Abstract
BACKGROUND AND PURPOSE: The prevalence of antiplatelet drug resistance among patients who undergo cerebrovascular stent placement is unknown. We aimed to assess the feasibility of monitoring antiplatelet drug effects in a single-center cohort undergoing cerebrovascular stent placement.
MATERIALS AND METHODS: We prospectively collected medical, laboratory, and radiographic data on patients who underwent cerebrovascular stent placement. We used the rapid platelet function assay-aspirin (RPFA-ASA) to calculate aspirin reaction units (ARU) and the P2Y12 assay to calculate P2Y12 reaction units and percentage platelet inhibition. Aspirin resistance was defined as ARU > 550, whereas clopidogrel resistance was defined as percentage platelet inhibition < 40%.
RESULTS: Among 76 patients, stent indications were the following: wide-neck aneurysm (57, 75.0%), symptomatic intracranial stenosis (12, 15.7%), carotid stenosis (5, 6.6%), and vertebral stenosis (2, 2.6%). For aspirin, the median dosage per week was 1300 mg and median ARU was 410. Among 71 patients on aspirin, 3 patients (4.2%) were resistant; there was a significant inverse correlation between aspirin dose and ARU (r = −0.31, P = .01). Among 55 patients on clopidogrel, the median dosage per week was 525 mg with a mean platelet inhibition of 43.2%. Twenty-eight patients (51.9%) were clopidogrel-resistant. In a multivariable linear regression model, age older than 55 years (b = −16.3, P = .020) and diabetes (b = −26.8, P = .015) were inversely related to percentage platelet inhibition.
CONCLUSIONS: Using point-of-care tests, we found that aspirin resistance is relatively uncommon, whereas clopidogrel resistance occurred in half of patients undergoing cerebrovascular stent placement. Further studies should focus on ideal doses, timing, and duration of antiplatelet therapy for cerebrovascular stent placement.
Although there is widespread use of endovascular stents in the treatment of coronary and peripheral arterial disease, the use of cerebrovascular stents has only emerged during the past decade. Indications include extracranial and intracranial large-artery stenosis and endovascular treatment of wide-neck cerebral aneurysms. Antithrombotic therapy is often used to combat the risk of stent thrombosis and re-stenosis associated with bare metal stents. Following percutaneous coronary intervention, aspirin and clopidogrel are routinely considered “standard of care.” On the basis of the current American Heart Association guidelines, dual antiplatelet therapy is recommended for 1 month following bare metal coronary stent placement and for up to 6–12 months for drug-eluting stents.1 Extrapolating from this clinical practice, combination antiplatelet therapy has also been increasingly used in patients undergoing cerebrovascular stent placement, for which higher rates of re-stenosis have been reported.2–4 However, little data exist to guide this practice.
Given the importance of platelet inhibition in the prevention of in-stent thrombosis and re-stenosis, there is a great incentive to ensure that adequate antiplatelet effects are achieved in such high-risk patients. Platelet inhibition from aspirin and clopidogrel varies broadly, and some patients are low responders or are classified as being “resistant.” In coronary patients undergoing stent placement, significant proportions have aspirin and clopidogrel resistance.5,6 There are no data on responses to aspirin and clopidogrel among patients who undergo cerebrovascular stent placement. Therefore, in a single-center prospective cohort by using point-of-care platelet function assays, we aimed to test the feasibility of monitoring antiplatelet drug effects, determine the prevalence of aspirin and clopidogrel resistance, and identify predictors of decreased antiplatelet response.
Patients and Methods
Between May 2005 and August 2006, we prospectively collected demographic, medical, serologic, and radiographic data on patients who underwent endovascular stent placement at our institution for various clinical indications, including vessel remodeling for wide-neck intracranial aneurysms and revascularization of extracranial and intracranial stenoses. Collected data also included aspirin and clopidogrel doses in the week before the procedure, duration of antiplatelet therapy, and clinical and angiographic outcomes at 6 months. The study was approved by the institutional review board.
