Abstract
BACKGROUND AND PURPOSE: Frontostriatal circuits involving the caudate nucleus have been implicated in frontotemporal lobar degeneration (FTLD). We assessed caudate nucleus volumetrics in FTLD and subtypes: frontotemporal dementia (FTD, n = 12), semantic dementia (SD, n = 13), and progressive nonfluent aphasia (PNFA, n = 9) in comparison with healthy controls (n = 27) and subjects with Alzheimer disease (AD, n = 19).
MATERIALS AND METHODS: Diagnoses were based on accepted clinical criteria. Manual volume measurement of the head and body of the caudate, excluding the tail, was conducted on T1-weighted brain MR imaging scans, using a published protocol, by a single analyst blinded to the diagnosis.
RESULTS: Paired t tests (P < .05) showed that the right caudate nucleus volume was significantly larger than the left in controls and PNFA. No hemispheric asymmetry was found in AD, FTD, and SD. Across the groups, there was a positive partial correlation between the left caudate nucleus volume and Mini-Mental State Examination (MMSE) scores (r = 0.393, n = 76, P = .001) with higher left caudate volumes associated with higher MMSE scores. Multivariate analysis of covariance was used to assess the statistical significance between the subject groups (AD, FTD, SD, PNFA, and controls) as independent variables and raw right/left caudate volumes at the within-subject level (covariates: age and intracranial volume; P < .05). Control volume was largest, followed by AD (93% of control volume), SD (92%), PNFA (79%), and FTD (75%).
CONCLUSIONS: Volume of the head and body of the caudate nucleus differs in subtypes of FTLD, due to differential frontostriatal dysfunction in subtypes being reflected in structural change in the caudate, and is correlated with cognition.
Frontotemporal lobar degeneration (FTLD), as currently conceptualized, is considered to comprise 3 main subtypes on the basis of clinical features: frontotemporal dementia (FTD), semantic dementia (SD), and progressive nonfluent aphasia (PNFA). FTLD is considered to comprise specific lesions of frontal, temporal, and associative neural pathways, with relative differences determining the clinical manifestations. Putatively, these subtypes should represent different neuropathologic processes. As in other neurodegenerative dementia processes, the neuropathology is progressive neuronal loss, manifesting as brain atrophy. Therefore, there has been recent interest in the pattern of brain atrophy as a method for aiding in the subtyping of FTLD. MR imaging has been used to examine the neuropathologic basis of FTLD in vivo.
Schroeter et al1 recently performed a quantitative meta-analysis of both structural and functional imaging of 267 subjects with FTLD and 351 controls. They demonstrated the existence of characteristic patterns of activation and atrophy for each of the subtypes of FTLD. Most structural imaging studies in the meta-analysis comprised relatively small numbers of each of the subtypes of FTLD, ranging from 6 to 18 subjects, whereas controls or comparators with Alzheimer disease (AD) were more numerous (20–64). The small groups raise issues about the robustness of the findings, which, of course, are pooled to assess significance in a meta-analysis. Nonetheless, some characteristic patterns of largely cortical activity and atrophy have been found. The regions implicated include the following: the prefrontal cortex, temporal lobe, amygdala, and caudate or lentiform nucleus.1 Thus, the proposed neuropathophysiology of FTLD may involve frontostriatal connections.
The striatum (caudate nucleus and putamen) serves as an entry point for afferent information from the periphery, as well as for afferents and efferents for functionally segregated regions of the cortex.2 Meta-analysis of functional magnetic imaging studies demonstrated connectivity implied by coactivation in the dorsolateral prefrontal cortex, anterior cingulate cortex, insula, and inferior frontal gyrus, all key cortical regions potentially implicated in FTLD.1,3 Using diffusion tensor tractography, Leh et al2 demonstrated the extensive nature of caudate nucleus connections with the prefrontal cortex, inferior and middle temporal gyrus, frontal eye fields, cerebellum, and thalamus.
Due to loss of afferent or efferent inputs from cortical atrophy, there may be resultant reduction in the volume of the striatum and, in particular, the caudate nucleus. Frequent and sometimes severe atrophy of the caudate was indeed noted long ago in FTLD.4–6 However, the observations were made on postmortem specimens from cases with, mostly, a long duration of illness. Measurements, when taken, were made on unrefined ordinal scales. Moreover, no attempt was made to correlate caudate atrophy with patterns of cortical atrophy. Given that subtypes of FTLD have different patterns of cortical atrophy, we hypothesized that there may be differential reduction in the volume of the caudate nucleus across the different subtypes of FTLD.
We aimed to use manual tracing of the caudate nucleus, the gold standard for volumetric measurement, by using a published reliable and valid protocol, to conduct robust between-group comparisons of caudate nucleus volume with reference to intracranial volume.7,8
Materials and Methods
Participants
Participants were recruited retrospectively from the Memory Clinic at the Karolinska University Hospital, Huddinge, Stockholm, Sweden. Routine dementia assessment was conducted in all participants. The study was approved by the local ethics committee.
The 80 subjects who participated were the following: 34 patients with FTLD (12 with FTD, 13 with SD, 9 with PNFA), 19 with AD, and 27 in a control group (Table 1).
