Abstract
BACKGROUND AND PURPOSE: Although the prognostic value of perfusion MR imaging in various gliomas has been investigated, that in high-grade astrocytomas alone has not been fully evaluated. The purpose of this study was to evaluate retrospectively whether the tumor maximum relative cerebral blood volume (rCBV) on pretreatment perfusion MR imaging is of prognostic value in patients with high-grade astrocytoma.
MATERIALS AND METHODS: Between January 1999 and December 2002, 49 patients (30 men, 19 women; age range, 23–76 years) with supratentorial high-grade astrocytoma underwent MR imaging before the inception of treatment. The patient age, sex, symptom duration, neurologic function, mental status, Karnofsky Performance Scale, extent of surgery, histopathologic diagnosis, tumor component enhancement, and maximum rCBV were assessed to identify factors affecting survival. Kaplan-Meier survival curves, the logrank test, and the multivariate Cox proportional hazards model were used to evaluate prognostic factors.
RESULTS: The maximum rCBV was significantly higher in the 31 patients with glioblastoma multiforme than in the 18 with anaplastic astrocytoma (P < .03). The 2-year overall survival rate was 67% for 27 patients with a low (≤2.3) and 9% for 22 patients with a high (>2.3) maximum rCBV value (P < .001). Independent important prognostic factors were the histologic diagnosis (hazard ratio = 9.707; 95% confidence interval (CI), 3.163–29.788), maximum rCBV (4.739; 95% CI, 1.950–11.518), extent of surgery (2.692; 95% CI, 1.196–6.061), and sex (2.632; 95% CI, 1.153–6.010).
CONCLUSION: The maximum rCBV at pretreatment perfusion MR imaging is a useful clinical prognostic biomarker for survival in patients with high-grade astrocytoma.
High-grade astrocytomas (WHO class III or IV) are the most common primary neoplasms of the central nervous system; patients with this disease have a poor prognosis.1–3 The median survival is approximately 3 years for anaplastic astrocytoma (AA) and 1 year for glioblastoma multiforme (GBM).1–5 The prognosis of these tumors varies from patient to patient despite identical histopathologic diagnoses; the reasons for this difference are not fully understood.
Although histopathologic assessment remains the reference standard for determining the glioma grade, sampling error, large range of WHO classification and grading systems, inter- and intrapathologist variability, and difference in biology within WHO grades of gliomas may result in inadequate evaluation of the entire tumor.6–9 Thus, for a final brain tumor diagnosis, imaging studies may provide information in addition to the pathologic assessment.
Perfusion MR imaging yields physiologic information on in vivo tumor neovascularity and angiogenesis in terms of the relative cerebral blood volume (rCBV).10–14 Tumor angiogenesis is an important factor that defines the biologic aggressiveness of astrocytomas,15 and perfusion MR imaging may provide a means for characterizing the vascularity of gliomas, thereby overcoming some of the limitations of histopathologic evaluation. Perfusion MR imaging has been used to grade gliomas, and because higher vascularity corresponds to a higher tumor grade, high-grade gliomas can be expected to manifest high rCBV values.10–13 Although the prognostic value of perfusion MR imaging in various gliomas has been investigated,16–20 that in high-grade astrocytomas alone has not been fully evaluated. Our hypothesis was that the rCBV of the tumor seen on pretreatment perfusion MR imaging is of prognostic value in patients with high-grade astrocytoma. The purpose of our study was to verify the hypothesis by using the MR imaging data of our institution and long-term follow-up results.
Materials and Methods
Prior written informed consent for routine MR imaging studies and treatment had been obtained from all patients. Our retrospective study was approved by the institutional review board of our hospital; the requirement for informed patient consent was waived. To protect patient privacy, we removed all identifiers from our records at the completion of our analysis.
Study Population
Between January 1999 and December 2002, 67 patients with newly diagnosed high-grade supratentorial gliomas underwent postoperative radiation therapy in conjunction with chemotherapy. Of these, 49 (30 men, 19 women; age range, 23–76 years; mean, 56 years) had supratentorial high-grade astrocytomas and fulfilled our inclusion criteria: 1) a histopathologic diagnosis of AA (class III) or GBM (class IV) according to WHO criteria; 2) absence of other previous or concurrent malignant diseases; 3) availability of digital pretreatment MR imaging data, including perfusion MR imaging, for review; 4) the presence of solid tumor components available for rCBV analysis; 5) no corticosteroid administration before MR imaging examination; and 6) the absence of gross blood products or infratentorial tumor components adversely affecting rCBV analysis. We excluded 18 patients: 14 had histologically identified oligodendroglial components. In 2, digital data from pretreatment perfusion MR imaging were unavailable, and 2 presented with MR imaging evidence of intratumoral hemorrhage.
