Abstract
BACKGROUND AND PURPOSE: Although accumulating evidence suggests the presence of microbleeds as a risk factor for intracerebral hemorrhage (ICH), little is known about its significance in anticoagulated patients. The aim of this study was to determine whether the presence of microbleeds is associated with recurrent hemorrhagic stroke in patients who had received warfarin following atrial fibrillation–associated cardioembolic infarction.
MATERIALS AND METHODS: A total of 87 consecutive patients with acute recurrent stroke, including 15 patients with ICH and 72 patients with cerebral infarction, were enrolled in this study. International normalized ratios (INRs), vascular risk factors, and imaging characteristics, including microbleeds on T2*-weighted MR images and white matter hyperintensity (WMH) on T2-weighted MR images, were compared in the 2 groups.
RESULTS: Microbleeds were noted more frequently in patients with ICH than in patients with cerebral infarction (86.7% versus 38.9%, P = .0007). The number of microbleeds was larger in patients with ICH than in patients with cerebral infarction (mean, 8.4 versus 2.1; P = .0001). INR was higher in patients with ICH than in patients with cerebral infarction (mean, 2.2 versus 1.4; P < .0001). The frequency of hypertension was higher in patients with ICH than in patients with cerebral infarction (86.7% versus 45.8%, P = .0039). Multivariate analysis revealed that the presence of cerebral microbleeds (odds ratio, 7.383; 95% confidence interval, 1.052–51.830) was associated with ICH independent of increased INR and hypertension.
CONCLUSION: The presence of cerebral microbleeds may be an independent risk factor for warfarin-related ICH, but more study is needed because of strong confounding associations with elevated INR and hypertension.
One of the major complications of warfarin treatment following atrial fibrillation–related cardioembolic infarction is the occurrence of intracerebral hemorrhage (ICH). With advancing age, the incidence of both atrial fibrillation–related cardioembolic infarction and warfarin-related ICH increases.
Cerebral microbleeds detected by gradient-echo T2*-weighted MR imaging, which are shown as signal-intensity loss, represent hemosiderin deposit1,2 and are associated with occurrence of ICH.3–19 Although accumulating evidence suggests that the presence of microbleeds is a risk factor for ICH in patients treated by antiplatelet therapy13,20 and hemorrhagic complications of anticoagulation in patients with prior ICH and atrial fibrillation have been reported,21 little is known about the significance of microbleeds in anticoagulated patients because, to our knowledge, no studies have focused on the association between cerebral microbleeds and anticoagulation therapy in a large number of patients. On the other hand, previous studies focusing on radiographic characteristics have shown that the presence of microangiopathy (leukoaraiosis) detected by CT is a risk factor for warfarin-related ICH.22 However, considering the close association between cerebral microbleeds and leukoaraiosis (white matter hyperintensity [WMH]),7,8,11,14–16,19,23,24 one could hypothesize that cerebral microbleeds, which represent bleeding from small vessels, may be more closely associated with ICH than WMH is. Therefore, the present study was performed to determine whether the presence of microbleeds is associated with recurrent hemorrhagic stroke in patients who have received warfarin treatment following atrial fibrillation–associated cardioembolic infarction.
Materials and Methods
We prospectively evaluated inpatients with acute recurrent stroke who had received warfarin treatment following nonvalvular atrial fibrillation–associated cardioembolic infarction and who had undergone MR imaging studies in our hospital during the period from September 2002 to August 2007. Patients with valvular infarcts were excluded because they might have received a different anticoagulation regimen, and patients with arterial-origin infarcts were also excluded because they might not have been anticoagulated. Patients with hemorrhagic stroke or hemorrhagic conversion of the initial infarction were also excluded. Diagnosis of acute recurrent stroke was made on the basis of neurologic signs and symptoms and results of neuroradiologic examinations. Stroke was classified into ICH and ischemic stroke. ICH was diagnosed by CT, and acute ischemic stroke was confirmed by diffusion-weighted imaging (DWI) and apparent diffusion coefficient maps.