All patients underwent cerebral angiography. Catheterization of the target vessel for intervention was done by using either a 6F Envoy guide catheter (Cordis, Miami Lakes, Fla) or a Shuttle-SL guide sheath (Cook, Bloomington, Ind). Heparin was administered until activated clotting time was between 250 and 300 seconds. The self-expanding stents (Neuroform and Wingspan, Boston Scientific, Natick, Mass; Acculink, Guidant, St. Paul, Minn) were deployed by using a single-operator technique over Synchro 14 microguidewire (Boston Scientific). The vascular access site was then closed with the Angio-Seal closure device (St. Jude Medical, Minnetonka, Minn).
We used the VerifyNow rapid platelet function assay-aspirin (RPFA-ASA) (Accumetrics, San Diego, Calif) to calculate aspirin reaction units (ARU) and the P2Y12 assay (VerifyNow) to calculate P2Y12 reaction units and percentage platelet inhibition immediately before the endovascular procedure. Aspirin resistance or low response was defined as ARU ≥ 550, whereas clopidogrel resistance or low response was defined as percentage platelet inhibition ≤40%.
Univariable statistical methods (analysis of variance [ANOVA] and correlation tests) were performed to test associations between demographic, clinical, angiographic, and treatment variables and percentage platelet inhibition as a continuous variable. Those variables with P < .20 on univariable testing were entered into multivariable models by using stepwise linear regression to yield b coefficients, 95% confidence intervals, and P values for predictors of percentage platelet inhibition. Significance was defined as P < .05. All statistics were performed by using the Statistical Package for the Social Sciences 14.0 (SPSS, Chicago, Ill).
Results
Among 76 consecutive patients who underwent cerebrovascular stent placement during the study period, the indications for stent placement were the following: wide-neck aneurysm (57, 75.0%), symptomatic intracranial stenosis (12, 15.7%), carotid stenosis (5, 6.6%), and vertebral stenosis (2, 2.6%). Fifty-six (73.7%) were on combined aspirin and clopidogrel at the time of stent placement, whereas 4 were taking clopidogrel alone (5.3%) and 16 were taking aspirin alone (21.0%). Most of those who received aspirin (64.8%) and clopidogrel (71.2%) were loaded within 1 week of stent placement. There were no acute stent thrombosis or stenosis and 1 intraoperative aneurysmal rupture. At 6 months, 2 patients had symptomatic re-stenoses presenting with transient ischemic attacks (1 patient taking aspirin plus clopidogrel and 1, aspirin alone), and 1 patient had transient episodes without documented re-stenosis.
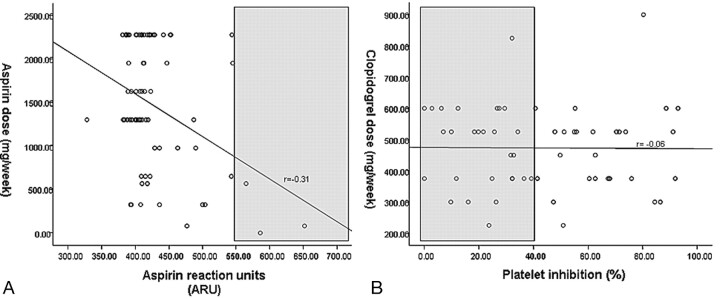
In the 71 patients on aspirin in whom ARU was measured (98.6%), the median dosage per week was 1300 mg and the median ARU was 410. Only 3 patients (4.2%) had ARU values >550 or aspirin low response; a strong inverse relationship (r = −0.31, P = .01) with aspirin dose was noted (Fig 1A). For clopidogrel, the median dosage per week was 525 mg and the mean platelet inhibition was 43.2 ± 26.6% among the 55 patients in whom percentage platelet inhibition was measured (91.7%). Twenty-eight patients (50.9%) had platelet inhibition ≤40% or clopidogrel resistance, but no correlation (r = −0.06, P = .64) with dose was seen (Fig 1B). In a subset of 39 patients who had clopidogrel loading within 1 week of the procedure, the median cumulative dosage before the stent placement was 325 mg and mean platelet inhibition was 44.3 ± 26.0%. Among these patients, 20 (51.3%) were low responders (platelet inhibition ≤40%).