Two authors (R.M., Y.H.) reviewed the patients’ medical records. On the basis of the tumor location and the patient's performance status, a surgical option, resection or biopsy, was chosen by the neurosurgeons (J.-i.K., H.N.). The tumor was resected to the greatest extent possible in all patients. Histopathologic diagnoses were based on WHO criteria and were reached consensually by 2 neuropathologists (J.-i.K., K.I., with 27 and 25 years of experience, respectively) who were blinded to the MR imaging data. All 49 patients underwent postoperative external-beam radiation therapy. Our protocol for the treatment of high-grade astrocytoma consists of 60 Gy of radiation for patients with GBM and 54 Gy for patients with AA, administered by way of conventional fractionation (ie, 2 Gy daily for 5 consecutive days, in conjunction with 3D conformal treatment planning). The extent of the radiation field was determined by conventional MR imaging findings. The initial field for both tumors was defined as the peritumoral edema +2 cm and was prescribed 40 Gy. The boost field for GBM and AA was defined as the resection cavity and residual solid tumor +2 cm and was prescribed 60 Gy and 54 Gy, respectively. Nitrosourea-based chemotherapy (procarbazine/1-[4-amino-2-methyl-5-pyrimidinyl]-methyl-3-[2-chloroethyl]-3-nitrosourea/ vincristine) was administered concurrently with radiation therapy.
All patients were followed for the evaluation of tumor control after postoperative radiation therapy by neurosurgeons. Follow-up included physical, neurologic, and MR imaging examinations. If tumor recurrence was documented, salvage surgery, additional chemotherapy, and/or additional radiation therapy was considered.
MR Imaging Examinations
All MR imaging studies were performed on a 1.5T superconducting imaging unit (Magnetom Vision; Siemens, Erlangen, Germany). Conventional MR images, including T1-weighted (TR/TE, 670/14 ms; NEX, 1), T2-weighted (TR/TEeff, 3600/96 ms; NEX, 2; echo-train length, 7), contrast-enhanced T1-weighted (TR/TE, 670/14 ms; NEX, 1) images, and perfusion MR images, were obtained during the same examination. Perfusion MR imaging was with a gradient-echo echo-planar imaging sequence during the first pass of a standard-dose (0.1 mmol/kg) bolus of gadopentetate dimeglumine (Magnevist; Nihon Schering, Osaka, Japan). We used 10 sections that covered the area from the upper to the lower margin of the lesion based on T2-weighted and fluid-attenuated inversion recovery images. The scanning parameters were the following: TR/TE, 2000/54 msec; band width, 926 Hz per pixel; FOV, 210 × 230 mm; matrix, 128 × 128; section thickness, 5 mm; section gap, 1 mm. In all patients, images were obtained every 2 seconds at each section location in 80 seconds. An MR imaging–compatible power injector was used for the intravenous administration of contrast material through the right antecubital vein at a rate of 3 mL/s; this was followed by a 20-mL bolus of saline delivered at the same flow rate.
rCBV Measurements and Analysis
To analyze the rCBV from the perfusion MR imaging data, we used built-in software (Siemens Medical Systems) featuring standard algorithms.12,21,22 Because the CBV must be expressed relative to an internal reference, we normalized it by expressing ratios relative to values measured in the normal contralateral white matter. We referred to these relative values as rCBV. In the evaluation of tumor vascularity by perfusion MR imaging, the maximum rCBV in the tumor is reportedly the most reliable parameter for interobserver and intraobserver reproducibility.23 Therefore, we used maximum rCBV as a representative parameter of tumor angiogenesis.
Solid tumor components with or without contrast enhancement on conventional MR imaging and rCBV maps were identified consensually by 2 neuroradiologists (T.H., M.K., with 18 and 17 years of brain MR imaging experience, respectively). They were blinded to clinical and histopathologic patient data. They placed four to eight 50-mm2 regions of interest within solid tumor components on the CBV maps. Maximum rCBV values were selected for analysis. To place the region of interest correctly inside the solid portion of the tumor while avoiding volume-averaging with normal vessels that influence rCBV values, they carefully inspected conventional MR images and dynamic image sets from the arterial to the venous phase.
