Abstract
BACKGROUND AND PURPOSE: Although the hyperattenuated middle cerebral artery sign is known to be related to acute infarction, the volume of clot associated with it is not known. We investigated whether the presence or absence of hyperattenuated artery sign (HAS) on noncontrast CT (NCCT) can predict the thrombus volume.
MATERIALS AND METHODS: We enrolled 90 consecutive patients with acute infarction who underwent both 5- and 1.25-mm NCCT and CT angiography (CTA). HAS was determined on 5-mm NCCT retrospectively. According to the location of thrombi, the patients were classified into ICA (ICA terminus/ICA and others), M1 (M1/both M1 and M2), and M2 (M2) groups. Thrombus volumes were measured by 1.25-mm NCCT and were compared between patients with and without HAS.
RESULTS: Occlusion of major arteries was seen on CTA in 78 patients. HAS was found in 46 patients (59.0%). The mean thrombus volume was significantly larger in patients with HAS than in those without except for the M2 group (ICA group: [n = 14], 188.7 ± 122.5 mm3 versus 39.4 ± 12.1 mm3 [P = .022]; M1 group: [n = 42], 128.1 ± 119.2 versus 56.8 ± 32.5 [P = .005]; M2 group: [n = 22], 34.7 ± 32.2 versus 20.0 ± 20.0 [P = .18]). Thrombus volumes determined by receiver operating characteristic curve analysis were 52.36 mm3 in the ICA group (sensitivity, 90.9%; specificity, 100%) and 53.96 mm3 in the M1 group (sensitivity, 88.0%; specificity, 58.8%).
CONCLUSION: Thrombus volumes were significantly larger in patients with HAS than in those without in ICA and M1 occlusions. The detection of HAS may provide an idea concerning rapid and dichotomized estimation of thrombus volume, which may be helpful for treatment decisions in potential candidates for thrombolysis.
Although hyperattenuated artery sign (HAS) on noncontrast CT (NCCT) represents the presence of the thrombus in acute ischemic stroke,1,2 many patients with the thrombus do not show the HAS. In previous studies using NCCT with ≥5-mm thickness, it has been reported that the HAS presents in 5%–50% of patients with the thrombus in the M1 segment of the middle cerebral artery (MCA)3–5 and in approximately 15% with the thrombus in the M2 segment.5,6 The volume of the thrombus may affect outcomes. Patients with HAS in the M1 segment had more severe ischemia and poorer outcome than those without.5,7 In addition, rapid estimation of the thrombus volume and its location may be helpful in determining treatment techniques, particularly in candidates for thrombolytic treatment.8 The volume of the thrombus, as well as its location, can be one of the factors determining the presence of HAS. However, the relationship between the thrombus volume and the presence of HAS is unknown. This may be partly due to difficulty in estimation of thrombus volume.
Recently, more sensitive detection of thrombus and measurement of its volume in patients with acute ischemic stroke became feasible by using thin-section NCCT.9,10 Therefore, we hypothesized that the relationship between the thrombus volume and the presence of HAS on conventional 5-mm CT could be determined with measurement of thrombus volume by using thin-section NCCT, and also the best threshold of thrombus volume for predicting the presence of HAS could be determined. The purpose of this study was to investigate whether the presence or absence of HAS can be used in the prediction of thrombus volume.
Materials and Methods
Patients
Between June 2003 and March 2007, 90 consecutive patients (31 women, 59 men; mean age, 66.9 years) who presented with acute MCA territory ischemic stroke within 6 hours of symptom onset were enrolled in this study. All patients had acute infarction, which was confirmed with follow-up diffusion-weighted imaging within 2 days after presentation.
CT Acquisition and Effective Dose Estimation
All patients underwent both 5- and 1.25-mm NCCT (LightSpeed Plus; GE Healthcare, Milwaukee, Wis), according to a predetermined protocol. Conventional axial 5-mm NCCT was performed first with the following parameters: 120 kVp; 250 mA; rotation time, 0.8 seconds; FOV, 25 cm. Twenty-eight to 30 images were obtained per examination according to the patient’s head size. Helical 1.25-mm NCCT was obtained after 5-mm CT, with the following parameters: 120 kVp; 250 mA; rotation time, 0.8 second; pitch, 0.75; FOV, 25 cm; matrix, 512 × 512; pixel size, 0.49 × 0.49 mm. The scan was obtained parallel to the plane of the inferior orbital rim to the basion and ended 7.5 cm above it to reduce the radiation dose. All images of 1.25-mm NCCT were reconstructed at 0.6-mm thickness. Last, CT angiography (CTA) was performed with the same parameters as those of helical 1.25-mm NCCT. Effective radiation dose was estimated by using the ImPACT CT dose calculator, Version 0.99x (www.impactscan.org/ctdosimetry; Impact Performance Assessment of CT, Bence Jones Offices, St. George’s Hospital, London, UK) and the National Radiologic Protection Board SR-250 datasets. The estimated effective radiation doses of conventional axial 5-mm NCCT (30 images) and helical 1.25-mm NCCT were 1.2 and 1.6 mSv, respectively. The institutional review board approved this study.
