Abstract
BACKGROUND AND PURPOSE: While brain MR imaging is routinely performed, the MR imaging assessment of spinal cord pathology in multiple sclerosis (MS) is less frequent in clinical practice. The purpose of this study was to determine whether measurements of medulla oblongata volume (MOV) on routine brain MR imaging could serve as a biomarker of spinal cord damage and disability in MS.
MATERIALS AND METHODS: We identified 45 patients with MS with both head and cervical spinal cord MR imaging and 29 age-matched and sex-matched healthy control subjects with head MR imaging. Disability was assessed by the expanded disability status scale (EDSS) and ambulation index (AI). MOV and upper cervical cord volume (UCCV) were manually segmented; semiautomated segmentation was used for brain parenchymal fraction (BPF). These measures were compared between groups, and linear regression models were built to predict disability.
RESULTS: In the patients, MOV correlated significantly with UCCV (r = 0.67), BPF (r = 0.45), disease duration (r = −0.64), age (r = −0.47), EDSS score (r = −0.49) and AI (r = −0.52). Volume loss of the medulla oblongata was −0.008 cm3/year of age in patients with MS, but no significant linear relationship with age was found for healthy control subjects. The patients had a smaller MOV (mean ± SD, 1.02 ± 0.17 cm3) than healthy control subjects (1.15 ± 0.15 cm3), though BPF was unable to distinguish between these 2 groups. MOV was smaller in patients with progressive MS (secondary- progressive MS, 0.88 ± 0.19 cm3 and primary-progressive MS, 0.95 ± 0.30 cm3) than in patients with relapsing-remitting MS (1.08 ± 0.15 cm3). A model including both MOV and BPF better predicted AI than BPF alone (P = .04). Good reproducibility in MOV measurements was demonstrated for intrarater (intraclass correlation coefficient, 0.97), interrater (0.79), and scan rescan data (0.81).
CONCLUSION: MOV is associated with disability in MS and can serve as a biomarker of spinal cord damage.
Multiple sclerosis (MS) is a multifactorial disease with a strong neurodegenerative component associated with progressive atrophy of the brain and spinal cord.1 Previous studies have shown involvement of the spinal cord in more than 80% of the patients with clinically definite MS.2–5 Atrophy of the spinal cord is thought to originate mainly from neurodegenerative changes, especially of the cervical segment,4,6–10 which results in impairment of motor function.11–16 In contrast, brain atrophy correlates well with neuropsychologic impairment.1 The most severe and debilitating physical disability in MS seems to be of spinal origin. Therefore, it has been suggested that measurements of upper spinal cord volume provides information regarding disease progression that is complementary to the assessment of brain atrophy.
Although head MR imaging is routinely performed in patients with MS, spinal cord MR imaging takes additional time and is therefore performed only on specific indications, both in the clinic and research.
The medulla oblongata is the most caudal part of the brain stem and is continuous with the spinal cord. It contains nuclei that are important for autonomic control such as respiration, heart rate, blood pressure and reflexes, and white matter (WM) tracts that connect the rostral and caudal parts of the central nervous system (CNS). Ventral, dorsal, and lateral funiculi in the lower medulla oblongata are continuous with those of the spinal cord.
The medulla oblongata is generally included in the field of view (FOV) of routine brain MR images of patients with MS. We set out to explore the feasibility and clinical relevance of volumetric measures of the lower medulla oblongata and hypothesized that these measures will reflect spinal cord atrophy. The relative ease to obtain such measurements from routinely performed clinical MR imaging examinations of the head makes this a potentially strong candidate for a biomarker of spinal cord damage and disability. In this work we present the results of a retrospective analysis of MR imaging-derived medulla oblongata volume (MOV) in MS to establish the relationship to spinal cord, its correlation with clinical disability and the reproducibility of this metric.