All patients were examined by using a 1.5T clinical MR imaging unit (Magnetom Symphony; Siemens, Erlangen, Germany), and the whole brain was scanned with a section thickness of 5 mm and a 1.5-mm intersection gap. The imaging protocol consisted of axial T2-weighted spin-echo sequences (TR/TE, 3800/99 ms; FOV, 220 × 220; matrix, 256 × 512), axial T2*-weighted gradient-echo sequences (TR/TE, 800/26 ms; flip angle, 20°; FOV, 230 × 230; matrix, 192 × 256), and DWI with single-shot echo-planar spin-echo sequences (TR/TE, 5300/135 ms; FOV, 196 × 261; matrix, 80 × 128; b-values, 0 and 1000 s/mm2). Patients who were unable to be evaluated by MR imaging because of artifacts were excluded.
Microbleeds were defined as homogeneous round signal-intensity-loss lesions on T2*-weighted MR images, excluding lesions in the globus pallidum and the subarachnoid space, which are likely to represent calcification and adjacent pial blood vessels, respectively. Intracerebral lesions with a hemorrhagic component associated with tumors, arteriovenous malformations, cavernomas, and abscesses were also excluded. WMH on T2-weighted images was graded by using the scoring system of Fazekas et al25 into 4 grades: grade 0 = absent, 1 = punctate, 2 = early confluent, and 3 = confluent. WMH of grade 2 or 3 was regarded as advanced WMH. MR images were evaluated by 2 of the authors (H.N., H.U.) separately without knowledge of the patients’ clinical profiles, and the number of microbleeds and the grading scores of WMH were determined by consensus.
In all patients, international normalized ratios (INRs) were evaluated by using blood taken before starting acute therapy. Hypertension was defined as systolic blood pressure of ≥140 mm Hg and diastolic blood pressure of ≥90 mm Hg before recurrence of stroke or occurring in patients currently undergoing medical treatment for hypertension. Diabetes mellitus was defined as a glycosylated hemoglobin A1c concentration of >5.8% or occurring in patients currently using hypoglycemic agents. Hypercholesterolemia was defined as a total cholesterol level of ≥220 mg/dL or occurring in patients currently undergoing cholesterol-lowering therapy. Whether patients had undergone antiplatelet therapy before occurrence of stroke was recorded.
All values are expressed as means ± SD. Among ICH and cerebral infarction, the χ2 test for independence was used for comparison of sex ratio, hypertension, diabetes mellitus, hypercholesterolemia, antiplatelet therapy, microbleeds, and advanced WMH; the Student t test was used for comparison of age and INRs; and the Mann-Whitney U test was used for comparison of the number of microbleeds. Logistic regression analysis was used to assess the relationships of ICH with the following variables: age, sex, INR, hypertension, diabetes mellitus, hypercholesterolemia, antiplatelet therapy, advanced WMH, and microbleeds.
Results
The study population consisted of 87 patients with acute recurrent stroke, including 72 patients (49 men and 23 women; mean age, 74.5 ± 11.7 years) with cerebral infarction and 15 patients (8 men and 7 women; mean age, 77.1 ± 9.6 years) with ICH. The patients’ backgrounds are summarized in Table 1. No significant difference was found in age, sex ratio, or frequencies of advanced WMH, diabetes mellitus, hypercholesterolemia, or antiplatelet therapy between the cerebral infarction and ICH groups. In contrast, microbleeds were noted more frequently in patients with ICH than in patients with cerebral infarction (86.7% versus 38.9%, P = .0007). The number of microbleeds was larger in patients with ICH than in patients with cerebral infarction (range, 0–54 versus 0–38; mean, 8.4 versus 2.1; P = .0001). Representative images of a patient with ICH and a patient with recurrent cardioembolic infarction are shown in Figs 1 and 2, respectively. INR was higher in patients with ICH than in patients with cerebral infarction (mean, 2.2 versus 1.4; P < .0001). The frequency of hypertension was higher in patients with ICH than in patients with cerebral infarction (86.7% versus 45.8%, P = .0039). Multivariate analysis revealed that increased INR (odds ratio, 5.119; 95% confidence interval [CI], 1.614–16.239), presence of cerebral microbleeds (odds ratio, 7.383; 95% CI, 1.052–51.830), and hypertension (odds ratio, 13.599; 95% CI, 1.653–111.898) were independently associated with ICH (Table 2).