Table 1:
Demographic features of patients and controls
| Control | FTD | SD | PNFA | AD | |
|---|---|---|---|---|---|
| No. | 27 | 12 | 13 | 9 | 19 |
| Sex (M:F) | 7:20 | 3:10 | 5:9 | 3:6 | 7:12 |
| Age (yr) | 61.1 (53–78) | 59.45 (42–72) | 63.77 (52–77) | 64.9 (57–78) | 61.8 (56–75) |
| MMSE | 28.7 (25–30) | 20.83 (10–30)* | 22.92 (5–29)* | 22.5 (15–28)† | 23.1 (7–29)* |
| Disease duration (yr) | – | 1.65 (0,25–3,4) | 3.90 (1,3–7,7)‡ | 3.56 (0,06–8,13) | 2.87 (0–4,97) |
Note:—FTD indicates frontotemporal dementia; SD, semantic dementia; PNFA, progressive nonfluent aphasia; AD, Alzheimer disease; MMSE, MIni-Mental State Examination.
*,†,‡ Kruskal-Wallis test.
P < .01 compared with controls.
P < .05 compared with controls.
P < 01 compared with FTD.
AD diagnoses were based on clinical criteria including the Diagnostic and Statistical Manual of Mental Disorders-IV-TR (text revision) and the International Classification of Diseases and Related Health Problems-10.9,10 Subjects with FTLD were diagnosed according to consensus diagnostic criteria for FTLD syndromes presented by Neary et al.11 Medical notes on all subjects included in this study were reviewed by an experienced neurologist (C.A.) to prevent inclusion of any wrongly diagnosed patient.
All subjects included in the studies went through the standard investigation procedure for patients referred to the memory clinic. The clinical diagnosis was determined at a multidisciplinary consensus conference with physicians, neuropsychologists, speech-language pathologists, and nurses.
The medical examination included information about history from a close informant, as well as assessment of somatic, neurologic, and psychiatric status. Laboratory investigation of blood, CSF, and urine (including vitamin B12 and folic acid levels and thyroid gland function) was performed. Neuroradiologic examination consisted of MR imaging of the brain and single-photon emission CT imaging of cerebral blood flow. Routine electroencephalography registration and neuropsychologic and speech-language examinations were performed.
The standard psychometric battery at the Memory Clinic included the following: Mini-Mental State Examination (MMSE)12; tests of immediate and delayed recall (Auditory-Verbal Learning Test, 196413; Complex Figure Test retention14,15; logical memory, Wechsler Memory Scale16; working memory, Wechsler Adult Intelligence Scale [WAIS] [WAIS Digit Span]17; visuomotor tracking and attention [Trail Making Test A and B; WAIS Digit Symbol])17,18; 2D and 3D figure reproduction15,19; 2D construction (WAIS Block Design)17; mental calculation19; general knowledge (WAIS Information)17; and conceptualization (WAIS Similarities).17
The speech-language assessment targeted motor speech function (sequential speech motion rate),20 picture naming (Boston Naming Test21 plus 10 “famous faces”), repetition,22 verb (action) fluency,23 noun (animal) fluency,24 and automatic-versus-controlled word sequence production (months forward and backward). Single-word reading was assessed to target alexia, whereas spelling to dictation and 2 cross-case transcription tasks were performed to assess agraphia.25 The Pyramid and Palm Trees Test was administered to check for associative agnosia.26 Spontaneous speech fluency was rated on the ordinal scale of the Western Aphasia Battery.27
FTLD
Clinical criteria for the subtypes of FTLD were based on international consensus criteria.11 The subtypes included the following: FTD, SD, and PNFA. Only patients with a primary degenerative cerebral process were selected, excluding patients with signs of cerebrovascular or systemic disorders. Patients with FTLD at different stages of the disease were included.
AD
Participants with AD displayed the development of multiple cognitive deficits, including memory impairment and 1 or more of aphasia, apraxia, or agnosia, plus disturbance in executive functioning. This presented as an illness of gradual onset, with continuing decline from previous levels of functioning. These symptoms were not due to another dementing process or psychiatric disorder.
Controls
The control group comprised individuals who were found, after careful assessment, not to fulfill criteria for FTLD, AD, or any other cognitive disorder but were sometimes forgetful in everyday life. Objective impairment was ruled out through comprehensive neuropsychologic assessment; impairment was defined as performance ≥1.5 SD units below the mean on any cognitive test. Accordingly, controls had no objective cognitive impairment by definition. To further minimize the risk of including participants with neurodegenerative disease in very early stages, we included only those participants who did not deteriorate over a minimum of 2 years of follow-up.
Imaging
Image Acquisition.
T1-weighted MR imaging scans were acquired on a 1.5T Magnetom Vision Plus scanner (Siemens Medical Systems, Erlangen, Germany). A 3D magnetization-prepared rapid acquisition of gradient echo pulse sequence (TR, 11.4 ms; TE, 4.4 ms; TI, 300 ms; flip angle, 10°; NEX, 1) was used to obtain 72 contiguous coronal 2.5-mm sections with a 512 × 144 matrix and 230-mm FOV.