Statistical Analysis
To evaluate the relationship between maximum rCBV and the glioma grade, we subjected the maximum rCBV values in different histopathologically diagnosed gliomas (AA versus GBM) to the unpaired Student t test. Survival was measured from the time of the pretreatment MR imaging study to the time of death or last follow-up. Follow-up ranged from 2–72 months (median, 24 months). To determine the relationship between maximum rCBV and patient survival, we compared the maximum rCBV values with survival times. Furthermore, we compared the survival curves on the basis of the histopathologic diagnosis of the tumor and the maximum rCBV.
We assessed the relationship between patient survival and prognostic factors determined from clinical and MR imaging data. Receiver operating characteristic analysis (ROC) was used to determine the optimal maximum rCBV cutoff for predicting the 2-year survival. For estimating the rCBV cutoff, the 2-year survival was used because the median follow-up in this study was 2 years. Prognostic factors included the patient age (≤49 versus ≥50 years), sex (male versus female), duration of symptoms (≤3 versus >3 months), neurologic function (able to work versus confined to home or hospitalized), mental status (normal versus abnormal), Karnofsky performance scale (KPS) score (≤80 versus 90–100), extent of surgery (biopsy versus partial or total resection), histopathologic diagnosis (AA versus GBM), enhancement of tumor components (present versus absent), and the maximum rCBV (equal or less cutoff value versus more cutoff value). Survival curves were calculated by using the Kaplan-Meier method; overall differences were analyzed with the logrank test. We used the multivariate Cox proportional hazards model to adjust for the influence of prognostic factors. Statistical analyses were performed with computer software (StatView, Version 5.0; SAS Institute, Cary, NC; StatFlex, Version 5.0; Artec, Osaka, Japan). For all analyses, P < .05 was considered to denote a significant difference.
Results
Patient Data
Patient data and prognostic factors are shown in Table 1. There were 18 patients with AA and 31 with GBM: 15 underwent biopsy, 19 underwent partial, and 15 underwent gross total resection. On conventional MR images, all GBM and 5 of 18 AA manifested enhanced components. The maximum rCBV values of all tumors ranged from 0.56 to 14.1 (mean, 3.03 ± 2.79), and the mean maximum rCBV was significantly higher in patients with GBM (3.90 ± 3.19) than in those with AA (1.56 ± 0.69, P = .0038).
Table 1:
Prognostic factors in patients with high-grade astrocytoma
| Prognostic Factor | No. of Patients (N = 49) | Overall Survival* (%) | P Value† |
|---|---|---|---|
| Age (yr) | |||
| ≤49 | 16 | 63 | .011 |
| ≥50 | 33 | 30 | |
| Sex | |||
| Male | 30 | 27 | .029 |
| Female | 19 | 63 | |
| Histologic diagnosis | |||
| AA | 18 | 89 | <.001 |
| GBM | 31 | 13 | |
| Neurologic function‡ | |||
| Work | 19 | 68 | .002 |
| Home or hospital | 30′ | 23 | |
| Mental status | |||
| Normal | 38 | 47 | .019 |
| Abnormal | 11 | 18 | |
| Symptom duration (mo) | |||
| ≤3 | 39 | 39 | .567 |
| <3 | 10 | 50 | |
| KPS score | |||
| ≤80 | 28 | 21 | .003 |
| 90–100 | 21 | 67 | |
| Extent of surgery | |||
| Biopsy | 15 | 27 | .024 |
| Resection | 34 | 47 | |
| Tumor enhancement | |||
| Present | 36 | 22 | <.001 |
| Absent | 13 | 92 | |
| rCBV | |||
| ≤2.3 | 27 | 67 | <.001 |
| <2.3 | 2 | 9 |
Note:—AA indicates anaplastic astrocytoma; GBM, glioblastoma multiforme; KPS, Karnofsky performance scale; rCBV, relative cerebral blood volume.
Data are 2-year overall survival rates, expressed as percentages.
P values were calculated by using the logrank test.
Neurologic function sufficient to enable the patient to work or limited so that the patient is either confined to the home or hospitalized.