Materials and Methods
Patient Selection
For this retrospective analysis, patients were selected from the general patient population and the Comprehensive Longitudinal Investigation of Multiple Sclerosis at the Brigham and Women's Hospital study at the Partners MS Center in Boston. A data base search was conducted in October 2004. At that time, the data base contained 251 patients who fulfilled our inclusion criteria: both a head and spinal cord MR imaging examination performed on the same day, paired with a clinical visit that was less than 30 days before or later than the MR imaging scan. Of these 251 patients, 50 with good quality imaging studies (head MR images encompassing the cranial cavity from the foramen magnum to upper convexity without major image artifacts) were chosen randomly to be the subjects of this retrospective study. Of 50 patients, 4 were excluded because they received corticosteroids up to 30 days before the MR imaging examination, a potential confounder of atrophy measures. One patient was excluded because of failure to fulfill the diagnostic criteria of MS according to the International Panel criteria.17,18 The group of 45 patients with MS was composed of 32 with relapsing-remitting (RR) MS, 8 with secondary- progressive (SP) MS, and 5 with primary-progressive (PP) MS.19,20
Within 1 month of the MR imaging examination, every patient underwent a full neurologic examination and a clinical evaluation, including the expanded disability status scale (EDSS)21 and ambulation index (AI),22 by a neurologist who specializes in MS. Although all patients were participating in various research studies and drug trials, 13 patients were receiving no disease-modifying treatment during the period of the study because of their early or mild disease course. Thirteen patients were treated with glatiramer acetate, 11 with interferon beta 1a, 3 with corticosteroids (but not within the last month), 3 with cyclophosphamide, 1 with interferon beta 1b, and 1 with intravenous immunoglobulin. The patients were compared with an age-matched and sex-matched group of 29 healthy subjects. To ascertain the reproducibility of the measurement technique, we also evaluated the MR images of 20 patients from a separate study on segmentation reproducibility.23 These patients had 2 head MR imaging examinations on the same day. Between the 2 scans, each patient was brought outside the scanning area, simulating a serial study with repositioning error. This study was performed with Institutional Review Board approval.
Image Acquisition
MR images were acquired on a 1.5T system (Signa; GE Healthcare, Milwaukee, Wis). Axial dual-echo spin-echo images covering the whole brain were obtained in all subjects by use of a standard birdcage coil with a section thickness of 3 mm. The TR, TE1, and TE2 were 3000, 30, and 80 ms, respectively; the FOV was 24 cm; and the acquisition matrix was 256 × 192 with a nominal in-plane pixel size of 0.94 × 0.94 mm. The 45 patients had, in addition, a T2-weighted scan of the cervical spinal cord with use of the following parameters: The TR was between 3200 and 6350 ms, and the TE was 120 ms. A total of 32 sections were acquired with 1-mm gaps, an acquisition matrix of 256 × 256, and an FOV of 24 cm with a nominal in-plane pixel size of 0.47 × 0.47 mm. Axial T2-weighted fast spin-echo images of the spinal cord were acquired with use of a surface neck coil covering the whole cervical spine.
Image Analysis
MOV and Upper Cervical Cord Volume (UCCV).
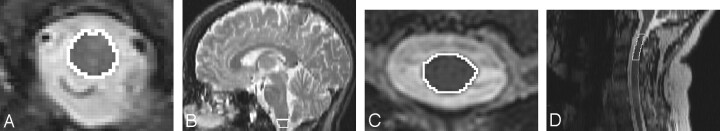
We performed all volumetric measurements of the lower medulla oblongata and the cervical spinal cord using 3D Slicer (www.slicer.org) by manually segmenting those structures on the original axial images (Fig 1). The MOV was measured on 3 contiguous sections, with the lowest section starting at the level of the foramen magnum. We limited our measurement to the most distal portion of the medulla oblongata to capture loss of white matter fibers while avoiding gray matter structures located in the proximal medulla oblongata. The sagittal reconstruction was used to find the opisthion, which is the midpoint on the posterior margin of the foramen magnum. This structure was located in the axial plane and used as the distal starting point for the measurement.
Fig 1.
Outline of the medulla oblongata and upper cervical cord in the axial and sagittal planes. Axial (A) and sagittal (B) outline of the medulla oblongata and axial (C) and sagittal (D) outline of the upper cervical cord.