Determination of HAS on NCCT
In a retrospective manner, a neuroradiologist assessed CTA images to determine artery occlusion (internal carotid artery [ICA], M1, and M2 segments of the MCA). A neurologist and another neuroradiologist who were not involved in assessment of CTA independently reviewed the 5-mm NCCT scans to find HAS. HAS was defined as a hyperattenuated lesion compared with the attenuation of the contralateral arteries and adjacent parenchyma and was determined subjectively. The anterior cerebral artery was not evaluated for HAS. Disagreements were settled by consensus. According to the location of the thrombus (HAS), the patients were classified into the ICA (ICA terminus/ICA and others), M1 (M1/both M1 and M2), and M2 (M2) groups.
Thrombus Volume Measurement
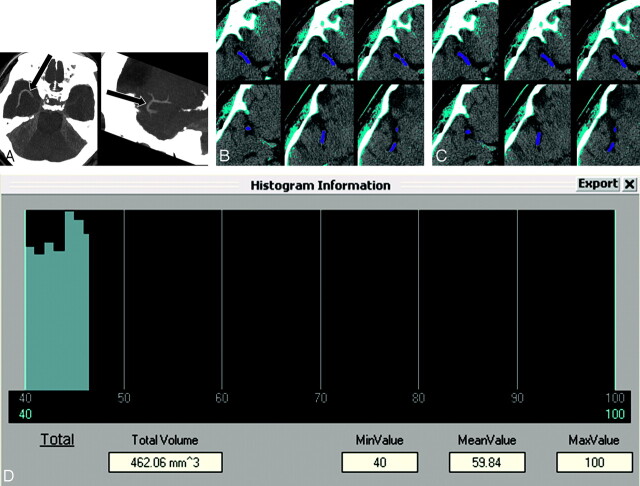
A neuroradiologist who was not involved in determination of the presence of HAS measured thrombus volume on 0.6-mm NCCT. All Digital Imaging and Communication in Medicine data of patients who showed a thrombus on 1.25-mm NCCT were transferred to commercial 3D software (Rapidia 2.8, Infinitt, Seoul, Korea). All pixels were segmented with a threshold of 50–100 HU to select the thrombus. We drew a region of interest around the thrombus and performed 3D region growing. Region of interest dilation to the margin of the thrombus was performed at a threshold of 40–100 HU. We did not perform segmentation with a threshold of 40–100 HU first, because normal brain parenchyma could be selected with this threshold. Therefore, we chose a threshold of 50–100 HU to select thrombus alone and subsequently dilated pixels to approximate the margin of the thrombus. The volume was calculated with the number of voxels (Fig 1).
Fig 1.
A representative case showing the procedure of thrombus volume measurement. A, Maximum intensity projection images of thin-section noncontrast CT in a patient with occlusion in both M1 and M2 segments on the right (arrows). B, All pixels are segmented with a threshold of 50–100 HU to select the thrombus (azure areas). After selecting the function of 3D region growing, we drew a region of interest around the thrombus on an axial image and then automatically segmented the whole thrombus (indigo areas). Note that the thrombus does not approximate the border of the probable thrombus. C, Pixel dilation with a single iteration for the segmented thrombus is performed at a threshold of 40–100 HU. The thrombus approximates its border more than does the thrombus on B (indigo areas). D, The volume and mean Hounsfield unit of the thrombus are calculated. MinValue indicates minimal value; MeanValue, mean value; MaxValue, maximal value.
Data Analyses
We compared thrombus volumes between patients with and without HAS by using the Mann-Whitney U test. Thrombus volumes were also compared among the groups (ICA, M1, and M2 groups) by using the Kruskal-Wallis one-way analysis of variance test, followed by a post hoc test. Comparison of a National Institutes of Health Stroke Scale (NIHSS) score between the groups was done by t test. The relationship between thrombus location and the presence or absence of HAS was determined by the χ2 test. The thrombus volumes predicting the presence of HAS were determined by receiver operating characteristic (ROC) curve analysis. A P value of less than .05 was considered to indicate statistical significance. All the statistical analyses were performed with statistical software (Statistical Package for the Social Sciences, Version 12; SPSS, Chicago, Ill).
