD, Bryan AJ, et al. Prevention of spinal cord ischaemia during descending thoracic and thoracoabdominal aortic surgery. Eur J Cardiothorac Surg 2001;19:203–13 [DOI] [PubMed] [Google Scholar]
- 7.Ogino H, Sasaki H, Minatoya K, et al. Combined use of Adamkiewicz artery demonstration and motor-evoked potentials in descending and thoracoabdominal repair. Ann Thorac Surg 2006;82:592–96 [DOI] [PubMed] [Google Scholar]
- 8.Heinemann MK, Brassel F, Herzog T, et al. The role of spinal angiography in operations on the thoracic aorta: myth or reality? Ann Thorac Surg 1998;65:346–51 [DOI] [PubMed] [Google Scholar]
- 9.Williams GM, Roseborough GS, Webb TH, et al. Preoperative selective intercostal angiography in patients undergoing thoracoabdominal aneurysm repair. J Vasc Surg 2004;39:314–21 [DOI] [PubMed] [Google Scholar]
- 10.Kieffer E, Fukui S, Godet G, et al. Spinal cord arteriography: a safe adjunct before descending thoracic or thoracoabdominal aortic aneurysmectomy. J Vasc Surg 2002;25:262–68 [DOI] [PubMed] [Google Scholar]
- 11.Savader SJ, William GMs, Trerotola SO, et al. Preoperative spinal artery localization and its relationship to postoperative neurologic complications. Radiology 1993;189:165–71 [DOI] [PubMed] [Google Scholar]
- 12.Minatoya K, Karck M, Hagl C, et al. The impact of spinal angiography on the neurological outcome after surgery on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg 2002;74:1870–72 [DOI] [PubMed] [Google Scholar]
- 13.Yamada N, Takamiya M, Kuribayashi S, et al. MRA of the Adamkiewicz artery: a preoperative study for thoracic aortic aneurysm. J Comput Assist Tomogr 2000;24:362–68 [DOI] [PubMed] [Google Scholar]
- 14.Hyodoh H, Kawaharada N, Akiba H, et al. Usefulness of preoperative detection of artery of Adamkiewicz with dynamic contrast-enhanced MR angiography. Radiology 2005;236:1004–09 [DOI] [PubMed] [Google Scholar]
- 15.Kawaharada N, Morishita K, Hyodoh H, et al. Magnetic resonance angiographic localization of the artery of Adamkiewicz for spinal cord blood supply. Ann Thorac Surg 2004;78:846–52 [DOI] [PubMed] [Google Scholar]
- 16.Kawaharada N, Morishita K, Fukada J, et al. Thoracoabdominal or descending aortic aneurysm repair after preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography. Eur J Cardiothorac Surg 2002;21:970–74 [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka K, Niinuma H, Ohira A, et al. MR angiography and CT angiography of the artery of Adamkiewicz: noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. RadioGraphics 2003;23:1215–25 [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka K, Niinuma H, Ehara S, et al. MR angiography and CT angiography of the artery of Adamkiewicz: state of the art. RadioGraphics 2006;26:S63–73 [DOI] [PubMed] [Google Scholar]
- 19.Takase K, Sawamura Y, Igarashi K, et al. Demonstration of the artery of Adamkiewicz at multi-detector row helical CT. Radiology 2002;223:39–45 [DOI] [PubMed] [Google Scholar]
- 20.Takase K, Akasaka J, Sawamura Y, et al. Preoperative MDCT evaluation of the artery of Adamkiewicz and its origin. J Comput Assist Tomogr 2006;30:716–22 [DOI] [PubMed] [Google Scholar]
- 21.Kudo K, Terae S, Asano T, et al. Anterior spinal artery and artery of Adamkiewicz detected by using multi-detector row CT. AJNR Am J Neuroradiol 2003;24:13–17 [PMC free article] [PubMed] [Google Scholar]
- 22.Thron AK. Vascular Anatomy of the Spinal Cord: Neuroradiological Investigations and Clinical Syndromes. Vol7 . New York, NY: Springer-Verlag;1988. :3–65 [Google Scholar]
- 23.Koshino T, Murakami G, Morishita K, et al. Does the Adamkiewicz artery originate from the larger segmental arteries? J Thorac Cardiovasc Surg 1999;117:898–905 [DOI] [PubMed] [Google Scholar]
- 24.Nojiri J, Matsumoto K, Kato A, et al. The Adamkiewicz artery: demonstration by intra-arterial computed tomographic angiography. Eur J Cardiothorac Surg 2007;31:249–55 [DOI] [PubMed] [Google Scholar]