Fig 1.
A, Scatterplot of aspirin reaction units (x-axis) versus aspirin dose (y-axis). There is a strong inverse correlation (P = .01). Gray area indicates aspirin resistance. B, Scatterplot of platelet inhibition (x-axis) versus clopidogrel dose (y-axis). There is no correlation (P = .64). Gray area indicates clopidogrel resistance.
Using ANOVA statistics (Table 1), percentage platelet inhibition was found to be lower in patients older than 55 years of age (37.2 versus 53.0, P = .031) and in those with diabetes (21.8 versus 45.8, P = .036), hypertension (35.1 versus 48.2, P = .077), or hypercholesterolemia (34.4 versus 46.2, P = .153) or in those taking statins (33.1 versus 47.0, P = .085) or angiotensin converting enzyme receptor inhibitors (ACE-I) (31.1 versus 45.0, P = .199). It was also lower among those with posterior circulation or multiple stents compared with those with anterior circulation location (28.7 versus 53.4, P = .029). Among laboratory results, percentage platelet inhibition was inversely correlated with baseline glucose and creatinine levels and directly correlated to pre-stent platelet count. Percentage platelet inhibition did not differ by sex, dose of clopidogrel, timing of clopidogrel loading, dose of aspirin, coronary artery disease, indication for stent placement, or other medications. There was no association between percentage platelet inhibition and re-stenosis or clinical outcomes at 6 months.
Table 1:
Univariable ANOVA of clinical variables as predictors of platelet inhibition
| Platelet Inhibition (%) | Parameter Estimate (b) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | – | −0.25 | .303 |
| Age groups (No.) | −15.8 | .031 | |
| <55 years (21) | 53.0 | ||
| >55 years (34) | 37.2 | ||
| Sex (No.) | −2.3 | .755 | |
| Women (34) | 44.1 | ||
| Men (21) | 41.8 | ||
| Medical history | |||
| Hypertension (No.) | −13.1 | .077 | |
| No (34) | 48.2 | ||
| Yes (21) | 35.1 | ||
| Diabetes (No.) | −24.0 | .036 | |
| No (49) | 45.8 | ||
| Yes (6) | 21.8 | ||
| Hypercholesterolemia (No.) | −11.8 | .153 | |
| No (41) | 46.2 | ||
| Yes (14) | 34.4 | ||
| Coronary artery disease (No.) | 2.7 | .789 | |
| No (47) | 43.6 | ||
| Yes (8) | 40.9 | ||
| Prior stroke (No.) | 6.7 | .414 | |
| No (40) | 41.4 | ||
| Yes (15) | 48.1 | ||
| Prior TIA (No.) | −11.6 | .288 | |
| No (48) | 44.7 | ||
| Yes (7) | 33.1 | ||
| Statin use (No.) | −13.9 | .085 | |
| No (40) | 47.0 | ||
| Yes (15) | 33.1 | ||
| ACE-I use (No.) | −13.5 | .199 | |
| No (48) | 45.0 | ||
| Yes (7) | 31.5 | ||
| Beta blocker use (No.) | −4.0 | .684 | |
| No (46) | 43.9 | ||
| Yes (9) | 39.9 | ||
| Ca-channel blocker use (No.) | 6.9 | .671 | |
| No (52) | 42.8 | ||
| Yes (3) | 49.7 | ||
| Diuretic use (No.) | −5.1 | .471 | |
| No (49) | 44.1 | ||
| Yes (6) | 35.7 | ||
| Aspirin use (No.) | −8.4 | .562 | |
| No (3) | 52.0 | ||
| Yes (52) | 47.7 | ||
| Timing of clopidogrel initiation (No.) | −0.8 | .889 | |
| <3 days (14) | 43.2 | ||
| 3–7 days (25) | 44.9 | ||
| >7 days (16) | 40.7 | ||
| Laboratory values | |||
| Creatinine (mg/dL) | – | −22.7 | .175 |
| Glucose (mg/dL) | – | −0.21 | .035 |
| Hemoglobin (g/dL) | – | −0.55 | .825 |
| Platelet count (1,000/μL) | – | 0.07 | .190 |
| Angiographic features | |||
| Stent indication (No.) | −6.0 | .490 | |
| Aneurysm (42) | 45.1 | ||
| Stenosis (13) | 39.1 | ||
| Stent location (No.) | −14.7 | .029 | |
| MCA/ACA/PcomA (19) | 53.4 | ||
| Other (36) | 38.7 | ||
| Outcomes | |||
| Re-stenosis at 6 months (No.) | 7.8 | .774 | |
| No (54) | 43.1 | ||
| Yes (1) | 50.9 | ||
| Stroke or TIA at 6 months (No.) | −4.8 | .806 | |
| No (53) | 43.4 | ||
| Yes (2) | 38.6 |
Note:— –indicates data not available; TIA, transient ischemic attack; Ca, calcium; MCA, middle cerebral artery; ACA, anterior cerebral artery; PcomA, posterior communicating artery; No., number.