Survival Analysis
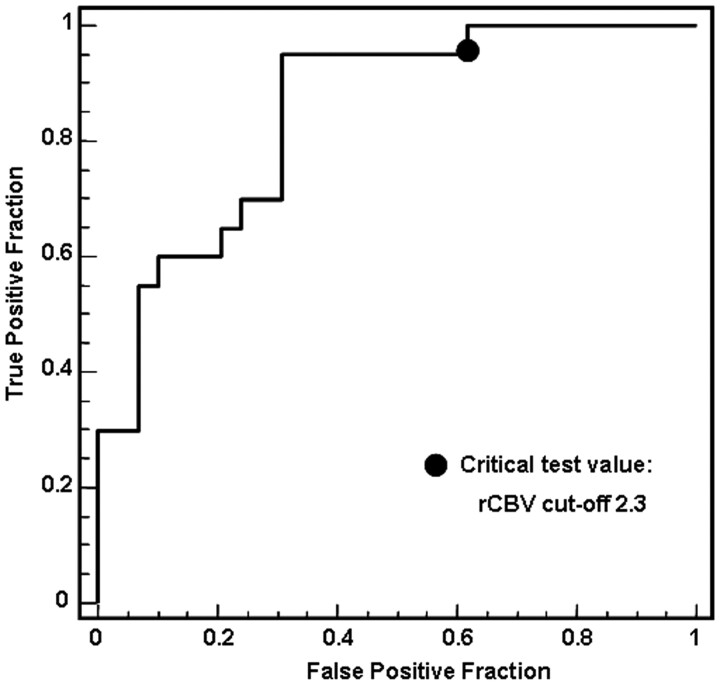
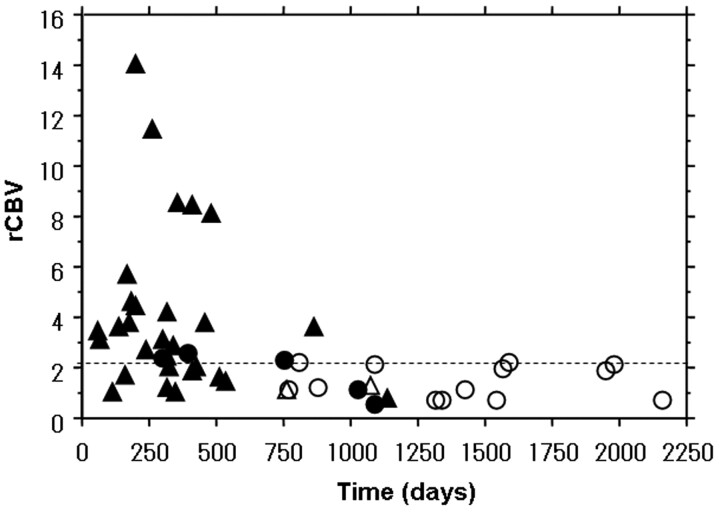
Of the 49 patients, 15 were alive at the most recent follow-up. The ROC analysis demonstrated that the optimal maximum rCBV cutoff for predicting the 2-year survival was 2.3 (sensitivity, 95%; specificity, 68%; positive predictive value, 66%; negative predictive value, 90%) (Fig 1). The relationship between maximum rCBV and survival time is shown in Fig 2. The 2-year overall survival rate for all 49 patients was 41% (n = 20); it was significantly lower for patients with GBM (4/31, 13%) than for those with AA (16/18, 89%; P < .001). Eighteen of 27 (67%) patients with low (≤2.3) and 2 of 22 (9%) with high (>2.3) maximum rCBV survived for 2 years (P < .001) (Table 1).
Fig 1.
ROC curves to determine the optimal maximum rCBV cutoff for predicting the 2-year survival. At a critical test cutoff value of rCBV = 2.3, sensitivity and specificity for distinguishing the 2-year survival are 95% and 68%, respectively. Area under the curve is 0.829.
Fig 2.
The relationship between maximum rCBV values and survival time in 49 patients with high-grade astrocytoma. A cutoff value of 2.3 for maximum rCBV (dotted line) was determined on the basis of an ROC analysis result to best discriminate patients with and without 2-year survival. ○ indicates surviving patients with AA; •, patients with AA who died; ▵, surviving patients with GBM; ▴, patients with GBM who died.