Image Analysis.
Volumetric analysis was performed by using HERMES (Nuclear Diagnostics, Stockholm, Sweden). Preprocessing of imaging data was performed by interpolation of the images to render them orthogonal in orientation (cubic voxels), followed by alignment via automated rigid-body registration28 to a customized local reference brain (in anterior/posterior commissure orientation), for ease of tracing in the same orientation and format.
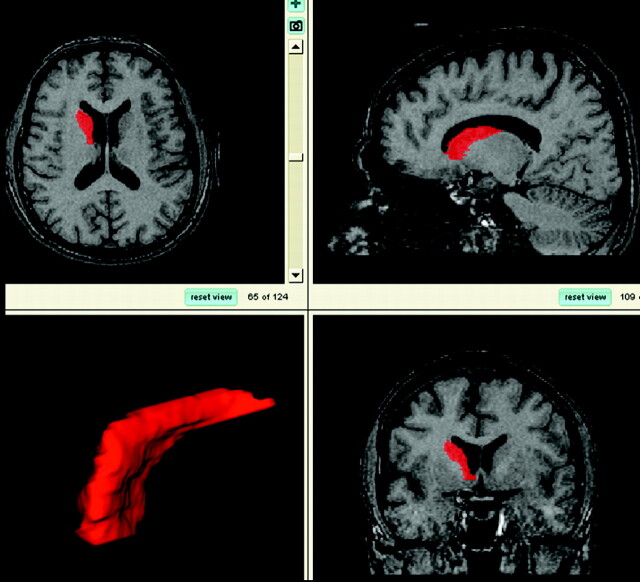
Using custom-designed software, Morphy-Display Scaled (designed by LS), 1 experienced tracer (J.C.L.L.) analyzed all brain MR imaging scans blinded to all clinical information (including diagnosis) as follows: On the basis of reference images, a standardized manual tracing protocol was used to trace and quantify the volume of the caudate via tracing its axial outline serially through successive images. All portions of the caudate nucleus were included until the tail curved ventrally to border the lateral atrium of the lateral ventricles; here it was excluded from measurements (Fig 1).7
Fig 1.
Views of the traced caudate nucleus and 3D reconstruction. Images from top left, clockwise: axial view, sagittal view, coronal view, and 3D reconstruction of the caudate.
Volumes obtained were assessed for covariance or normalized in reference to total intracranial volume (see below). Total intracranial volume (ICV) was measured as follows: ICV was traced on coronal sections by a stereologic point-counting technique manually tracing the intracranial volume. Every fourth section was traced. The starting point was randomly chosen between 1 and 4, the first sections at the most anterior end of the brain. The landmarks for delineation and protocol were based on those used by Eritaia et al.8
Reliability of image analysis was assessed by using intraclass correlations performed via Statistica (Statsoft Scandinavia, Uppsala, Sweden). The intrarater class correlation (ICC) 1-way single-measure reliability, was evaluated by repeating right and left caudate measurements on 10 scans (20 comparisons) and was 0.98.29 Inter-rater mean class correlation on 10 scans (20 comparisons) with another experienced tracer ICC (1,k) 1-way single-measure reliability, was 0.95.29
Statistical Analysis
Statistical analysis was performed by using the Statistical Package for the Social Sciences 15.0 (SPSS, Chicago, Ill). Paired t tests were used to assess hemispheric differences in caudate volume within subject groups with the significance level set at <.05. Partial correlation was used to explore the relationship between caudate nucleus volume and MMSE scores across all groups, while controlling for age and ICV. Four subjects had missing MMSE values and were excluded from analysis. Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity, and homoscedasticity. Multivariate analysis of covariance (MANCOVA) was used to test statistical significance between the subject groups (AD, FTD, SD, PNFA, and controls) as independent variables and raw right and left caudate volumes at the within-subject level. Using SPSS, we satisfied checks of assumption of normality, linearity, homogeneity of variances and regression slopes, and reliable measurements of covariates for the data, as a prerequisite for MANCOVA. Covariates used in the MANCOVA were age and intracranial volume. However, MMSE score was not included as a covariate in the MANCOVA because the number of missing values significantly reduced the sample of the PNFA (from 9 to 6) and SD (from 13 to 12) groups for the MANCOVA. The significance level was set at <.05.
Results
Demographic Data
Although not specifically age-matched, the groups did not differ significantly in mean age (Table 1). Similarly, MMSE scores were significantly different from those of controls, but not across the dementia diagnoses. Illness duration was significantly different for the SD group versus the FTD group.
Within-Group Comparisons of Hemispheric Caudate Volume
The results of the paired t tests within group comparisons, with strength of the significance measured as P and power measured as eta-squared, are summarized in Table 2. Within controls, there was hemispheric asymmetry of caudate nucleus volume with the right caudate nucleus volume significantly larger than the left at P = .000, eta-squared (the sum of squares between groups divided by total sum of squares) 0.441. Within the PNFA group, there was hemispheric asymmetry of caudate nucleus volume, with the right caudate nucleus volume significantly larger than the left at P = .003, eta-squared 0.668. Within the other groups of AD, FTD, and SD, no hemispheric asymmetry was found.
