Abstract
BACKGROUND AND PURPOSE: Perfusion imaging sequences are an important part of imaging studies designed to provide information to guide therapy for treatment of cerebrovascular disease. The purpose of this study was to perform a meta-analysis of the medical literature on perfusion imaging to determine its role in clinical decision making for patients with acute cerebral ischemia.
MATERIALS AND METHODS: We searched MEDLINE by using a strategy that combined terms related to perfusion imaging with terms related to acute cerebral ischemia and brain tumors. We identified 658 perfusion imaging articles and classified them according to the clinical usefulness criteria of Thornbury and Fryback. We found 59 articles with promise of indicating usefulness in clinical decision making. We devised and implemented a clinical decision making scoring scale more appropriate to the topic of acute cerebral ischemia.
RESULTS: Several articles provided important insights into the physiologic processes underlying acute cerebral ischemia by correlation of initial perfusion imaging deficits with clinical outcome or ultimate size of the infarct. However, most articles showed relatively low relevance to influencing decisions in implementing treatment.
CONCLUSION: Most perfusion imaging articles are oriented toward important topics such as optimization of imaging parameters, determination of ischemia penumbra, and prediction of outcome. However, information as to the role of perfusion imaging in clinical decision making is lacking. Studies are needed to demonstrate that use of perfusion imaging changes outcome of patients with acute cerebral ischemia.
This study is the culmination of work performed in response to a request for applications submitted by the Neuroradiology Education and Research Fund of the American Society of Neuroradiology (ASNR) for a systematic review of the medical literature regarding the usefulness of perfusion imaging in neuroradiology. The authors were charged with the task of examining the evidence for a substantive role for perfusion imaging in evaluation of the brain with a focus on understanding the place of perfusion imaging in medical decision making. We found that most published work has focused on 2 subjects, cerebrovascular disease and brain neoplasms, and 2 techniques, CT perfusion imaging and MR perfusion imaging. This study presents the results of our meta-analysis solely with regard to cerebrovascular disease. The results of the meta-analysis of perfusion imaging in imaging of brain tumors will be provided in a separate report.
The goal of the meta-analysis was to determine the extent to which perfusion imaging figures affect clinical decision making and influence patient outcomes. It is important to note that the goal was not to establish whether perfusion imaging yields important information that advances our understanding of the physiologic processes of cerebral infarction or whether perfusion imaging might provide data that could determine likelihood of success of stroke therapy.
Materials and Methods
Search of the Medical Literature
We performed this study from January 2006 to December 2006. We searched MEDLINE by using a search strategy that combined terms related to perfusion scanning with those related to acute cerebral ischemia and brain tumors. The procedural concept included the medical subject heading (MeSH) terms: MR angiography, MR imaging, diffusion MR imaging and tomography, x-ray computed, regional blood flow, or cerebrovascular circulation. Because there is no precise MeSH term for perfusion imaging, we empirically developed a list of phrases used to describe perfusion imaging and used these terms as text string searches. We implemented the acute cerebral ischemia disease concept using MeSH terms cerebrovascular accident or ischemic attack, transient, hypoxia-ischemia, brain, and searching for the text string acute cerebral ischemia. During the peer review and revision of this manuscript, we updated the literature search using the same search terms through March 2008, selected relevant citations, and added 2 articles.
Review of Abstracts
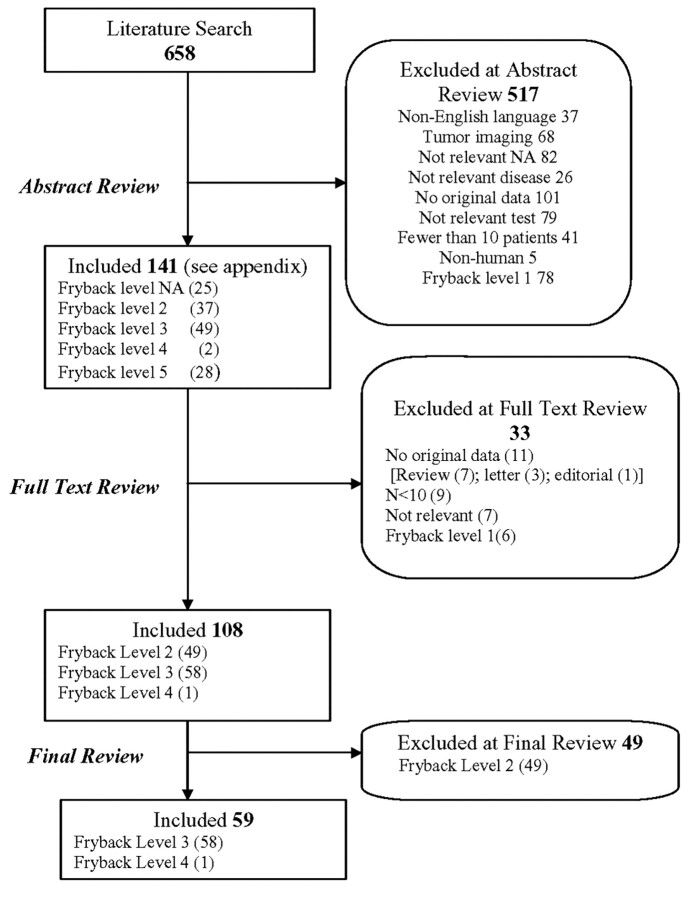