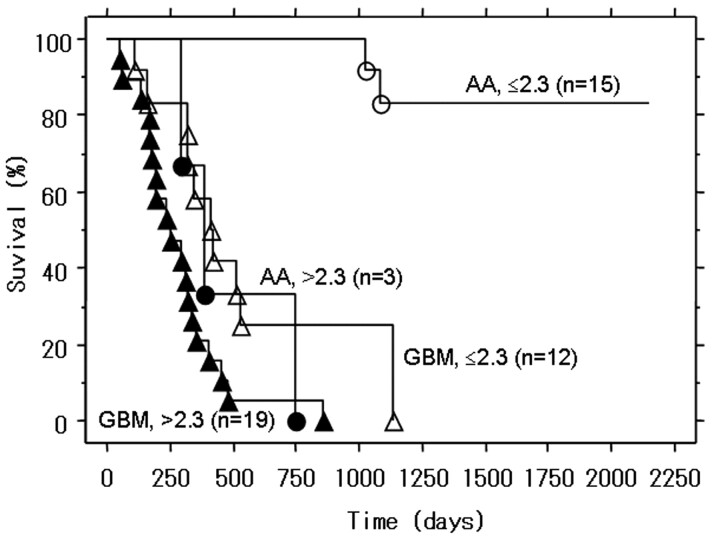
The survival curves according to the histopathologic diagnosis and maximum rCBV are shown in Fig 3. Of the 31 patients with GBM, 19 (61%) manifested high rCBV values; only 1 of these patients (5%) survived for 2 years compared with 4 of 12 (25%) patients with a low maximum rCBV value. The 2-year overall survival rate was significantly lower for patients with GBM with high maximum rCBV than with low maximum rCBV values (P = .013). Among the 18 patients with AA, 3 manifested high and 15, low maximum rCBV values; 33% (n = 1) of patients with high and all patients with low maximum rCBV survived for 2 years (Figs 3–5). The 2-year overall survival rate was significantly lower for patients with AA with high maximum rCBV than for those with low maximum rCBV values (P < .001).
Fig 3.
Kaplan-Meier survival curves for patients with GBM or AA with low rCBV (≤2.3) or high rCBV (>2.3). For patients with GBM and AA, the overall survival rate was significantly lower for patients with high rCBV (>2.3) than for those with low rCBV (≤2.3) (P = .013 and P < .001, respectively). ○ indicates surviving patients with AA; •, patients with AA who died; ▵, surviving patients with GBM; ▴, patients with GBM who died.
Fig 5.
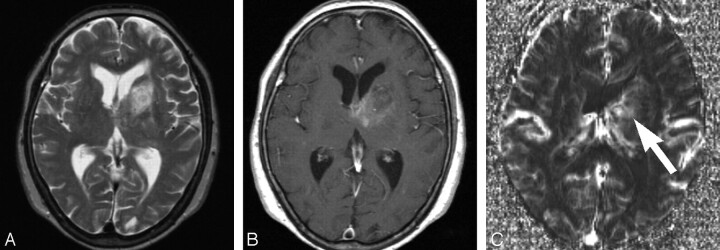
Transverse MR images obtained in a 43-year-old woman with anaplastic astrocytoma. A and B, T2-weighted (A) (TR/TE, 3600/96 ms) and contrast-enhanced T1-weighted (B) (TR/TE, 670/14 ms) images showing a heterogeneous signal-intensity lesion with patchy enhanced areas in the left temporal lobe. C, Transverse rCBV map showing slight high-perfusion areas (arrow) in the medial portion of the temporal lesion. The maximum rCBV value within the tumor is 1.9. This patient survived 65 months after the initial MR imaging study.
Univariate analysis revealed that the significant factors were patient age (P = .011), sex (P = .029), histologic diagnosis (P < .001), neurologic function (P = .002), mental status (P = .019), KPS score (P = .003), extent of surgery (P = .024), tumor enhancement (P < .001), and rCBV (P < .001) (Table 1). Multivariate Cox proportional hazards model results are shown in Table 2. The most important factor was the histologic diagnosis (hazard ratio = 9.707; 95% confidence interval (CI), 3.163–29.788), followed by the maximum rCBV (hazard ratio = 4.739; 95% CI, 1.950–11.518). The extent of surgery and sex were also significant independent factors related to survival.