In a stepwise multivariable linear regression model (Table 2), with percentage platelet inhibition as the dependent variable and indication for stent placement and location of the stent (anterior circulation versus other), age older than 55 years, diabetes, hypertension, hypercholesterolemia, statin use, ACE-I use, and baseline glucose, creatinine, and platelet levels as independent variables, only age older than 55 years (b = −16.3, P = .020) and history of diabetes (b = −26.8, P = .015) were significantly and inversely related to percentage platelet inhibition.
Table 2:
Multivariable linear regression model for dependent variable (percentage platelet inhibition) with the following dependent variables: indication for stenting (wide-neck aneurysm vs stenosis), location of stent (anterior circulation vs other), age >55 years, diabetes, hypertension, hypercholesterolemia, statin use, ACE-I use, and baseline glucose, creatinine, and platelet levels (n = 55)
| Unstandardized Coefficients |
Standardized Coefficients |
P Value | 95% CI for b |
||||
|---|---|---|---|---|---|---|---|
| b | Std. Error | b | t | Lower Bound | Upper Bound | ||
| (Constant) | 56.84 | 5.55 | 10.249 | .000 | 45.708 | 67.964 | |
| Diabetes mellitus | −26.83 | 10.61 | −0.319 | −2.529 | .015 | −48.128 | −5.540 |
| Age <55 years | −16.33 | 6.81 | −0.303 | −2.399 | .020 | −30.001 | −2.673 |
Note:—CI indicates confidence interval; Std. Error, standard error.
Discussion
Using point-of-care tests for platelet function, we observed that >50% of patients undergoing cerebrovascular stent placement might be low responders to clopidogrel and have inadequate platelet inhibition (defined as ≤40%). Clopidogrel resistance was unrelated to dose or timing of clopidogrel load before stent placement. Because no standardized definition of clopidogrel resistance exists, prior reported estimates of prevalence have varied greatly from 0% to 44%.7 By contrast, aspirin resistance or low response was inversely related to aspirin dosage but only occurred in 3 patients, all of whom were treated with 81 mg daily.
Demographic and medical factors that contributed to a low response to clopidogrel loading included older age (>55 years), diabetes, hypertension, hypercholesterolemia, location of stent placement, and baseline levels of glucose, creatinine, and platelets. In addition, medication interactions such as statin therapy and ACE-I use, were associated with an impaired antiplatelet effect of clopidogrel. In multivariable models, only diabetes and age older than 55 years were independently predictive of poor platelet inhibition in clopidogrel-treated patients.
This is the first study of clopidogrel and aspirin resistance among patients undergoing cerebrovascular stent placement and suggests that point-of-care aggregometry is feasible. Two prior small studies addressed aggregometry testing for abciximab during cerebrovascular stent placement.8,9 In coronary literature, there have been many reports of clopidogrel and aspirin resistance, with the finding that high poststent platelet reactivity may be a predictor of recurrent coronary events and stent thrombosis.6,10–13 In our study, we were unable to correlate percentage platelet inhibition to stent thrombosis, re-stenosis, or any clinical outcome. However, because follow-up management was not standardized, subsequent dosage and duration of antiplatelet drug therapy may have been influenced by aggregometry results at the time of stent placement.