After obtaining a list of 658 articles that were potentially relevant, we performed an initial review of abstracts using 2 pairs of raters (Fig 1). We first identified and removed non-English language articles (n = 37). We next deleted 68 studies on tumor as well as articles that were otherwise not relevant (n = 82) or were not related to acute cerebrovascular disease (n = 26). Other reasons for excluding articles on the basis of solely review of abstracts included lack of original data (including review articles; n = 101), imaging techniques that did not evaluate perfusion or a perfusion technique that is not in widespread use for stroke imaging (eg, single-photon emission CT) (n = 79), study population of less than 10 patients (n = 41), and nonhuman studies (n = 5). Then, still on the basis of abstracts alone, we classified articles according to the Fryback and Thornbury hierarchy.1 This hierarchy is a well-accepted 6-tiered model that ranks diagnostic assessment studies, according to the concept of effectiveness, into the following levels: level 1, technical efficacy; level 2, diagnostic accuracy efficacy; level 3, diagnostic thinking efficacy; level 4, therapeutic efficacy; level 5, patient outcome efficacy; and level 6, societal efficacy. When more than 1 of the 6 categories could be assigned, we chose the highest category.
Fig 1.
Flowchart depicting our review of the literature.
Fryback Criteria for Articles on Cerebrovascular Disease
To understand application of the Fryback criteria to perfusion imaging articles about acute cerebral ischemia, it is worth providing examples to illustrate each category. A study that simply assessed the technical quality of images (eg, signal-to-noise ratio of MR perfusion images) would be an example of a Fryback level 1 study. An example of a Fryback level 2 study is one that correlates the presence or absence of neurologic deficits with the presence and location of perfusion imaging deficit. A study in which perfusion imaging changed the probable diagnosis in patients is an example of an article in the Fryback level 3 category. An example of a Fryback level 4 article is one that shows that patients with a mismatch between perfusion imaging deficits and diffusion-weighted imaging (DWI) deficits were given thrombolytic therapy more often than those in whom there was no mismatch. A Fryback level 5 article exemplifies patients who underwent perfusion imaging who were shown to have a better clinical outcome (and not just a more accurate diagnosis or more appropriate treatment). Societal efficacy (Fryback level 6) might be demonstrated by showing that the marginal cost of perfusion imaging results in reduced disability.
A tally of the Fryback classification of abstracts was as follows: 78 citations for level 1, 37 citations for level 2, 49 citations for level 3, 2 citations for level 4, 28 for level 5, and none for level 6 (Table 1). We excluded level 1 articles on the belief that they would not provide information valuable for the measurement of patient outcomes (Fig 1). These Fryback assignments were preliminary because they were based solely on review of abstracts and not on the entire article. This finding brought the total of articles excluded during the abstract review process to 517, with 141 articles remaining for review of the entire article (as opposed to review of solely the abstract).
Table 1.
Initial (abstract) and final (full-text) consensus Fryback level assignments
| Fryback Level | Abstract Screen (no.) | Full-Text Review Consensus Reassignment | Change (no.) | Final (no.) |
|---|---|---|---|---|
| 1 | 78 | Not further reviewed | +6 | 84 |
| 2 | 37 | 5 excluded | +12 | 49 |
| 1 reassigned to level 1 | ||||
| 6 reassigned to level 3 | ||||
| 3 | 49 | 12 excluded | +9 | 58 |
| 1 reassigned to level 1 | ||||
| 19 reassigned to level 2 | ||||
| 1 reassigned to level 4 | ||||
| 4 | 2 | Both reassigned to level 3 | −1 | 1 |
| 5 | 28 | 2 excluded | −28 | 0 |
| 5 reassigned to level 2 | ||||
| 21 reassigned to level 3 | ||||
| 6 | 0 | None | 0 | 0 |
| NA* | 25 | 8 excluded | −25 | 0 |
| 4 assigned to level 1 | ||||
| 13 assigned to level 3 |
Additional articles reviewed during final manuscript preparation; not initially assigned.
Review of Full Text
We next read the full text of 141 articles, which consisted of 116 included from abstract review and another 25 published in 2007 and 2008 identified in an update search. A list of these articles can be found in the appendix that accompanies the on-line version of this article. Each article was re-evaluated according to the inclusion and exclusion criteria described in Fig 1, and we reached a consensus, through group discussion, on the final Fryback level assignment (Table 1). These decisions are described in Fig 1 and Table 1. At the conclusion of the full-text review of all articles, 49 had been scored as Fryback level 2, 58 as Fryback level 3, and 1 scored as Fryback level 4 (Tables 1 and 2). The 49 Fryback level 2 articles were removed from further consideration because their effect on clinical decision making was deemed not of sufficient importance. We then examined the remaining 59 articles (Table 2) in greater detail with regard to their usefulness in clinical decision making.