Table 2:
Multivariate analysis of specific prognostic factors
| Prognostic Factor | Hazard Ratio* (95% CI) | P Value |
|---|---|---|
| Age ≥50 years | NA | .814 |
| Sex: male | 2.632 (1.153–6.010) | .021 |
| Histologic diagnosis of GBM | 9.707 (3.163–29.788) | <.001 |
| Neurologic function: home or hospital† | NA | .435 |
| Abnormal mental status | NA | .228 |
| KPS score ≤ 80 | NA | .150 |
| Extent of surgery: biopsy | 2.692 (1.196–6.061) | .016 |
| Tumor enhancement: present | NA | .726 |
| rCBV > 2.3 | 4.739 (1.950–11.518) | <.001 |
Note:—NA indicates not applicable; GBM, glioblastoma multiforme; KPS, Karnofsky performance scale; rCBV, relative cerebral blood volume.
Hazard ratio was not calculated when P ≥ .05.
Neurologic function is limited so that the patient is either confined to the home or hospitalized.
Discussion
Our study suggests that the tumor rCBV on pretreatment MR imaging is an independent important prognostic factor in patients with high-grade astrocytoma. The predictive value of perfusion MR imaging for the prognosis of patients with glioma has been documented.16–20 Lev et al16 reported that in low- and high-grade gliomas, elevated rCBV was a stronger predictor of survival than the degree of enhancement. According to Tzika et al,17 in pediatric patients with low- or high-grade glioma, the rCBV value was useful for distinguishing between progressive and stable tumors. Law et al18 suggested that rCBV measurements in low-grade gliomas correlated more accurately with time to progression than the initial histopathologic grading of the tumor. Others19,20 claimed that rCBV on perfusion MR imaging was not predictive of the prognosis of patients with glioma. Oh et al,19 who performed a survival analysis of 28 patients with glioblastoma, found that rCBV had no predictive value with respect to the prognosis. Their cutoff value for rCBV was 1.4, but it is not clear whether they used maximum rCBV in their analysis. Multivariate analysis of another 27 adult patients with low- or high-grade glioma20 showed that rCBV provided no prognostic information different from that yielded by histopathologic study; however, the type of glioma was not specified and the maximum rCBV value in the region of interest was not measured. Histopathologic microvessel attenuation is an independent prognostic indicator in patients with astroglial brain tumors.15 We suggest that areas with the maximum rCBV are the sites of the highest glioma grade within heterogeneous tumors and are thus the important sites for diagnosis and prognosis.
In our patients, the 2-year overall survival rate was significantly higher for patients with low (≤2.3) than with high (>2.3) maximum rCBV, irrespective of whether the tumor was AA or GBM. Unlike histopathologic findings, maximum rCBV values derived from perfusion MR imaging may make it possible to predict the survival of patients with high-grade astrocytoma. The progression of AA to GBM is a key prognostic factor,1 though there is considerable variation in the time to progression (mean, 2 years).24 We suspect that AA with a high (>2.3) maximum rCBV value may undergo genetic changes reflecting a higher degree of malignancy. Survival was significantly longer in patients with GBM with low rather than high maximum rCBV values. Although the presence of microvascular proliferation is a histopathologic hallmark of GBM, in our study population the maximum rCBV values varied considerably among our patients with GBM. Our results suggest that the degree of microvascular proliferation in GBM may affect the prognosis.
In patients with high-grade astrocytoma, pretreatment and treatment-related prognostic variables include patient age, sex, symptom duration, neurologic function, mental status, KPS score, extent of surgery, histopathologic diagnosis, tumor-component enhancement, location of the tumor, and the dose and extent of radiation.5,25–30 In our univariate analysis of the 10 items, all items except symptom duration had a significant difference. In our multivariate analysis of the 9 items, the most important prognostic factor was the histopathologic diagnosis followed by the maximum rCBV, the extent of surgery, and patient sex. Although histopathology is primarily used to determine the WHO tumor grade, the maximum rCBV value may provide additional valuable information. Because perfusion MR imaging facilitates assessment of the entire tumor and the identification of the intratumoral areas with the highest microvascular attenuation, it may be helpful for selecting the biopsy targets with the highest information yield and may aid in the selection of appropriate treatment strategies. Thus, this technique may prevent histologic misdiagnosis (eg, sampling error) and may demonstrate biologic differences (eg, genetic change) in the tumor. AA harboring components manifesting a high maximum rCBV may be associated with a poor prognosis and may require the same aggressive treatment as GBM.