Table 1:
Background of the patients
| Characteristics | ICH (n = 15) | Ischemic Stroke (n = 72) | P Value |
|---|---|---|---|
| Male sex, no. (%) | 8 (53.3) | 49 (68.1) | .28 |
| Age (yr), mean (SD) | 77.1 (9.6) | 74.6 (11.6) | .43 |
| Frequency of MBs, no. (%) | 13 (86.7) | 28 (38.9) | .0007 |
| No. of MBs, no., mean (SD) | 8.4 (14.0) | 2.1 (2.1) | .0001 |
| WMH, no. (%) | 8 (53.3) | 23 (31.9) | .12 |
| Antiplatelet therapy, no. (%) | 4 (26.7) | 18 (25.0) | .89 |
| Hypertension, no. (%) | 13 (86.7) | 33 (45.8) | .0039 |
| Diabetes mellitus, no. (%) | 4 (26.7) | 18 (25.0) | .89 |
| Hypercholesterolemia, no. (%) | 3 (20.0) | 14 (19.4) | .96 |
| INR, mean (SD) | 2.20 (1.13) | 1.41 (0.51) | <.0001 |
Note:—MBs indicates microbleeds; WMH, white matter hyperintensity; INR, international normalized ratio.
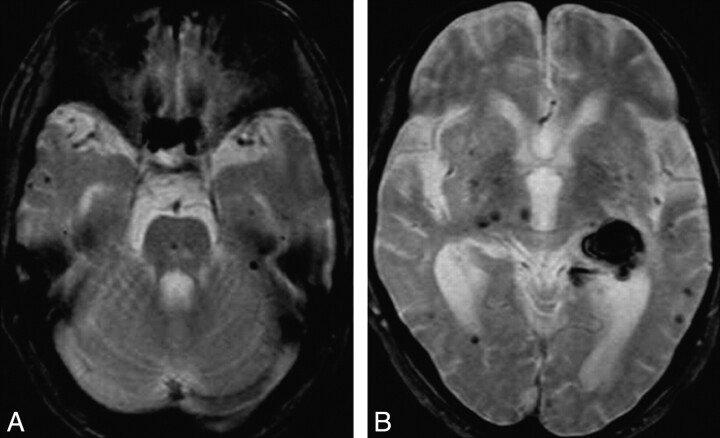
Fig 1.
MR images of a patient with intracerebral hemorrhage in the left thalamus. T2*-weighted images reveal multiple foci of signal-intensity loss (microbleeds) in the brain stem, thalamus, and cerebral hemispheres.
Fig 2.
MR images of a patient with recurrent cardioembolic infarction. A, DWI shows hyperintense signal-intensity alterations in the right frontal lobe, indicating acute cerebral infarction. B and C, T2*-weighted images do not show cerebral microbleeds.
Table 2:
Logistic regression analysis
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age | 1.06 | 0.982–1.144 | .1352 |
| INR | 5.119 | 1.614–16.239 | .0056 |
| Sex | 0.967 | 0.194–4.816 | .9677 |
| MBs | 7.383 | 1.052–51.830 | .0443 |
| WMH | 1.234 | 0.227–6.722 | .8077 |
| Antiplatelet therapy | 0.662 | 0.105–4.178 | .6603 |
| Hypertension | 13.599 | 1.653–111.898 | .0152 |
| Diabetes mellitus | 1.424 | 0.175–11.613 | .7414 |
| Hypercholesterolemia | 2.06 | 0.301–14.106 | .4617 |
Note:—MBs indicates microbleeds; WMH, white matter hyperintensity; INR, international normalized ratio.
Discussion
The present study demonstrated that the presence of cerebral microbleeds was associated with ICH independent of increased INR and hypertension in patients with cerebral infarction following atrial fibrillation–related cardioembolic infarction. Although some studies have been performed to determine whether the use of antiplatelet therapy is associated with the occurrence of ICH in patients with microbleeds,13 this is the first study aimed at determining whether the presence of microbleeds is associated with recurrent hemorrhagic stroke in patients who had undergone warfarin treatment following atrial fibrillation–associated cardioembolic infarction.