Table 2.
Categories used to classify articles according to degree of influence on decision making
| Category | |
|---|---|
| 1 | Perfusion used to guide treatment |
| 2 | Predict clinical outcome (suggestive evidence that PWI can help triage patients) |
| 3 | Predict final infarct size |
| 4 | Determine effects of therapy on perfusion abnormalities |
| 5 | Characterize infarct types or to better understand infarct-like processes |
| 6 | As a comparison with DWI but no outcome measure |
| 7 | Compare perfusion with an imaging technique other than MR |
| 8 | Solely as an entry criterion |
| 9 | Although perfusion data were recorded, analysis of perfusion data was not a goal of the study |
Note:—PWI indicates perfusion-weighted imaging; DWI, diffusion-weighted imaging.
Development of a New Classification Scheme
We encountered many issues that highlighted the difficulty in classifying radiology articles according to the Fryback criteria, which are outlined in the Discussion. Therefore, we proposed new categories into which articles could be classified, which were more descriptive of uses of perfusion imaging in the articles studied and more flexible than the Fryback classification. These categories allowed us to render the subject matter into terms that were more familiar to the radiologic community and provide a more specific set of descriptors of the clinical import of the perfusion imaging component.
Results
During our initial review of articles, we found that the authors often did not explain the role of perfusion imaging, requiring the reader to infer this role after careful reading. Furthermore, after analysis, we found that perfusion imaging was used in 9 major ways. On that basis, we divided articles on acute cerebral ischemia into 9 categories on the basis of how perfusion imaging was used (Table 2), reflecting a wide variety of uses of perfusion imaging. The categories are stratified according to likelihood that perfusion imaging would influence decision making, with category 1 having the highest degree of influence on clinical decision making.
Explanation of Categories of Articles with Use of Perfusion Imaging for Acute Cerebral Ischemia
Category 1 consisted of articles in which perfusion imaging was used to prospectively guide therapy.2 In such studies, we expected that physicians would obtain perfusion imaging data before making a treatment decision and use the data (alone or with DWI) to choose a treatment. In an optimal setting, such a study would not only use perfusion imaging to decide treatment but would also show that decisions made by use of perfusion imaging data provided better outcomes than decisions made without perfusion imaging data.
Articles in categories 2 and 3 have less influence on decision making than those in category 1. Articles in category 2 correlated perfusion imaging findings with clinical outcome,3–28 and those in category 3 compared perfusion imaging findings with final size of the infarct on subsequent imaging studies.5,6,14,19,29–44 Articles in category 2 and category 3 served the dual functions of validating perfusion imaging as a test and providing information that could be used for prognosis but not use of perfusion imaging in decision making.
The category 4 articles were defined as those that showed the effect of therapy on perfusion imaging deficits but did not attempt to correlate such changes with patient outcome (category 2) or final size of the infarct (category 3).45,46 Articles in category 4 shed more light on the effects of thrombolytic therapy than on a role for use of perfusion imaging in medical decision making.
Categories 5 to 7 included articles about the physiologic aspects of infarcts but did not provide correlation of perfusion imaging with an outcome. Articles in category 5 primarily described perfusion imaging to characterize infarct types or to better understand infarcts.47–52 Those in category 6 compared the size of perfusion imaging deficits with the size of simultaneous abnormalities on DWI.53,54 Articles in category 7 compared CT and MR perfusion imaging results with perfusion imaging results obtained by use of other techniques.55,56
Articles in categories 8 and 9 had the lowest effect on clinical decision making. Category 8 consisted of a single article in which perfusion imaging is used solely as an entry criterion for a study.57 Articles in category 9 were those in which perfusion imaging was recorded but analysis of perfusion imaging data was not a goal of the study.58–60
Representative Articles from Acute Cerebral Ischemia Perfusion Imaging Categories
Articles in category 1.
We found only 1 article that we deemed appropriate for category 1.2 The study, although retrospective, provided a potential prospective role for use of perfusion imaging in decision-making in the setting of acute cerebral ischemia. The investigators retrospectively analyzed perfusion versus diffusion imaging differences in 62 patients with acute cerebral ischemia; in 16 of these patients, large perfusion imaging abnormalities were seen, but DWI abnormalities were either absent or very small. Furthermore, in 15 patients, the perfusion imaging finding was the only imaging evidence for acute ischemia. The authors did not specifically state that perfusion imaging was actually used in the cases to make clinical decisions; however, they noted that if DWI and conventional MR imaging findings alone had been used to guide treatment, those 16 patients might have been inappropriately treated, raising a possible venue in which the perfusion imaging findings might be used to guide treatment. However, this conclusion is open to debate; neurologists commonly provide thrombolytic therapy to patients on the basis of clinical findings, even in the presence of a DWI study with normal results. In such centers, the decision to administer thrombolytic therapy is more heavily weighted by clinical findings than by DWI and perfusion imaging findings.