In our study, sex was an independent prognostic factor, and female patients had better prognosis than male patients. Some studies have shown that female patients with GBM have a better prognosis than their male counterparts28–30 and that hormones or tumor-suppressor genes on the X chromosome may be associated with their longer survival.31,32
There are some limitations in our study. First, we did not evaluate the location of the tumor, such as eloquent brain. In our study, the location of the tumor was supratentorial in all patients. Although this factor may affect patient survival,25,26 to our knowledge, its effect on survival has not been established. Polin et al33 reported that laterality of high-grade gliomas is not an independent prognostic factor for predicting survival or functional outcome. Second, the dose and extent of radiation were not assessed in this study. Our protocol for the treatment of high-grade astrocytoma consists of 60 Gy of radiation for patients with GBM and 54 Gy for patients with AA. The extent of the radiation field for the GBM and AA groups was determined in the same fashion as described previously. Therefore, we believe that these effects are small. Third, we did not evaluate whether additional salvage surgery, chemotherapy, and radiation affected the survival of each patient. Although these additional treatments might have affected the results, we tried to perform the optimal treatment in each patient. Fourth, proved prognostic factors such as patient age and KPS were not found to be significant in our multivariate analysis. The reason for the result in the multivariate analysis is not clear. However, these factors were found to be significant in our univariate analysis.
Fifth, there is the possibility of histopathologic misdiagnosis attributable to sampling error at pathologic examination. When only a few small tissue samples are assessed, particularly from stereotactic biopsy, suboptimal sampling may result in inaccurate glioma grading because of the histologic heterogeneity of tumor tissues.6,7 However, because only 15 of our 49 patients (30%) were biopsied, we postulate that the incidence of histopathologic misdiagnosis was low in our series. Sixth, we used a gradient-echo dynamic-susceptibility contrast technique in our perfusion MR imaging study. This technique tends to be more sensitive to larger vessels than the spin-echo dynamic-susceptibility contrast technique.34 We inspected not only conventional MR images but also dynamic image sets from the arterial to the venous phase. Because the region of interest was placed correctly inside the solid portion of the tumor and because we carefully avoided volume averaging with normal large vessels, we think that only tumor-specific vessels were assessed.
In conclusion, our long-term follow-up study of patients with high-grade astrocytoma showed that the maximum rCBV value on pretreatment MR imaging scans is useful as a clinical biomarker for predicting the survival of these patients. The presence of an intratumoral area with a high maximum rCBV value (>2.3) may be predictive of a poor prognosis. The combined assessment of histopathologic and perfusion MR imaging findings obtained before the inception of treatment may be useful to determine optimal management strategies in patients with high-grade astrocytoma.
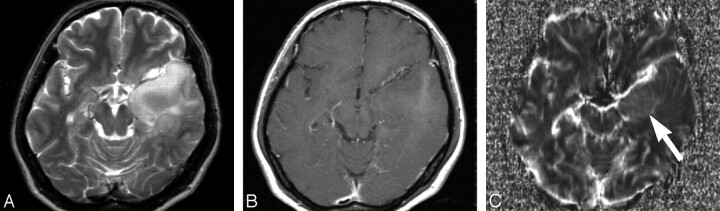
Fig 4.
Transverse MR images obtained in a 71-year-old woman with anaplastic astrocytoma. A and B, T2-weighted (A) (TR/TE, 3600/96 ms) and contrast-enhanced T1-weighted (B) (TR/TE, 670/14 ms) images showing a heterogeneous signal-intensity lesion with slightly enhanced areas in the left basal ganglia. C, Transverse rCBV map showing intratumoral high-perfusion areas (arrow). The maximum rCBV value within the tumor is 2.4. This patient died 25 months after the initial MR imaging study.