Although it has been established that warfarin treatment is effective for preventing cerebral embolism arising from atrial fibrillation, the most serious adverse event in atrial fibrillation is hemorrhage complication. An excess incidence of major bleeding (7% per year) in patients with nondisabling cerebral ischemia of presumed arterial origin and treated with oral anticoagulation led to early termination of the Stroke Prevention in Reversible Ischemia Trial (SPIRIT).26 As for radiographic characteristics as risk factors for warfarin-related ICH, the presence of advanced leukoaraiosis has been reported.22,27 Gorter27 assessed independent predictors of hemorrhage in 651 anticoagulated patients after cerebral ischemia in SPIRIT and reported that leukoaraiosis detected by CT, intensity of anticoagulation, and age older than 65 years are independent risk factors for bleeding in anticoagulated patients. Smith et al22 compared radiographic and clinical characteristics of 26 patients with warfarin-related ICH following ischemic stroke with those of 56 controls, and they reported that the presence and severity of leukoaraiosis detected by CT were independent risk factors for poststroke warfarin-related ICH.
These results indicate that warfarin may trigger ICH in patients with severe disturbance of cerebral small arteries. In contrast, our results showed that the presence of microbleeds, but not advanced WMH, was an independent risk factor of poststroke warfarin-related ICH. This discrepancy may be explained by the pathologic difference between microbleeds and WMH. For instance, though both microbleeds and WMH are associated with small-vessel diseases and often coexist, microbleeds are a bleeding-prone small-vessel disease and WMH is an ischemic small-vessel disease. In fact, some previous studies have shown that the presence of microbleeds is a predictor of ICH in patients with no or mild leukoaraiosis.7,19 On the other hand, it was also revealed that patients with advanced leukoaraiosis but without microbleeds tend to be associated with ischemic stroke rather than ICH.19 In a study using 3 autopsied brains, microbleeds were revealed to be associated with hemosiderin deposit that had been caused by a rupture of arteriosclerotic microvessels, supporting the notion that microbleeds are associated with fragility of blood vessels.1 INR in the ICH group was much higher than that in the cerebral infarction group, and INR was an independent risk factor for ICH in our study. Excess anticoagulation itself may have resulted in the occurrence of ICH, whereas hemorrhagic diathesis may have predisposed to microbleeds and ICH with the use of warfarin.
The results of the present study also showed that the frequency of hypertension was higher in patients with ICH than in patients with cerebral infarction and was an independent risk factor of warfarin-related ICH. Although it has been shown that hypertension is a major risk factor of microbleeds1,4,15,18,28,29 and ICH, it remains to be clarified whether hypertension management in the presence of microbleeds prevents the occurrence of ICH. Strict management of hypertension in anticoagulated patients with microbleeds is important in preventing the occurrence of ICH, but further studies are needed to confirm this.
Our study has some limitations. Because we could see the hematoma in patients with ICH when evaluating microbleeds on T2*-weighted MR images, this study was not a completely blind trial; therefore, bias could not be avoided. In addition, the duration of anticoagulant medication was not recorded in the present study. It remains to be determined whether the duration of anticoagulant medication affects the presence of microbleeds and the occurrence of ICH. It would also be informative to have included a control group of patients who were not treated with warfarin to estimate to what extent warfarin increases the risk of ICH in patients with microbleeds above their untreated incidence. In addition, patients with arterial embolic strokes who are not anticoagulated would be an additional and important group. Further studies are needed to clarify such issues.
Conclusions
The results of the present study suggest that evaluation of the presence of cerebral microbleeds as a risk factor for ICH in patients being treated with warfarin deserves study in a blind and controlled prospective therapeutic trial.
Footnotes
This work was partially supported by research grants from the Ministry of Health, Labor and Welfare, Japan, and from the Smoking Research Foundation, Japan.