Articles in category 2.
In this category, perfusion imaging was used (alone or in conjunction with other tests) to predict patient outcome. The 26 articles in this category are listed in on-line Table 1.3–28 Two approaches have been used with regard to predictors. First, some studies have used serial perfusion imaging during the first 1 to 4 days after acute cerebral ischemia, in which the change in perfusion imaging (as evidence of reperfusion) was used as the predictor of later clinical outcome. Some of these studies were prospective clinical trials of thrombolytic treatment in which all patients had evidence of perfusion-diffusion mismatch at entry.3,9,10 Because the perfusion imaging data were serial in nature, by definition they were retrospectively analyzed after therapy was administered. Thus, although the studies were prospective, only the first perfusion imaging dataset could have influenced decision making. Other studies in category 2 were solely retrospective ones; as such, the role of initial perfusion imaging data in directing treatment decisions at the time of patient presentation was not described.7,15,17,20,23,26,27 These studies provide support that a perfusion-diffusion mismatch, on occasion, can be valuable information but do not prove that such information must be obtained before therapy or that it changes patient outcome. In actual fact, perfusion imaging data are not usually available to treating physicians, and so its actual impact on clinical decision making is limited.
Second, some studies used perfusion imaging data at the time of initial presentation of acute cerebral ischemia to predict later clinical outcome.3,8,11–13,16,18,21,22,24,25,28 Such studies describe the size of perfusion imaging deficit, perfusion-diffusion mismatch, and size of DWI deficit. In some cases, studies compared the predictive ability of different parameters in an attempt to identify the strongest predictors of later clinical outcome. Three studies evaluated the independent association in a multivariable model and found that the size of the initial DWI abnormality was a better predictor than the size of perfusion imaging abnormality or perfusion-diffusion mismatch to predict later neurologic outcome.8,16,23
Studies of both types support the contention that the physiologic changes depicted on perfusion imaging studies are related to clinical outcome. However, in the clinical setting, treating physicians are likely to be more interested in using perfusion imaging techniques to determine the presence or absence of a perfusion-diffusion mismatch rather than to predict outcome. As such, the studies in category 2, although validating perfusion imaging as an imaging technique, show clinical efficacy of perfusion imaging solely if one accepts a perfusion-diffusion mismatch as an indication for therapy. However, from the standpoint of actual efficacy assessment, because the articles in category 2 do not compare patient outcomes against patients who did not undergo perfusion imaging, clinical efficacy is not shown in these articles.
Articles in category 3.
The common feature of articles in category 3 was the correlation of perfusion imaging data primarily with final infarct size or lesion growth on subsequent imaging studies. The 20 articles in this category are listed in on-line Table 2.5,6,14,19,29–44 In aggregate, these articles suggest that hemodynamic parameters can show regions of brain tissue that are ischemic and likely to progress to infarction. Although this information helps one to understand the physiologic changes in ischemic tissue and may provide some insights into conversion of ischemic (and potentially salvageable) regions into infarcted tissue, this information is of limited use in deciding on treatment in individual cases. Stated differently, it is not clear what added value perfusion imaging would offer in such circumstances.
Articles in category 4.
The 2 articles45,46 in category 4 differ from those in categories 2 and 3 because, though they perform serial perfusion imaging scans, they do not correlate serial perfusion imaging findings with clinical outcome or final infarct size. Nonetheless, articles in this category do provide information about the effect of therapy on regions that are abnormal on perfusion imaging (and therefore presumably ischemic). In 1 such article, perfusion imaging abnormalities on studies after therapy were considerably smaller than on initial imaging, indicating that (at least by imaging criteria) therapy can provide substantial reduction in ischemic deficits.45 Another article described perfusion imaging changes on serial imaging studies in a group treated with intravenous tissue plasminogen activator (tPA) and an untreated control group.46 The size of the perfusion imaging deficit was measured 5 times: at baseline, 3 other times in the first week, and again at 30 days. In this article, both treated and untreated patients had high rates of perfusion imaging normalization, thereby not showing a clear superiority of intravenous-tPA compared with no therapy.
Discussion
As mentioned in the Materials and Methods section, we found several difficulties in applying the Fryback criteria to the medical literature on perfusion imaging. These difficulties can be summarized as follows. First, few articles had the classic study designs (eg, sensitivity and specificity estimates) usually found in articles categorized by use of Fryback criteria. Second, most articles were not designed to study an outcome, such as a clinical end point. Unfortunately, most the studies were not designed to show how patient outcome was improved by the diagnostic information. Finally, depending on one's perspective, an article could be designated into more than 1 Fryback category. As a result of these difficulties, we designed and implemented a novel classification scheme (outlined in Materials and Methods) that seemed to be much more appropriate for consideration of perfusion articles according to the concept of effectiveness.