Acknowledgments
We thank Junji Shiraishi, PhD, for his valuable advice in ROC analysis.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. World Health Organization Classification of Tumours of the Central Nervous System: Astrocytic Tumours. Lyon, France: IARC Press;2007. :13–52
- 2.Gupta T, Sarin R. Poor-prognosis high-grade gliomas: evolving an evidence-based standard of care. Lancet Oncol 2002;3:557–64 [DOI] [PubMed] [Google Scholar]
- 3.Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet 2003;361:323–31 [DOI] [PubMed] [Google Scholar]
- 4.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002;359:1011–18 [DOI] [PubMed] [Google Scholar]
- 5.Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90–06. Int J Radiat Oncol Biol Phys 1998;40:51–55 [DOI] [PubMed] [Google Scholar]
- 6.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 2001;3:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilles FH, Brown WD, Leviton A, et al. Limitations of the World Health Organization classification of childhood supratentorial astrocytic tumors: Children Brain Tumor Consortium. Cancer 2000;88:1477–83 [PubMed] [Google Scholar]
- 8.Coons SW, Johnson PC, Scheithauer BW, et al. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer 1997;79:1381–93 [DOI] [PubMed] [Google Scholar]
- 9.Aldape K, Simmons ML, Davis RL, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer 2000;88:2342–49 [PubMed] [Google Scholar]
- 10.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994;191:41–51 [DOI] [PubMed] [Google Scholar]
- 11.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity in gliomas. AJR Am J Roentgenol 1998;171:1479–86 [DOI] [PubMed] [Google Scholar]
- 12.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999;211:791–98 [DOI] [PubMed] [Google Scholar]
- 13.Wong JC, Provenzale JM, Petrella JR, et al. Perfusion MR imaging of brain neoplasms. AJR Am J Roentgenol 2000;174:1147–57 [DOI] [PubMed] [Google Scholar]
- 14.Cha S, Johnson G, Wadghiri YZ, et al. Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med 2003;49:848–55 [DOI] [PubMed] [Google Scholar]
- 15.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 1996;77:362–72 [DOI] [PubMed] [Google Scholar]
- 16.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 2004;25:214–21 [PMC free article] [PubMed] [Google Scholar]
- 17.Tzika AA, Astrakas LG, Zarifi MK, et al. Spectroscopic and perfusion magnetic resonance imaging predictors of progression in pediatric brain tumors. Cancer 2004;100:1246–56 [DOI] [PubMed] [Google Scholar]
- 18.Law M, Oh S, Babb JS, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology 2006;238:658–67 [DOI] [PubMed] [Google Scholar]
- 19.Oh J, Henry RG, Pirzkall A, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging 2004;19:546–54 [DOI] [PubMed] [Google Scholar]
- 20.Mills SJ, Patankar TA, Haroon HA, et al. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol 2006;27:853–58 [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen BR, Belliveau JW, Vevea JM, et al. Perfusion imaging with NMR contrast agents. Magn Reson Med 1990;14:249–65 [DOI] [PubMed] [Google Scholar]
- 22.Rosen BR, Belliveau JW, Buchbinder BR, et al. Contrast agents and cerebral hemodynamics. Magn Reson Med 1991;19:285–92 [DOI] [PubMed] [Google Scholar]
- 23.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 2002;224:797–803 [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Sato K, Biernat W, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res 1997;3:523–30 [PubMed] [Google Scholar]
- 25.Kowalczuk A, Macdonald RL, Amidei C, et al. Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery 1997;41:1028–36 [DOI] [PubMed] [Google Scholar]
- 26.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190–98 [DOI] [PubMed] [Google Scholar]
- 27.Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 2003;99:467–73 [DOI] [PubMed] [Google Scholar]
- 28.Sant M, van der Sanden G, Capocaccia R. Survival rates for primary malignant brain tumours in Europe. Eur J Cancer 1998;34:2241–47 [DOI] [PubMed] [Google Scholar]
- 29.Shinojima N, Kochi M, Hamada J, et al. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg 2004;101:219–26 [DOI] [PubMed] [Google Scholar]
- 30.Magrini SM, Ricardi U, Santoni R, et al. Patterns of practice and survival in a retrospective analysis of 1722 adult astrocytoma patients treated between 1985 and 2001 in 12 Italian radiation oncology centers. Int J Radiat Oncol Biol Phys 2006;65:788–99 [DOI] [PubMed] [Google Scholar]
- 31.Plunkett RJ, Lis A, Barone TA, et al. Hormonal effects on glioblastoma multiforme in the nude rat model. J Neurosurg 1999;90:1072–77 [DOI] [PubMed] [Google Scholar]
- 32.Seki Y, Suico MA, Uto A, et al. The ETS transcription factor MEF is a candidate tumor suppressor gene on the X chromosome. Cancer Res 2002;62:6579–86 [PubMed] [Google Scholar]
- 33.Polin RS, Marko NF, Ammerman MD, et al. Functional outcomes and survival in patients with high-grade gliomas in dominant and nondominant hemispheres. J Neurosurg 2005;102:276–83 [DOI] [PubMed] [Google Scholar]
- 34.Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 2006;239:632–49 [DOI] [PubMed] [Google Scholar]