Results
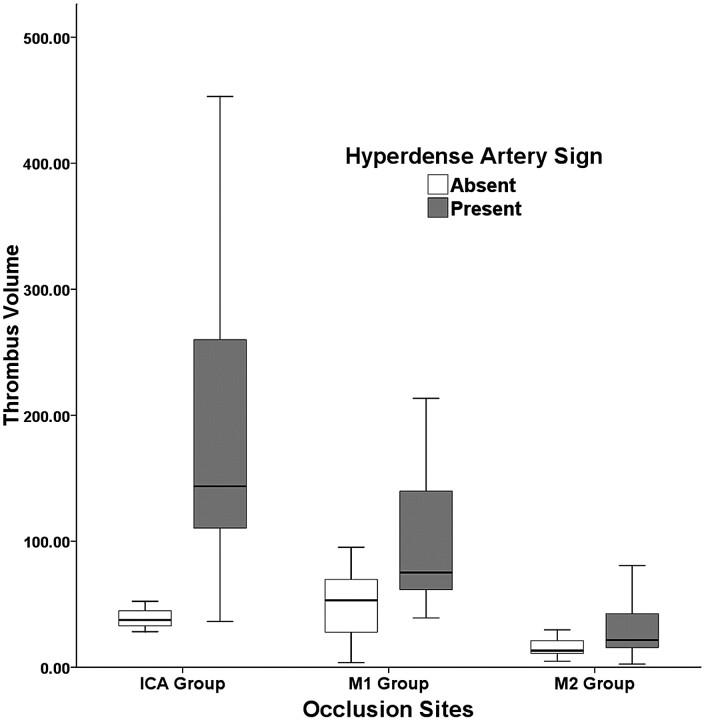
Occlusion of major arteries (ICA terminus, M1, and M2) was identified on CTA in 78 patients. HAS was found in 46 (59.0%) of 78 patients, 11 (78.6%) in the ICA group (n = 14), 25 (59.5%) in the M1 group (n = 42), and 10 (45.5%) in the M2 group (n = 22). Interobserver reliability was excellent in the determination of HAS (κ = 0.806). The mean thrombus volume, which was measured in 78 patients with an occlusion, was significantly larger in patients with HAS than in those without, except for those in the M2 group (Table 1, Fig 2). The mean thrombus volume in the M2 group was significantly smaller than those in ICA and M1 groups (P < .0001), but there was no difference between the latter groups (Table 2). The presence or absence of HAS was not related to the location of the thrombus (P > .05) (Table 2). The thrombus volumes for predictability of the presence of HAS by ROC curve analysis were 52.36 mm3 in the ICA group (sensitivity, 90.9%; 95% confidence interval [CI], 58.7–98.5; specificity, 100%; 95% CI, 30.5–100.0) and 53.96 mm3 (sensitivity, 88.0%; 95% CI, 68.8–97.3; specificity, 58.8%; 95% CI, 33.0–81.5) in the M1 group.
Table 1:
Thrombus volumes in patients with and without HAS
| ICA Group (n = 14) |
M1 Group (n = 42) |
M2 Group (n = 22) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HAS (+) (n = 11) | HAS (–) (n = 3) | P* | HAS (+) (n = 25) | HAS (–) (n = 17) | P* | HAS (+) (n = 10) | HAS (–) (n = 12) | P* | |
| Mean thrombus volume (mm3) | 188.7 ± 122.5 | 39.4 ± 12.1 | .022 | 128.1 ± 119.2 | 56.8 ± 32.5 | .005 | 34.7 ± 32.2 | 20.0 ± 20.0 | .18 |
Note:—+ indicates presence; –indicates absence.
Mann-Whitney U test.
Fig 2.
Thrombus volume comparison according to the presence or absence of hyperdense artery sign.
Table 2:
Thrombus volume comparison according to the occlusion site
| ICA group (n = 14) | M1 group (n = 42) | M2 group (n = 22) | P | |
|---|---|---|---|---|
| Mean thrombus volume (mm3) | 156.7 ± 125.0 | 94.1 ± 95.2 | 26.7 ± 26.7 | <.001* |
| HAS (present/absent) | 11/3 (78.6%) | 25/17 (59.5%) | 10/12 (45.5%) | >.05† |
Kruskal-Wallis one-way ANOVA test.
χ2 test.
We compared the baseline NIHSS scores between patients with HAS in the ICA or M1 (ICA or M1 group, n = 36) and those in the M2 (M2 group) or without HAS (n = 54). The mean NIHSS score was significantly higher in patients in the former (16.06 ± 5.1) than the latter groups (12.76 ± 6.9) (P = .01).
Discussion
HAS is an indicator of the presence of the thrombus in the artery. The detection of HAS has been particularly useful in patients with stroke during the hyperacute stage, during which the parenchymal CT changes are not obvious, by providing information of potential targets for thrombolytic treatment and diagnostic clues of infarction. This study showed that the presence of HAS can provide additional information by indicating the presence of a larger thrombus when these patients were compared with patients without HAS.