References
- 1.Tanaka A, Ueno Y, Nakayama Y, et al. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke 1999;30:1637–42 [DOI] [PubMed] [Google Scholar]
- 2.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–42 [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg SM, O'Donnell HC, Schaefer PW, et al. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology 1999;53:1135–38 [DOI] [PubMed] [Google Scholar]
- 4.Roob G, Lechner A, Schmidt R, et al. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke 2000;31:2665–69 [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita T, Okudera T, Tamura H, et al. Assessment of lacunar hemorrhage associated with hypertensive stroke by echo-planar gradient-echo T2*-weighted MRI. Stroke 2000;31:1646–50 [DOI] [PubMed] [Google Scholar]
- 6.Hermier M, Nighoghossian N, Derex L, et al. MRI of acute post-ischemic cerebral hemorrhage in stroke patients: diagnosis with T2*-weighted gradient-echo sequences. Neuroradiology 2001;43:809–15 [DOI] [PubMed] [Google Scholar]
- 7.Kim DE, Bae HJ, Lee SH, et al. Gradient-echo magnetic resonance imaging in the prediction of hemorrhagic vs ischemic stroke: a need for the consideration of the extent of leukoaraiosis. Arch Neurol 2002;59:425–29 [DOI] [PubMed] [Google Scholar]
- 8.Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol 2003;24:88–96 [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell CS, Saver JL, Villablanca JP, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 2002;33:95–98 [DOI] [PubMed] [Google Scholar]
- 10.Nighoghossian N, Hermier M, Adeleine P, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke 2002;33:735–42 [DOI] [PubMed] [Google Scholar]
- 11.Kato H, Izumiyama M, Izumiyama K, et al. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke 2002;33:1536–40 [DOI] [PubMed] [Google Scholar]
- 12.Fan YH, Zhang L, Lam WW, et al. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke 2003;34:2459–62 [DOI] [PubMed] [Google Scholar]
- 13.Wong KS, Chan YL, Liu JY, et al. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003;60:511–13 [DOI] [PubMed] [Google Scholar]
- 14.Naka H, Nomura E, Wakabayashi S, et al. Frequency of asymptomatic microbleeds on T2*-weighted MR images of patients with recurrent stroke: association with combination of stroke subtypes and leukoaraiosis. AJNR Am J Neuroradiol 2004;25:714–19 [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SH, Bae HJ, Kwon SJ, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology 2004;62:72–76 [DOI] [PubMed] [Google Scholar]
- 16.Jeong SW, Jung KH, Chu K, et al. Clinical and radiologic differences between primary intracerebral hemorrhage with and without microbleeds on gradient-echo magnetic resonance images. Arch Neurol 2004;61:905–09 [DOI] [PubMed] [Google Scholar]
- 17.Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology 2006;66:165–71 [DOI] [PubMed] [Google Scholar]
- 18.Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke 2006;37:550–55 [DOI] [PubMed] [Google Scholar]
- 19.Naka H, Nomura E, Takahashi T, et al. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol 2006;27:830–35 [PMC free article] [PubMed] [Google Scholar]
- 20.Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy: recent data and ideas. Stroke 2005;36:1588–93 [DOI] [PubMed] [Google Scholar]
- 21.Eckman MH, Rosand J, Knudsen KA, et al. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–16 [DOI] [PubMed] [Google Scholar]
- 22.Smith EE, Rosand J, Knudsen KA, et al. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology 2002;59:193–97 [DOI] [PubMed] [Google Scholar]
- 23.Kwa VI, Franke CL, Verbeeten B Jr., et al. Silent intracerebral microhemorrhages in patients with ischemic stroke. Ann Neurol 1998;44:372–77 [DOI] [PubMed] [Google Scholar]
- 24.Hanyu H, Tanaka Y, Shimizu S, et al. Cerebral microbleeds in Binswanger's disease: a gradient-echo T2*-weighted magnetic resonance imaging study. Neurosci Lett 2003;340:213–16 [DOI] [PubMed] [Google Scholar]
- 25.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–56 [DOI] [PubMed] [Google Scholar]
- 26.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin: The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group. Ann Neurol 1997;42:857–65 [DOI] [PubMed] [Google Scholar]
- 27.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Neurology 1999;53:1319–27 [DOI] [PubMed] [Google Scholar]
- 28.Roob G, Schmidt R, Kapeller P, et al. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology 1999;52:991–94 [DOI] [PubMed] [Google Scholar]
- 29.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review. Brain 2007;130:1988–2003 [DOI] [PubMed] [Google Scholar]