Perfusion Imaging for Assessment of Cerebral Ischemia
In general, we found 3 levels of evidence that indicate that perfusion imaging is valuable for the evaluation of cerebral ischemia. First, we found evidence that a perfusion-diffusion mismatch correlates with poor clinical outcome.8,24 In itself, this finding does not indicate that perfusion imaging has a high impact on decision making. Second, on a higher level of impact on efficacy, we found evidence that treatment of large perfusion-diffusion imaging mismatches results in improved clinical outcome compared with untreated patients.3,9,10 On a third level, we found evidence that perfusion imaging provides information not available from other techniques (eg, by showing abnormalities not detectable on DWI).2,20 However, we did not find strong evidence in the published literature that addresses the issue of whether perfusion imaging has influenced the number of patients who would receive thrombolytic agents in any single trial. Nonetheless, an examination of clinical practice patterns would suggest that decisions about administration of thrombolytic agents are frequently made without perfusion imaging and that, commonly, perfusion imaging does not change clinical decisions. In fact, in most stroke centers, such decisions are routinely made without perfusion imaging.
We found a large number of studies in which perfusion imaging was used in some manner in an acute cerebral ischemia treatment algorithm.3,9,10,14 From the standpoint of efficacy assessment, it is important to distinguish articles in which perfusion imaging is an entry criterion from those in which the benefits of perfusion imaging in decision making are actually evaluated. The use of perfusion imaging as an entry criterion for acute cerebral ischemia trials may indeed be appropriate, but this practice assigns, rather than shows, a critically important role of perfusion imaging in decision making. To our knowledge, no study that prospectively assesses the efficacy of perfusion imaging in decision making in assessment of cerebral ischemia has been performed.
One of the major difficulties we encountered in our literature review is that the exact role of perfusion imaging in a study was often vague and was rarely reported as a hypothesis or explicitly stated in the Materials and Methods. Instead, typically patients were observed by use of 1 or more imaging techniques (including perfusion imaging), and the reader was left to interpret the role that perfusion imaging played in the study. Furthermore, studies were typically retrospective, and it was sometimes unclear whether perfusion imaging was used to guide treatment in any way or if (as was often the case) perfusion imaging results were simply compared with clinical or other imaging parameters in a post hoc manner.
Response of Perfusion Imaging Ischemic Deficits to Therapy
With regard to whether thrombolytic therapy produces decreases in perfusion imaging deficits, a few articles are especially relevant.3,9,10 Nonetheless, the exact importance of perfusion imaging in the treatment scheme in these articles is open to debate. These articles do not ask whether a beneficial outcome could have been reached without perfusion imaging. Stated differently, many of the articles in our category 2 can be taken as evidence that 1) thrombolytic therapy is effective in limiting infarct size and improving clinical outcome in selected cases3,9,10,17,20,24,26 and 2) patients with large perfusion imaging volumes tend to have a poorer outcome than those with a smaller perfusion imaging volume.8,24 Despite these important findings, these articles do not show that perfusion imaging has been used in a prospective manner to alter patient outcomes.
Gaps in the Medical Literature on Perfusion Imaging in Acute Cerebral Ischemia
We found systematic flaws in previous trials in the use of perfusion imaging for assessment of acute cerebral ischemia. First, we found no trials comparing patients undergoing perfusion imaging and those not undergoing perfusion imaging, which makes determining the added benefit of perfusion imaging difficult. Second, in many studies, it was not clear whether perfusion imaging analysis was done at the time of decision making or afterward. Third, acute cerebral ischemia imaging is frequently performed in a time-sensitive manner, and the time taken to perform perfusion imaging, analyze perfusion imaging data, and communicate results could delay treatment decisions. Given these concerns, it would seem important to record the additional time needed for perfusion imaging, as some authors have suggested.54 Unfortunately, this feature is rarely recorded in perfusion imaging articles.
The most convincing role of perfusion imaging we found is prediction of final infarct size. For instance, Warach et al14 indicated that the size of an initial perfusion imaging abnormality correlated most strongly with change (ie, growth) in infarct size. Although this information is of some usefulness in predicting outcome in individual patients, it does not allow one to triage patients to different treatment strategies. In other words, we know of no studies that have shown a better outcome in patients treated on the basis of a DWI-perfusion imaging mismatch compared with those who were treated on the basis of DWI alone. Sunshine et al2 showed that some perfusion imaging scans showed abnormalities in patients in whom no DWI abnormality was seen. However, these patients already had clinical symptoms of ischemia, raising a question of the added value of perfusion imaging to clinical decision making. Nonetheless, this single citation is the closest we have to an article showing that perfusion imaging provides additive information in the triage of patients for therapy.