Although we found no relationship between the dose or timing of clopidogrel administration and platelet inhibition, others have recently shown that higher dose clopidogrel loading (600 mg) can produce greater platelet inhibition in coronary patients.14–16 Differences in the studies may explain this discrepancy in results. The lack of loading with clopidogrel at the time of stent placement, methodologic differences, and varying sensitivities of aggregometry measurements may be responsible. These differences may also account for the relatively high rate of clopidogrel resistance in our patients. Furthermore, among cardiac patients, the addition of glycoprotein IIb/IIIa inhibitors may enhance the anti-platelet effects of clopidogrel alone.17 Higher dose clopidogrel regimens, peri-stent placement loading doses, and use of other adjunctive drugs have not been sufficiently tested in cerebrovascular patients and may be offset by concerns of intracranial hemorrhage.
The mechanisms of poor response to antiplatelet drug therapy have only recently been studied. Poor response to aspirin has been associated with inadequate dose, poor absorption, and noncompliance,18–20 though other intrinsic mechanisms may also exist.21,22 Regarding clopidogrel, poor response may be related to noncompliance, inadequate dosing or absorption,23 body mass index,24 genetic polymorphisms of cytochrome P450 3A4 and the P2Y12 receptor,25,26 and increased platelet activity related to an acute thrombotic event.27
We observed a significant association between older age and percentage platelet inhibition. Possible explanations are age-related decreases in drug absorption or in the activity of cytochrome P450 3A4, which is essential in the conversion of clopidogrel to its active form. In addition, drug-drug interactions, which are more common in the elderly, could impair its hepatic metabolism. The interaction of statins with clopidogrel has been recently suggested but still remains controversial.28,29 We were unable to substantiate an association between statin or other drug therapy and platelet inhibition on multivariable analysis.
There was a strong effect of diabetes on platelet activity. Prior studies have observed that patients with diabetes show increased platelet aggregation and activation and are more frequently aspirin and clopidogrel nonresponders than healthy patients,30,31 which may translate into increased ischemic event rates. There is accumulating evidence that platelet hyperactivity in patients with diabetes32 is mediated by insulin resistance and increased P2Y12 signaling.33 Other potential mechanisms include increased platelet turnover, altered platelet membrane structure, increased intracellular calcium, and abnormal glycation.32,34,35 A recent study suggested that 150-mg daily maintenance dosage of clopidogrel resulted in greater platelet inhibition than conventional 75-mg daily dosing in patients with diabetes.36
Our study was a consecutive series from a large academic center and the first of its type. Limitations of this study include its small sample size and nonrandom selection, which could lead to bias. The heterogeneity of the cohort is also a limitation, such that older patients with atherosclerotic disease differ in clinical profile from younger patients with wide-neck aneurysms. However, we adjusted for this possible confounder in the multivariable model by including age, atherosclerotic risk factors, and indication for stent placement. In addition, antiplatelet therapy, including the agents and doses, was not standardized in the study. There are, however, no clear guidelines regarding the appropriate choice and dosing for antiplatelet therapy in cerebrovascular stent placement.
Second, the study of accumetrics in clinical medicine is also limited by a lack of consensus on a standard definition of antiplatelet drug resistance. Furthermore, the assignment of a cutoff may be arbitrary because drug responsiveness is likely a continuous variable. Although we chose to adopt a previously defined cutoff (<40%) to report prevalence of clopidogrel low responders,5 we performed regression analyses by using percentage platelet inhibition as a continuous variable. Nevertheless, other studies have shown that a relative lack of platelet response based on pre- and poststent measurements, rather than a single absolute measurement, may be more predictive of clinically relevant outcomes.37 Third, the available aggregometry devices differ in sensitivity and reliability. The measurement of platelet inhibition by using the VerifyNow point-of-care system has shown excellent correlations with optical aggregometry (gold standard) for aspirin38 and clopidogrel,39 though P2Y12-independent pathways were not assessed by using this device. A final limitation is that the study was underpowered to detect effects of platelet activity on clinical outcomes.