Table 2:
Within-group comparisons of hemispheric caudate nucleus volume*
| Groups | Caudate Volume |
t-Value | df | Sig | Eta-Squared | |||
|---|---|---|---|---|---|---|---|---|
| R (SD) | R (SEM) | L (SD) | L (SEM) | |||||
| C (n = 27) | 3.976 (0.435) | 0.083 | 3.772 (0.426) | 0.082 | 4.527 | 26 | .000† | .441 |
| AD (n = 19) | 3.674 (0.595) | 0.137 | 3.551 (0.487) | 0.112 | 1.219 | 18 | .239 | |
| FTD (n = 12) | 3.055 (0.811) | 0.234 | 2.819 (0.524) | 0.151 | 1.200 | 11 | .255 | |
| SD (n = 13) | 3.698 (0.565) | 0.157 | 3.503 (0.598) | 0.166 | 1.572 | 12 | .142 | |
| PNFA (n = 9) | 3.323 (0.601) | 0.200 | 2.695 (0.539) | 0.179 | 4.282 | 8 | .003† | .668 |
Note:—C indicates control; R, right; L, left; SD (header), standard deviation; df, degrees of freedom; Sig, significance (2-tailed); SD (column 1), semantic dementia; SEM, standard error of the mean; AD, Alzheimer disease; FTD, frontotemporal dementia; PNFA, progressive nonfluent aphasia; Eta-Squared, the sum of squares between groups divided by total sum of squares.
All volumes are in cubic centimeters.
Significant at P < .05.
Partial Correlations of MMSE Scores with Caudate Nucleus Volume across Groups
Across all diagnostic groups and controls combined, there was a medium positive partial correlation between left caudate nucleus volume and MMSE scores (r = 0.393, n = 76, P = .001), with higher left caudate volumes associated with higher MMSE scores. Inspection of the zero-order correlation (r = 0.395) showed that controlling for age and ICV had little effect on the strength of the relationship between the variables. The coefficient of determination was 0.154, with MMSE scores explaining 15% of the variance of left caudate volume. Across all diagnostic groups and controls combined, there was a nonsignificant small positive partial correlation between right caudate nucleus volume and MMSE scores (r = 0.163, n = 76, P = .165). The partial correlations by groups are summarized in Table 3.
Table 3:
Partial correlations between caudate volume and MMSE scores by subject group*
| Volume | Control (n = 27) | AD (n = 19) | FTD (n = 12) | SD (n = 12) | PNFA (n = 6) | ALL (n = 76) |
|---|---|---|---|---|---|---|
| R Caudate | ||||||
| r | 0.018 | 0.193 | −0.574 | −0.067 | −0.904 | 0.163 |
| Sig | 0.930 | 0.457 | 0.083 | 0.855 | 0.096 | 0.165 |
| L Caudate | ||||||
| r | 0.251 | 0.136 | 0.062 | 0.412 | 0.319 | 0.393 |
| Sig | 0.227 | 0.602 | 0.865 | 0.236 | 0.861 | 0.001† |
Note:—ALL indicates across all groups: control, AD, FTD, SD, PNFA; R, right; L, left; r, partial correlation value; Sig, 2-tailed significance for partial correlation value; AD, Alzheimer disease; FTD, frontotemporal dementia; SD, semantic dementia; PNFA, progressive nonfluent aphasia.
Partial correlation controlled for age and ICV.
Partial correlations significant at P < .05.
Between-Group Comparisons of Caudate Nucleus Volume
MANCOVA was conducted to assess the volume of the caudate nucleus (right and left) in relation to the diagnostic subject groups (n = 80) (Table 1). The results of the repeated-measures MANCOVA comparisons are summarized in Table 4.