In retrospect, it is clear that the development of perfusion imaging for acute cerebral ischemia could have been better focused to provide information that is useful for clinical decision making. Instead, topics such as optimization of imaging protocols; determination of appropriate hemodynamic parameters; and correlation with clinical outcome, subsequent infarct size, and other imaging techniques have been explored. This lack of focus is, in part, responsible for the fact that, even after more than a decade, the use of perfusion imaging to assess cerebral ischemia in daily clinical practice is open to dispute, and it has not been deemed of enough significance to be incorporated into many major position papers on the topic. Conducting perfusion imaging trials with use of the guidelines of an efficacy assessment early on might have resolved the issues that are still faced.
Recommendations for Future Perfusion Imaging Trials for Acute Cerebral Ischemia
Most articles we examined did not include a study design in which the efficacy of perfusion imaging could be fully assessed. We now describe some study paradigms that could meet the needs for an adequate efficacy assessment. At this point, it is worth considering whether one should even develop studies to determine the efficacy of perfusion imaging in an assessment of patients with acute cerebral ischemia. One's stance toward this question is largely determined by one's attitude toward the state of acceptance of a perfusion-diffusion mismatch as a clinical parameter for treatment. If one takes the stance that such a mismatch can now be accepted as a valid factor determining the need to provide thrombolytic therapy, given that it is now an entry criterion for many ischemia therapy trials, then the efficacy of perfusion imaging in this clinical context may require no further evaluation.
If one takes the position that, despite use of perfusion imaging as an entry criterion for cerebral ischemia trials, further determination of efficacy is needed, then several possible studies are reasonable. One possibility would be to (either prospectively or retrospectively) determine what clinical decisions would be made without perfusion imaging and whether they differ from decisions that would have been made had perfusion imaging data been available. If the decisions substantially differed on the basis of whether perfusion imaging data were available or unavailable, then it would be safe to assume that perfusion imaging played a substantial role in determining therapy. Another method would be to randomly assign patients to a group in which perfusion imaging is performed (and used in triaging patients to a therapeutic regimen) or a group in which perfusion imaging is not performed. However, it is clear that such a paradigm would not likely be prospectively performed in future studies. Many treating physicians believe that the accumulated evidence of the importance of a DWI- perfusion imaging mismatch is well established enough (despite its limitations) that it would be unethical to randomize patients to not undergo perfusion imaging.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the encouragement and generous support provided for the study.
Footnotes
This study was funded by the Neuroradiology Education and Research Fund of the American Society of Neuroradiology.
Indicates article with supplemental on-line tables and appendix.
References
- 1.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Medical Decision Making 1991;11:88–94 [DOI] [PubMed] [Google Scholar]
- 2.Sunshine JL, Bambakidis N, Tarr RW, et al. Benefits of perfusion MR imaging relative to diffusion MR imaging in the diagnosis and treatment of hyperacute stroke. AJNR Am J Neuroradiol 2001;22:915–21 [PMC free article] [PubMed] [Google Scholar]
- 3.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–17 [DOI] [PubMed] [Google Scholar]
- 4.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008;7:299–309 [DOI] [PubMed] [Google Scholar]
- 5.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 2001;32:2021–28 [DOI] [PubMed] [Google Scholar]
- 6.Wintermark M, Reichhart M, Thiran J-P, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol 2002;51:417–32 [DOI] [PubMed] [Google Scholar]
- 7.Chalela JA, Alsop DC, Gonzalez-Atavales JB, et al. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke 2000;31:680–87 [DOI] [PubMed] [Google Scholar]
- 8.Derex L, Nighoghossian N, Hermier M, et al. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci 2004;225:3–9 [DOI] [PubMed] [Google Scholar]
- 9.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 2006;37:1227–31 [DOI] [PubMed] [Google Scholar]
- 10.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73 [DOI] [PubMed] [Google Scholar]
- 11.Kluytmans M, van Everdingen KJ, Kappelle LJ, et al. Prognostic value of perfusion- and diffusion-weighted MR imaging in first 3 days of stroke. European Radiol 2000;10:1434–41 [DOI] [PubMed] [Google Scholar]