Conclusions
Using a point-of-care platelet function test in patients undergoing cerebrovascular stent placement is feasible and may be a valuable tool in the prevention of stent-related complications. Given the potential consequences of in-stent thrombosis and restenosis, identifying those individuals with poor platelet inhibition to standard regimens may be of clinical importance and may help prevent cerebral ischemic events in this high-risk population. Our data suggest that older patients and those with diabetes mellitus are poor responders to clopidogrel and may require alternate approaches. Drawing from the extensive cardiology data, neurointerventional research should focus on the ideal doses, timing, choices, safety, and reliable measurement of antiplatelet drug therapy and should confirm the clinical relevance of aggregometry in cerebrovascular patients.
Footnotes
Abstract previously presented at: International Stroke Conference, February 20–21, 2007; San Francisco, Calif.
References
- 1.Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention). Circulation 2006;113:e166–286 [DOI] [PubMed] [Google Scholar]
- 2.SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke 2004;35:1388–92. Epub 2004 Apr 22 [DOI] [PubMed] [Google Scholar]
- 3.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the Wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke 2007;38:881–87. Epub 2007 Feb 8 [DOI] [PubMed] [Google Scholar]
- 4.Henkes H, Miloslavski E, Lowens S, et al. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology 2005;47:222–28 [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res 2005;115:101–08 [DOI] [PubMed] [Google Scholar]
- 6.Marcucci R, Paniccia R, Antonucci E, et al. Usefulness of aspirin resistance after percutaneous coronary intervention for acute myocardial infarction in predicting one-year major adverse coronary events. Am J Cardiol 2006;98:1156–59 [DOI] [PubMed] [Google Scholar]
- 7.Gurbel PA, Tantry US. Clopidogrel resistance? Thromb Res 2007;120:311–21 [DOI] [PubMed] [Google Scholar]
- 8.Lombard FW, Welsby IJ, Alexander MJ, et al. Thromboelastography detects inadequate response to abciximab therapy during stent-assisted cerebral aneurysm coil embolization complicated by stroke. Neurocrit Care 2006;4:32–34 [DOI] [PubMed] [Google Scholar]
- 9.Shenkman B, Schneiderman J, Tamarin I, et al. Testing the effect of GPIIb-IIIa antagonist in patients undergoing carotid stenting: correlation between standard aggregometry, flow cytometry and the cone and plate(let) analyzer (CPA) methods. Thromb Res 2001;102:311–17 [DOI] [PubMed] [Google Scholar]
- 10.Chen WH, Lee PY, Ng W, et al. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol 2004;43:1122–26 [DOI] [PubMed] [Google Scholar]
- 11.Gurbel PA, Lau WC, Bliden KP, et al. Clopidogrel resistance: implications for coronary stenting. Curr Pharm Des 2006;12:1261–69 [DOI] [PubMed] [Google Scholar]
- 12.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004;109:3171–75 [DOI] [PubMed] [Google Scholar]
- 13.Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005;46:1827–32 [DOI] [PubMed] [Google Scholar]
- 14.Gurbel PA, Bliden KP, Hayes KM, et al. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005;45:1392–96 [DOI] [PubMed] [Google Scholar]
- 15.Hochholzer W, Trenk D, Frundi D, et al. Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation 2005;111:2560–64 [DOI] [PubMed] [Google Scholar]
- 16.Kastrati A, von Beckerath N, Joost A, et al. Loading with 600 mg clopidogrel in patients with coronary artery disease with and without chronic clopidogrel therapy. Circulation 2004;110:1916–19 [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Bliden KP, Zaman KA, et al. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation 2005;111:1153–59. Epub 2005 Feb 28 [DOI] [PubMed] [Google Scholar]