Table 4:
AD comparison of MANCOVA results
| Corr. Model | Group No. | Depend | df | F | Sig. | Partial Eta-Squared | Observed Power | Covariates |
|---|---|---|---|---|---|---|---|---|
| AD Comparison | ||||||||
| Vs C | AD = 19, C = 27 | R | 3 | 2.824 | .050 | .168 | 0.637 | Age: 61.38 yr, ICV: 1401.93 |
| L | 3 | 3.893 | .015 | .218 | 0.789 | |||
| Vs FTD | AD = 19, FTD = 12 | R | 3 | 2.104 | .123 | .189 | 0.478 | Age: 60.86 yr, ICV: 1416.58 |
| L | 3 | 5.516 | .004 | .380 | 0.903 | |||
| Vs SD | AD = 19, SD = 13 | R | 3 | 1.588 | .214 | .145 | 0.371 | Age: 62.57 yr, ICV: 1424.31 |
| L | 3 | 1.173 | .338 | .112 | 0.280 | |||
| Vs PNFA | AD = 19, PNFA = 9 | R | 3 | 1.109 | .365 | .122 | 0.261 | Age: 62.77 yr, ICV: 1400.43 |
| L | 3 | 7.246 | .001 | .475 | 0.980 | |||
| FTLD Comparisons | ||||||||
| FTD vs C | FTD = 12, C = 27 | R | 3 | 9.787 | .000 | .455 | 0.478 | Age: 60.61 yr, ICV: 1402.59 |
| L | 3 | 14.492 | .000 | .554 | 0.903 | |||
| FTD vs SD | FTD = 12, SD = 13 | R | 3 | 2.301 | .107 | .247 | 0.499 | Age: 61.70 yr, ICV: 1431.60 |
| L | 3 | 3.146 | .047 | .310 | 0.644 | |||
| FTD vs PNFA | FTD = 12, PNFA = 9 | R | 3 | 0.664 | .586 | .105 | 0.160 | Age: 61.79 yr, ICV: 1401.14 |
| L | 3 | 0.741 | .542 | .116 | 0.175 | |||
| SD vs Control | FTD = 13, C = 27 | R | 3 | 3.436 | .027 | .223 | 0.724 | Age: 61.98 yr, ICV: 1409.12 |
| L | 3 | 2.834 | .052 | .191 | 0.631 | |||
| SD vs PNFA | SD = 13, PNFA = 9 | R | 3 | 1.970 | .155 | .247 | 0.422 | Age: 64.24 yr, ICV: 1413.09 |
| L | 3 | 4.040 | .023 | .402 | 0.279 | |||
| PNFA vs C | C = 27, PNFA = 9 | R | 3 | 9.479 | .000 | .471 | 0.993 | Age: 62.07 yr, ICV: 1388.86 |
| L | 3 | 18.153 | .000 | .630 | 1.000 |
Note:—Corr. Model indicates corrected model; Depend, dependent variable; df, degrees of freedom; F, F-statistic; Sig, significance; C, controls; R, right; L, left; FTD, frontotemporal dementia; SD, semantic dementia; PNFA, progressive nonfluent aphasia; ICV, total intracranial volume; Eta-Squared, the sum of squares between groups divided by total sum of squares.
AD Comparisons with FTLD Subgroups
There was a significant difference in raw left caudate nucleus volume between subjects with AD compared with controls: F(3,45) = 3.893, P = .015 (P < .05), partial eta-squared = 0.218. Right caudate nucleus volume approached a significant difference between AD and controls: F(3,45) = 2.824, P = .050, eta-squared = 0.168. There was a significant difference in raw left caudate nucleus volume between subjects with AD compared with FTD: F(3,30) = 5.516, P = .004 (P < .05), eta-squared = 0.380. There was a significant difference in raw left caudate nucleus volume between subjects with AD compared with PNFA: F(3,27) = 7.246, P = .001 (P < .05), eta-squared = 0.475.
FTLD Comparisons between Subgroups and with Controls
There was a significant difference in raw right caudate nucleus volume between subjects with FTD compared with controls: F(3,38) = 9.787, P = .000 (P < .05), eta-squared = 0.455. Left caudate nucleus volume was significantly different between FTD and controls F(3,38) = 14.492, P = .000, eta-squared = 0.554. Right caudate nucleus volume was significantly different between SD and controls: F(3,39) = 2.834, P = .027, eta-squared = 0.223. There was a significant difference in raw right caudate nucleus volume between subjects with PNFA compared with controls: F(3,35) = 9.479, P = .000 (P < .05), eta-squared = 0.471. Left caudate nucleus volume was significantly different between PNFA and controls F(3,35) = 18.153, P = .000, eta-squared = 0.630. There was a significant difference in raw left caudate nucleus volume between subjects with FTD compared with SD: F(3,24) = 3.146, P = .047 (P < .05), with a moderate-effect-sized eta-squared = 0.310. There was a significant difference in raw left caudate nucleus volume between subjects with PNFA compared with SD: F(3,21) = 4.040, P = .023 (P < .05), eta-squared = 0.420.
Comparison of Corrected Mean Caudate Volumes
The results of the group MANCOVA and mean caudate volumes for each group are displayed in Tables 5 and 6. Across the 5 diagnostic groups, both right and left caudate volumes were significantly different. There was a significant difference in raw right caudate nucleus volume between subjects by diagnosis (AD, control, FTD, PNFA, and SD): F(6,79) = 5.229, P = .000 (P < .05), eta-squared = 0.301. Left caudate nucleus volume was also significantly different between diagnostic groups: F(6,79) = 10.159, P = .000, eta-squared = 0.464.
Table 5:
Group MANCOVA*
| Corr. Model | Group No. | Depend | df | F | Sig. | Partial Eta- Squared | Observed Power | Covariates |
|---|---|---|---|---|---|---|---|---|
| Group | AD = 19, C = 27, FTD = 12, PNFA = 9, SD = 13 | R | 6 | 5.229 | .000 | .301 | 0.992 | Age: 61.88 yr, ICV: 1408.00 |
| L | 6 | 10.159 | .000 | .464 | 1.000 |
Note:—Corr. Model indicates corrected model; Depend, dependent variable; R, right; L, left; C, control; df, degrees of freedom; F, F-statistic; Sig, significance; AD, Alzheimer disease; FTD, frontotemporal dementia; PNFA, progressive nonfluent aphasia; SD, semantic dementia; ICV, total intracranial volume; Eta-Squared, the sum of squares between groups divided by total sum of squares.