- 12.Perkiö J, Soinne L, Ostergaard L, et al. Abnormal intravoxel cerebral blood flow heterogeneity in human ischemic stroke determined by dynamic susceptibility contrast magnetic resonance imaging. Stroke 2005;36:44–49 [DOI] [PubMed] [Google Scholar]
- 13.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53–59 [DOI] [PubMed] [Google Scholar]
- 14.Warach S, Pettigrew LC, Dashe JF, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol 2000;48:713–22 [PubMed] [Google Scholar]
- 15.Schellinger PD, Jansen O, Fiebach JB, et al. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke 2000;31:1318–28 [DOI] [PubMed] [Google Scholar]
- 16.Arenillas JF, Rovira A, Molina CA, et al. Prediction of early neurological deterioration using diffusion- and perfusion-weighted imaging in hyperacute middle cerebral artery ischemic stroke. Stroke 2002;33:2197–203 [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Parsons MW, Desmond PM, et al. The use of PWI and DWI measures in the design of “proof-of-concept” stroke trials. J Neuroimaging 2004;14:123–32 [PubMed] [Google Scholar]
- 18.Bruening R, Dichgans M, Berchtenbreiter C, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: decrease in regional cerebral blood volume in hyperintense subcortical lesions inversely correlates with disability and cognitive performance. AJNR Am J Neuroradiol 2001;22:1268–74 [PMC free article] [PubMed] [Google Scholar]
- 19.Hermier M, Nighoghossian N, Adeleine P, et al. Early magnetic resonance imaging prediction of arterial recanalization and late infarct volume in acute carotid artery stroke. J Cereb Blood Flow Metab 2003;23:240–48 [DOI] [PubMed] [Google Scholar]
- 20.Hillis AE, Wityk RJ, Beauchamp NJ, et al. Perfusion-weighted MRI as a marker of response to treatment in acute and subacute stroke. Neuroradiology 2004;46:31–39 [DOI] [PubMed] [Google Scholar]
- 21.Hillis AE, Wityk RJ, Tuffiash E, et al. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol 2001;50:561–66 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Karonen JO, Vanninen RL, et al. Acute ischemic stroke: predictive value of 2D phase-contrast MR angiography—serial study with combined diffusion and perfusion MR imaging. Radiology 2004;231:517–27 [DOI] [PubMed] [Google Scholar]
- 23.Nighoghossian N, Hermier M, Adeleine P, et al. Baseline magnetic resonance imaging parameters and stroke outcome in patients treated by intravenous tissue plasminogen activator. Stroke 2003;34:458–63 [DOI] [PubMed] [Google Scholar]
- 24.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol 2002;51:28–37 [DOI] [PubMed] [Google Scholar]
- 25.Rohl L, Geday J, Ostergaard L, et al. Correlation between diffusion- and perfusion-weighted MRI and neurological deficit measured by the Scandinavian Stroke Scale and Barthel Index in hyperacute subcortical stroke (< or = 6 hours). Cerebrovasc Dis 2001;12:203–13 [DOI] [PubMed] [Google Scholar]
- 26.Singer OC, Du Mesnil De Rochemont R, Foerch C, et al. Early functional recovery and the fate of the diffusion/perfusion mismatch in patients with proximal middle cerebral artery occlusion. Cerebrovasc Dis 2004;17:13–20 [DOI] [PubMed] [Google Scholar]
- 27.Uno M, Harada M, Yoneda K, et al. Can diffusion- and perfusion-weighted magnetic resonance imaging evaluate the efficacy of acute thrombolysis in patients with internal carotid artery or middle cerebral artery occlusion? Neurosurgery 2002;50:28–34 [DOI] [PubMed] [Google Scholar]
- 28.Yamada M, Yoshimura S, Kaku Y, et al. Prediction of neurologic deterioration in patients with lacunar infarction in the territory of the lenticulostriate artery using perfusion CT. AJNR Am J Neuroradiol 2004;25:402–08 [PMC free article] [PubMed] [Google Scholar]
- 29.Alsop DC, Makovetskaya E, Kumar S, et al. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke 2005;36:746–50 [DOI] [PubMed] [Google Scholar]
- 30.Eastwood JD, Lev MH, Azhari T, et al. CT perfusion scanning with deconvolution analysis: pilot study in patients with acute middle cerebral artery stroke. Radiology 2002;222:227–36 [DOI] [PubMed] [Google Scholar]
- 31.Kim EY, Na DG, Kim SS, et al. Prediction of hemorrhagic transformation in acute ischemic stroke: role of diffusion-weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol 2005;26:1050–55 [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy BD, Fox AJ, Lee DH, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 2006;37:1771–77 [DOI] [PubMed] [Google Scholar]
- 33.Schaefer PW, Ozsunar Y, He J, et al. Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol 2003;24:436–43 [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer PW, Roccatagliata L, Ledezma C, et al. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. AJNR Am J Neuroradiol 2006;27:20–25 [PMC free article] [PubMed] [Google Scholar]
- 35.Tan JC, Dillon WP, Liu S, et al. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007;61:533–43 [DOI] [PubMed] [Google Scholar]
- 36.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979–85 [DOI] [PubMed] [Google Scholar]