- 18.Maree AO, Curtin RJ, Dooley M, et al. Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol 2005;46:1258–63 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz KA, Schwartz DE, Ghosheh K, et al. Compliance as a critical consideration in patients who appear to be resistant to aspirin after healing of myocardial infarction. Am J Cardiol 2005;95:973–75 [DOI] [PubMed] [Google Scholar]
- 20.Tantry US, Bliden KP, Gurbel PA. Resistance to antiplatelet drugs: current status and future research. Expert Opin Pharmacother 2005;6:2027–45 [DOI] [PubMed] [Google Scholar]
- 21.Macchi L, Christiaens L, Brabant S, et al. Resistance in vitro to low-dose aspirin is associated with platelet PlA1 (GP IIIa) polymorphism but not with C807T(GP Ia/IIa) and C-5T Kozak (GP Ibalpha) polymorphisms. J Am Coll Cardiol 2003;42:1115–19 [DOI] [PubMed] [Google Scholar]
- 22.Undas A, Brummel K, Musial J, et al. Pl(A2) polymorphism of beta(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation 2001;104:2666–72 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen T, Frishman WH, Nawarskas J, et al. Variability of response to clopidogrel: possible mechanisms and clinical implications. Cardiol Rev 2006;14:136–42 [DOI] [PubMed] [Google Scholar]
- 24.Feher G, Koltai K, Alkonyi B, et al. Clopidogrel resistance: role of body mass and concomitant medications. Int J Cardiol 2007;120:188–92 [DOI] [PubMed] [Google Scholar]
- 25.Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 2004;109:166–71 [DOI] [PubMed] [Google Scholar]
- 26.Taubert D, Kastrati A, Harlfinger S, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost 2004;92:311–16 [DOI] [PubMed] [Google Scholar]
- 27.Soffer D, Moussa I, Harjai KJ, et al. Impact of angina class on inhibition of platelet aggregation following clopidogrel loading in patients undergoing coronary intervention: do we need more aggressive dosing regimens in unstable angina? Catheter Cardiovasc Interv 2003;59:21–25 [DOI] [PubMed] [Google Scholar]
- 28.Neubauer H, Gunesdogan B, Hanefeld C, et al. Lipophilic statins interfere with the inhibitory effects of clopidogrel on platelet function: a flow cytometry study. Eur Heart J 2003;24:1744–49 [DOI] [PubMed] [Google Scholar]
- 29.Serebruany VL, Midei MG, Malinin AI, et al. Absence of interaction between atorvastatin or other statins and clopidogrel: results from the interaction study. Arch Intern Med 2004;164:2051–57 [DOI] [PubMed] [Google Scholar]
- 30.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005;54:2430–35 [DOI] [PubMed] [Google Scholar]
- 31.Watala C, Golanski J, Pluta J, et al. Reduced sensitivity of platelets from type 2 diabetic patients to acetylsalicylic acid (aspirin): its relation to metabolic control. Thromb Res 2004;113:101–13 [DOI] [PubMed] [Google Scholar]
- 32.Winocour PD, Watala C, Perry DW, et al. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost 1992;68:577–82 [PubMed] [Google Scholar]
- 33.Ferreira IA, Mocking AI, Feijge MA, et al. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2006;26:417–22 [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal Ca(2+) homeostasis in diabetes mellitus. Am J Physiol Heart Circ Physiol 2001;280:H1480–89 [DOI] [PubMed] [Google Scholar]
- 35.Tschoepe D, Roesen P, Esser J, et al. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemost 1991;17:433–38 [DOI] [PubMed] [Google Scholar]
- 36.Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007;115:708–16. Epub 2007 Jan 29 [DOI] [PubMed] [Google Scholar]
- 37.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol 2005;46:1820–26. Epub 2005 Oct 21 [DOI] [PubMed] [Google Scholar]
- 38.Wang JC, Aucoin-Barry D, Manuelian D, et al. Incidence of aspirin nonresponsiveness using the Ultegra Rapid Platelet Function Assay-ASA. Am J Cardiol 2003;92:1492–94 [DOI] [PubMed] [Google Scholar]
- 39.Malinin A, Pokov A, Swaim L, et al. Validation of a VerifyNow-P2Y12 cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol 2006;28:315–22 [DOI] [PubMed] [Google Scholar]