Dependent factors: R and L caudate volume; independent factors: group: AD, control, FTD, PNFA, SD.
Table 6:
Estimated marginal means (from MANCOVA, Table 5)
| Dependent Variable: Caudate Volume | Group | Mean | Percentage Control | Std Error | 95% Confidence Intervals |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Right (cm3) | ||||||
| C | 3.982 | 100 | 0.109 | 3.764 | 4.200 | |
| AD | 3.666 | 92 | 0.130 | 3.407 | 3.925 | |
| SD | 3.685 | 92 | 0.159 | 3.369 | 4.002 | |
| PNFA | 3.402 | 85 | 0.193 | 3.019 | 3.786 | |
| FTD | 3.007 | 76 | 0.166 | 2.677 | 3.338 | |
| Left (cm3) | ||||||
| C | 3.785 | 100 | 0.093 | 3.599 | 3.970 | |
| AD | 3.544 | 94 | 0.110 | 3.324 | 3.764 | |
| SD | 3.476 | 92 | 0.135 | 3.207 | 3.745 | |
| PNFA | 2.754 | 73 | 0.164 | 2.428 | 3.080 | |
| FTD | 2.788 | 74 | 0.141 | 2.507 | 3.069 | |
Note:—Std Error indicates standard error of mean; Percentage Control, percentage of control volume; C, control; AD, Alzheimer disease; SD, semantic dementia; PNFA, progressive nonfluent aphasia; FTD, frontotemporal dementia.
MANCOVA corrected mean raw caudate volumes in the various clinical groups are displayed as a percentage of control caudate volumes in Table 6. The AD group was largest in mean volume of the diagnostic groups (93% of control volume), followed by the SD group (92%), the PNFA group (79%), and finally, the FTD group (75%).
Discussion
We have demonstrated that volume of the head and body of the caudate nucleus differs significantly and substantially (ranging from 93% to 75% of control caudate volumes) in subtypes of FTLD. We also found that caudate nucleus volume was correlated with cognition measured via MMSE score, with lower volume correlating with poorer cognition. This finding complements previous meta-analyses showing functional and structural change in the caudate within subtypes of FTLD as well as in cortical regions.1
Krishnamoorthy30 has postulated that increase in volume of brain structures associated with emotion, such as the amygdala, may reflect either a predilection for emotional reactivity or plasticity and growth of the structure due to hyperactivity. The corollary is that neurodegenerative processes may result in atrophy and underactivity of the structure. Therefore, the caudate may be reduced in volume due to underactivity and/or degeneration as part of the FTLD process. That smaller caudate nucleus volume was correlated with lower cognitive scores across the entire sample supports the possibility that cognitive dysfunction in FTLD may be based on such structural change.
Frontotemporal dementia is characterized by emotional and behavioral disturbances considered to arise from neuropathology involving frontal cognitive dysfunction, which may involve frontostriatal circuits.31–33 Frontostriatal circuits are crucial in human behavior and cognition, constituting significant neurodevelopmentally distinct pathways.34–36 These 5 circuits share commonalities in cytoarchitecture, with 3 circuits primarily arising from the prefrontal cortex serving roles in executive cognitive function and emotional and behavior regulation.35 The architecture of the 3 prefrontal circuits comprises the following: origin from the relevant prefrontal region, via the caudate or nucleus accumbens, via the globus pallidus, via the thalamus, and, thence, feedback to the prefrontal cortex. In summary, these are fronto-striato-pallido-thalamo-cortical circuits.
The dorsolateral prefrontal circuit (DLPFC) mediates problem solving, verbal/nonverbal fluency, and retrieval from memory and is linked to the limbic memory system. Clinical syndromes associated with DLPFC dysfunction are described as executive dysfunction and involve such higher order cognition. The orbitofrontal circuit (OFC) mediates inhibition and impulse control. Clinical syndromes associated with OFC dysfunction are described as emotional and social dysfunction and are characterized by deficits in social judgment and impulse control. The anterior cingulate (or ventromedial prefrontal) circuit (ACC) mediates motivation and initiation of behavior. Clinical syndromes associated with ACC dysfunction are described as apathy and loss of motivation, including akinetic mutism, and are characterized by a lack of motivation.35–37 Such clinical syndromes are seen in FTLD and may be due to atrophy in the relevant cortical regions as well as circuit dysfunction.31–33
Differential volumetrics of the caudate in subtypes of FTLD may be based on the degree of involvement of the caudate in the neuropathophysiology of the subtype. The key is the crucial role played by the caudate as a relay in all frontostriatal circuits associated with human behavior.34–36 Dysfunction in such circuits may be due to, or the result of, disruptions of connections or structures, including atrophy of afferents or efferents.35,38 Thus dysfunction can be reflected in, or due to, structural change in the circuit. Volumetric loss or atrophy in the caudate may result in, or be due to, emotional or cognitive dysfunction based on cortical inputs found in FTLD and its subtypes. As the degree of emotional or cognitive dysfunction due to frontostriatal pathology differs in subtypes, so may the volume of structures involved in the circuits vary, especially the caudate.