Brain CT has usually been performed with 5-mm thickness in evaluating patients with acute stroke. The ICA terminus and MCA are nonlinear in course and are <5 mm in diameter. Therefore, a small thrombus in them may not be identified due to the partial volume effect. However, the partial volume effect may be mitigated by thrombus size and arterial course. This was particularly true in occlusion of the ICA terminus, which had a larger diameter and more vertical course than that in the MCA in our study. The ICA terminus demonstrated a higher probability of HAS than the MCA when thrombus was present in it and had a very high predictability for the large volume (more than approximately 53 mm3) when HAS was present. Although this pattern was observed in the M1 group, a considerable overlap in the thrombus volume was present between patients with HAS and those without; this may be explained by a higher probability of the partial volume effect in the MCA.
Although intravenous (IV) recombinant tissue-type plasminogen activator (rtPA) treatment was a breakthrough for the effective recanalization of the occluded artery in patients with stroke, the recanalization rate following the treatment is known to be low, in that the thrombus can be successfully lysed in only approximately a quarter of patients treated with IV rtPA.8 The recanalization rate is further decreased in the occlusion of the proximal arteries (ICA terminus and MCA)8 due to their larger thrombus volumes.3,11 Rapid estimation of the thrombus location and volume at the time of initial evaluation may be helpful in determining treatment technique in candidates for thrombolysis. For example, in patients whose thrombus is thought to be large and/or located in the large artery, additional treatment with mechanical thrombectomy or intra-arterial treatment for IV thrombolysis may be considered from the beginning.
This study has a few limitations. First, we measured thrombus volume by using thin-section NCCT but did not validate this method. The volume of a pure red clot may be accurately measured in vitro by using thin-section NCCT because of its homogeneous nature. However, in vivo thrombus is heterogeneous in nature, precluding it from accurate measurement of its volume. Second, though areas containing calcium must have been excluded because the thrombus volume measurement was performed at a threshold of 50–100 HU, very small amounts of calcium might be included due to partial volume effect, which may result in overestimation of the thrombus size. Nevertheless, CT-based measurements may be used for estimation of the thrombus volume because thin-section CT is sensitive in the detection of the thrombus, whereas no method for exact measurement of the thrombus volume in the human brain arteries has been available, to our knowledge. Third, we did not analyze the relationship between thrombus volume and efficacy of fibrinolysis or clinical outcome due to different treatment methods among the patients. For this purpose, a further prospective study should be followed.
Conclusion
The thrombus volume was significantly larger in patients with HAS than in those without HAS in ICA and M1 occlusion, but not in those with M2 occlusion. Our data indicate that the presence of HAS on conventional 5-mm NCCT not only suggests the presence of thrombus but also may provide a simple and dichotomized estimation of the thrombus volume in patients with ICA or M1 occlusion.
Footnotes
This work was supported by the Korea Science and Engineering Foundation through the National Core Research Center for Nanomedical Technology (R15-2004 to 024-00000-0) and by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A060171).
References
- 1.Gacs G, Fox AJ, Barnett HJ, et al. CT visualization of intracranial arterial thromboembolism. Stroke 1983;14:756–62 [DOI] [PubMed] [Google Scholar]
- 2.Leys D, Pruvo JP, Godefroy O, et al. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke 1992;23:317–24 [DOI] [PubMed] [Google Scholar]
- 3.von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke 1992;23:646–52 [DOI] [PubMed] [Google Scholar]
- 4.Tomsick T, Brott T, Barsan W, et al. Thrombus localization with emergency cerebral CT. AJNR Am J Neuroradiol 1992;13:257–63 [PMC free article] [PubMed] [Google Scholar]
- 5.Barber PA, Demchuk AM, Hudon ME, et al. Hyperdense sylvian fissure MCA “dot” sign: a CT marker of acute ischemia. Stroke 2001;32:84–88 [DOI] [PubMed] [Google Scholar]
- 6.Leary MC, Kidwell CS, Villablanca JP, et al. Validation of computed tomographic middle cerebral artery “dot” sign: an angiographic correlation study. Stroke 2003;34:2636–40 [DOI] [PubMed] [Google Scholar]
- 7.Manelfe C, Larrue V, von Kummer R, et al. Association of hyperdense middle cerebral artery sign with clinical outcome in patients treated with tissue plasminogen activator. Stroke 1999;30:769–72 [DOI] [PubMed] [Google Scholar]
- 8.Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke 2007;38:192–93 [DOI] [PubMed] [Google Scholar]
- 9.Kim EY, Lee SK, Kim DJ, et al. Detection of thrombus in acute ischemic stroke: value of thin-section noncontrast-computed tomography. Stroke 2005;36:2745–47. Epub 2005 Nov 3 [DOI] [PubMed] [Google Scholar]
- 10.Kim EY, Heo JH, Lee SK, et al. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology 2006;67:1846–48 [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86 [DOI] [PubMed] [Google Scholar]