- 37.Wu O, Christensen S, Hjort N, et al. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using MRI. Brain 2006;129:2384–93 [DOI] [PubMed] [Google Scholar]
- 38.Butcher K, Parsons M, Baird T, et al. Perfusion thresholds in acute stroke thrombolysis. Stroke 2003;34:2159–64 [DOI] [PubMed] [Google Scholar]
- 39.Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours’ duration. Stroke 2004;35:1652–58 [DOI] [PubMed] [Google Scholar]
- 40.Thomalla GJ, Kucinski T, Schoder V, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke 2003;34:1892–99 [DOI] [PubMed] [Google Scholar]
- 41.Fiehler J, von Bezold M, Kucinski T, et al. Cerebral blood flow predicts lesion growth in acute stroke patients. Stroke 2002;33:2421–25 [DOI] [PubMed] [Google Scholar]
- 42.Grandin CB, Duprez TP, Smith AM, et al. Which MR-derived perfusion parameters are the best predictors of infarct growth in hyperacute stroke? Comparative study between relative and quantitative measurements. Radiology 2002;223:361–70 [DOI] [PubMed] [Google Scholar]
- 43.Schaefer PW, Hunter GJ, He J, et al. Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. AJNR Am J Neuroradiol 2002;23:1785–94 [PMC free article] [PubMed] [Google Scholar]
- 44.Shih LC, Saver JL, Alger JR, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003;34:1425–30 [DOI] [PubMed] [Google Scholar]
- 45.Lee S-K, Kim DI, Jeong E-K, et al. Postoperative evaluation of Moyamoya disease with perfusion-weighted MR imaging: initial experience. AJNR Am J Neuroradiol 2003;24:741–47 [PMC free article] [PubMed] [Google Scholar]
- 46.Marks MP, Tong DC, Beaulieu C, et al. Evaluation of early reperfusion and I.V. tPA therapy using diffusion- and perfusion-weighted MRI. Neurology 1999;52:1792–98 [DOI] [PubMed] [Google Scholar]
- 47.Allder SJ, Moody AR, Martel AL, et al. Differences in the diagnostic accuracy of acute stroke clinical subtypes defined by multimodal magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003;74:886–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerraty RP, Parsons MW, Barber PA, et al. Examining the lacunar hypothesis with diffusion and perfusion magnetic resonance imaging. Stroke 2002;33:2019–24 [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Shin T, Park JH, et al. Various patterns of perfusion-weighted MR imaging and MR angiographic findings in hyperacute ischemic stroke. AJNR Am J Neuroradiol 1999;20:613–20 [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Karonen JO, Vanninen RL, et al. Detecting the subregion proceeding to infarction in hypoperfused cerebral tissue: a study with diffusion and perfusion weighted MRI. Neuroradiology 2003;45:345–51 [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Laakso MP, Karonen JO, et al. Apolipoprotein E polymorphism and acute ischemic stroke: a diffusion- and perfusion-weighted magnetic resonance imaging study. J Cereb Blood Flow Metab 2002;22:1336–42 [DOI] [PubMed] [Google Scholar]
- 52.Liu Y-J, Chen C-Y, Chung H-W, et al. Neuronal damage after ischemic injury in the middle cerebral arterial territory: deep watershed versus territorial infarction at MR perfusion and spectroscopic imaging. Radiology 2003;229:366–74 [DOI] [PubMed] [Google Scholar]
- 53.Fiehler J, Knudsen K, Kucinski T, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke 2004;35:514–19 [DOI] [PubMed] [Google Scholar]
- 54.Sunshine JL, Tarr RW, Lanzieri CF, et al. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 1999;212:325–32 [DOI] [PubMed] [Google Scholar]
- 55.Karonen JO, Vanninen RL, Liu Y, et al. Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke. Ischemic penumbra predicts infarct growth. Stroke 1999;30:1583–90 [DOI] [PubMed] [Google Scholar]
- 56.Na DG, Ryoo JW, Lee KH, et al. Multiphasic perfusion computed tomography in hyperacute ischemic stroke: comparison with diffusion and perfusion magnetic resonance imaging. J Comput Assist Tomogr 2003;27:194–206 [DOI] [PubMed] [Google Scholar]
- 57.Ribo M, Molina CA, Rovira A, et al. Safety and efficacy of intravenous tissue plasminogen activator stroke treatment in the 3- to 6-hour window using multimodal transcranial Doppler/MRI selection protocol. Stroke 2005;36:602–06 [DOI] [PubMed] [Google Scholar]
- 58.Finnigan SP, Rose SE, Walsh M, et al. Correlation of quantitative EEG in acute ischemic stroke with 30-day NIHSS score: comparison with diffusion and perfusion MRI. Stroke 2004;35:899–903 [DOI] [PubMed] [Google Scholar]
- 59.Hillis AE, Wityk RJ, Barker PB, et al. Change in perfusion in acute nondominant hemisphere stroke may be better estimated by tests of hemispatial neglect than by the National Institutes of Health Stroke Scale. Stroke 2003;34:2392–96 [DOI] [PubMed] [Google Scholar]
- 60.Neumann-Haefelin T, du Mesnil de Rochemont R, Fiebach JB, et al. Effect of incomplete (spontaneous and postthrombolytic) recanalization after middle cerebral artery occlusion: a magnetic resonance imaging study. Stroke 2004;35:109–14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.