Frontostriatal dysfunction was not prominent in AD and was not present in controls. Accordingly, there was no significant difference in the volume of the caudate between these groups; and overall, these volumes should be the largest due to lack of involvement of the caudate in putative neuropathophysiology. The distinction between the FTLD subgroups is based on the relative degree of frontostriatal dysfunction, hence involvement of the caudate as a relay within frontostriatal circuits. The FTD group should have the greatest dysfunction due to greater involvement of frontostriatal pathology32; and it has, overall, the smallest caudate volume (75% of controls). Intermediate between these extremes are interposed the SD and PNFA groups. SD may involve less frontostriatal dysfunction because it clinically resembles AD and thus is not significantly different from AD (93% of controls) with respect to caudate volume (92% of controls). PNFA may display more frontostriatal dysfunction and more involvement of the caudate and thus is not significantly different from FTD with respect to caudate volume (79% of controls).
Previous studies have found that the right caudate nucleus is larger than the left in healthy controls, as was found in this study.39–41 However, hemispheric asymmetry was not present in any of the disease groups (AD, FTD, SD), except for the PNFA group. Absence of asymmetry may be due to the small size of the disease groups relative to the hemispheric comparisons within groups. The presence of between-group differences in only the left caudate nucleus volume, with the exception of comparisons with controls, suggests a possible lateralized neuropathologic process within the FTLD subtypes.
One limitation of this study is the use of a subjective-memory-complaint cohort as the controls. As a counterpoint, this group was comprehensively assessed for objective cognitive dysfunction, and those with objective changes were excluded. Given the exigencies to recruit subtypes of FTLD, AD, and controls, age and sex matching were not possible; however, apart from duration of illness and MMSE scores, there were no other significant differences between groups. There was also a preponderance of women in all groups studied. Adjustments were made via the MANCOVA for the covariates age and ICV, but not for MMSE, due to missing values for this variable. The reliability and validity of our manual tracing protocol has been independently peer-reviewed and published.7 Although we acknowledge that automated tracing protocols may have greater reliability, greater validity is achieved with expert observer tracing.7 Morphologic (shape)-related changes may be significant and are the subject of other related work in this sample.
The clinical implications of our findings relate to the neuropathophysiology of FTLD and the functional significance of the caudate nucleus. Decreased caudate volume is associated with poorer cognition and may serve as an indicator for dysfunction in associated structures, including frontostriatal circuits. The caudate nucleus shows a gradient of atrophy in FTLD correlating with the relative degree of frontostriatal dysfunction. Given this finding, further exploration of the structural and functional integrity of frontostriatal circuits as a substrate for cognitive and behavioral change is warranted.
Conclusion
We have found that the volume of the head and body of the caudate nucleus (excluding the tail) is significantly different across subtypes of FTLD. This may be due to relative differences in frontostriatal circuit dysfunction in the subtypes of FTLD, being reflected in structural change in a key relay in the circuits, the caudate nucleus. Caudate nucleus volume is correlated with cognition as assessed by MMSE scores across AD, FTLD, and controls, further supporting a role for the caudate in cognition.
Further studies on cortical regions in the same sample are in preparation. It will be interesting to explore the interrelationship of the caudate volume to cortical regions in subtypes of FTLD, morphologic changes via shape analysis, and correlations with neuropsychologic and behavioral features.
Footnotes
J.C.L.L. designed the study, developed the protocols, performed all caudate measurements and statistical analysis, and wrote the first draft of the paper. O.L. performed inter-rater reliability measurements and assisted with volumetric data extraction and analysis and stereologic intracranial volume measurements. B.B.Z. assisted with volumetric data extraction and analysis and stereologic intracranial volume measurements. P.Ö. performed clinical assessments for diagnosis of FTLD subtypes. C.A. performed neurologic assessments for diagnosis of FTLD subtypes. L.B. assisted with preprocessing of imaging data. L.S. wrote the software used in volumetric measurement. L.-O.W was the principal investigator. All authors contributed to the editing of the paper.
J.C.L. Looi gratefully acknowledges the Canberra Hospital Specialists Private Practice Trust Fund and ACT Health for leave, access to travel, accommodation, and incidentals support for the duration of this project. P. Östberg's contribution was supported by a grant from the Gun and Bertil Stohne Foundation.
References
- 1.Schroeter ML, Razcka K, Neumann J, et al. Towards a nosology for frontotemporal lobar degenerations: a meta-analysis involving 267 subjects. Neuroimage 2007;36:497–510 [DOI] [PubMed] [Google Scholar]
- 2.Leh SE, Ptito A, Chakravaty MM, et al. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett 2007;419:113–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 2007;16:1508–21 [DOI] [PubMed] [Google Scholar]
- 4.von Braunmühl A, Picksche K. In: Bumke O, ed. Handbuch der Geisteskrankheiten. Vol. 11. Part VII. Berlin, Germany: Springer-Verlag;1930. :673–715
- 5.von Bagh K. Klinische und pathologisch-anatomische Studien an 30 Fällen systematischer umschriebener Atrophie der Grosshirnrinde (Pickscher Krankheit): Annales Academiae Scientiarum Fennicae—Series A. V. Medica Anthropologica Helsinki. Helsinki, Finland: Suomalaisen Tiedeakatemia;1946
- 6.Lüers T, Spatz H. Picksche Krankheit (Progressive Umschriebene Groβhirnatrophie.). In: Lubarsch O, Rössle F, Henke F, eds. Handbuch der speziellen pathologischen anatomie und histologie. Vol. 13. Part 1A. Berlin, Germany: Springer-Verlag;1957. :614–715
- 7.Looi JCL, Lindberg O, Liberg B, et al. Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry Res 2008;163:279–88 [DOI] [PubMed] [Google Scholar]
- 8.Eritaia J, Wood SJ, Stewart GW, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med 2000;44:973–77 [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association;2000
- 10.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th Revision. Version for 2007. Available at: http://www.who.int/classifications/apps/icd/icd10online/. Accessed January 11,2007
- 11.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54 [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 13.Rey A. L'Examen Clinique en Psychologie. Paris, France: Presses Universitaires de France;1964
- 14.Rey A. L'examen psychologique dans les d'encephalopatie traumatique. Archives de Psychologie 1941;28:286–340 [Google Scholar]
- 15.Osterrieth PA. Le test de copie d'une figure complexe. Archives de Psychologie 1944;30:206–356 [Google Scholar]
- 16.Wechsler D. Wechsler Memory Scale: Manual. 3rd ed. San Antonio: The Psychological Corporation;1997
- 17.Wechsler D. Wechsler Adult Intelligence Scale, III. San Antonio: The Psychological Corporation;1997
- 18.Reitan RM. Validity of the Trail Making Test as an indicator of organic damage. Percept Mot Skills 1958;8:271–76 [Google Scholar]
- 19.Christensen AL. Luria's Neuropsychological Investigation. 2nd ed. Copenhagen, Denmark: Munksgaard;1979
- 20.Kent RD, Kent JF, Rosenbek JC. Maximum performance tests of speech production. J Speech Hear Disord 1987;52:367–87 [DOI] [PubMed] [Google Scholar]
- 21.Goodglass H, Kaplan E. The Boston Naming Test. Philadelphia: Lippincott Williams & Wilkins;2000
- 22.Lindström E, Werner C. A-ning: Neurolingvistisk Afasiundersökning. Stockholm, Sweden: Ersta utbildningsinstitut;1995
- 23.Piatt AL, Fields JA, Paolo AM, et al. Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J Clin Exp Neuropsychol 1999;21:435–43 [DOI] [PubMed] [Google Scholar]
- 24.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Feiberger;1983
- 25.Hughes JC, Graham N, Patterson K, et al. Dysgraphia in mild dementia of Alzheimer's type. Neuropsychologia 1997;35:533–45 [DOI] [PubMed] [Google Scholar]
- 26.Howard D, Patterson K. The Pyramids and Palm Trees Test. Bury St. Edmunds, UK: Thames Valley Test Company;1992
- 27.Kertesz A. The Western Aphasia Battery. San Antonio: The Psychological Corporation;1982
- 28.Woods RP, Grafton ST, Holmes CJ, et al. Automated image registration. I. General methods and intrasubject, intramodality validation. J Comput Assisted Tomogr 1998;22:139–52 [DOI] [PubMed] [Google Scholar]
- 29.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–28 [DOI] [PubMed] [Google Scholar]
- 30.Krishnamoorthy E. A differential role for the hippocampus and amygdala in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry 2007;78:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tekin S, Cummings JL. Frontal–subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 2002;53:647–54 [DOI] [PubMed] [Google Scholar]
- 32.Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol 2005;4:771–80 [DOI] [PubMed] [Google Scholar]
- 33.Levy B, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006;16:916–28 [DOI] [PubMed] [Google Scholar]
- 34.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–81 [DOI] [PubMed] [Google Scholar]
- 35.Cummings JL. Frontal subcortical circuits and human behavior. Arch Neurol 1993;5:873–80 [DOI] [PubMed] [Google Scholar]
- 36.Andrewes DG. Neuropsychology: From Theory to Practice. East Sussex, UK: Psychology Press;2001
- 37.Barker-Collo S, McCarthy D. Neuropsychological Assessment. In: Feigin VL, Bennett DA. eds. Handbook of Clinical Neuroepidemiology. New York: Nova Science;2007. :621–48
- 38.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 1995;20:91–127 [DOI] [PubMed] [Google Scholar]
- 39.Raz N, Torres IJ, Acker JD. Age, gender, and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiol Learn Mem 1995;63:133–42 [DOI] [PubMed] [Google Scholar]
- 40.Ifthikharuddin SF, Shrier DA, Numaguchi Y, et al. 2000: MR volumetric analysis of the human basal ganglia: normative data. Acad Radiol 2000;7:627–34 [DOI] [PubMed] [Google Scholar]
- 41.Looi JCL, Maller JM, Pagani M, et al. Caudate volumes in public transportation workers exposed to trauma in the Stockholm train system. Psychiatry Res Neuroimage In press [DOI] [PubMed]