To assess intrarater and interrater reproducibility, all medulla oblongata measurements for MS patients were done twice by the same investigator (Z.L.) and once by a second investigator (A.M.B.). Scan-rescan image sets were also analyzed by 1 investigator (O.F.). Three of these scan-rescan datasets had to be excluded because of artifacts or insufficient anatomic coverage. For blinding reasons, all MR imaging sets were performed anonymously before their analysis.
The upper cervical cord was outlined on 7 consecutive image sections, with the middle section at the level of the first intervertebral disk between the second and third vertebrae (C2 and C3; Fig). All medulla oblongata and upper cervical cord measurements were divided by the subject/population ratio of the intracranial cavity (ICC) to normalize these measures, thereby correcting for variation in subjects’ head size on the basis of the results of several previous reports.24–29
Brain Morphometry
For the measurement of the ICC and brain parenchymal fraction (BPF), we used template-driven segmentation plus partial volume effect correction (TDS+), which is a segmentation method that uses both signal intensity characteristics and anatomic locations to subdivide the brain into WM (normal-appearing WM and lesions), gray matter (GM), and CSF.30 TDS+ was carried out on the dual-echo images of the brain. The BPF was defined as BPF = (WM+GM)/(WM+GM+CSF) = 1 -CSF/ICC.
Statistical Analysis
We calculated the correlation coefficients among MOV, UCCV, BPF, disease duration, and age using the Pearson correlation coefficient. As the EDSS and AI are ordinal variables, we used the Spearman rank correlation coefficient to measure the relationship between these variables and MOV, UCCV, and BPF. The linear relationship between all of the factors was confirmed by the appropriate plots (data not shown). To assess the significance of differences between correlation coefficients, we used the 2-sided Meng test31 for all Pearson correlation coefficients and the test proposed by Choi32 for Spearman correlation coefficients. The Dunn-Sidak method was used to correct for multiple comparisons.33 In addition, we examined whether MOV or UCCV significantly improved the model fit for the relationship between clinical disability landmarks and BPF by use of logistic regression models. Cutoff values of >4 for EDSS and >3 for AI were used to classify patients into 2 groups because these cutoff values reflected clinically relevant changes in the patient's ambulatory capacity. The likelihood ratio test was used to select the best model.
We compared the age-related MOV change in patients with MS and healthy control subjects using the following linear regression model:
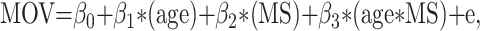 |
where MS is an indicator for disease status. If β3 is significantly different from 0, the rates of change in the healthy control subjects and the patients with MS are significantly different from each other.
Although the patients were mostly in the RR phase, the presence of a small sample of SPMS and PPMS patients allowed for a preliminary post hoc analysis. Because of the limited sample size, the Kruskal-Wallis and Mann-Whitney tests were used for the overall and pairwise comparisons. As above, to correct for multiple comparisons, we used the Dunn-Sidak method.
Finally, we calculated the intraclass correlation coefficient (ICcoeff)34 to determine the reproducibility of the MOV measurement. For the computation of the intrarater agreement and reproducibility of MOV measurement on scan-rescan images, a 1-way random model was used.34 For interrater agreement, we fit a 2-way random model using 1 observation per rater per scan. Although different guidelines exist for the interpretation of ICcoeff, we applied a scale35 in which an ICcoeff value of less than 0.40 indicates poor reproducibility; ICcoeff values in the range 0.40 to 0.75 indicate fair to good reproducibility; and an ICcoeff value of greater than 0.75 shows excellent reproducibility. We performed all statistical analyses using SPSS (version 14.0.1; SPSS, Chicago, Ill), StatXact (version 6; Cytel, Cambridge, Mass), and Matlab (version 2006b; Math Works, Natick, Mass).
Results
Demographics
Demographic and clinical characteristics of the 45 patients with MS and 29 healthy control subjects are shown in on-line Table 1. Patients and control subjects had similar distributions for age and sex. On-line Table 1 also compares demographic and clinical characteristics of the study's patient sample and the overall MS patient population of our clinic at the time of patient selection (October 2004). The patient sample seems to match our clinic's patient population reasonably well, with perhaps a slight underrepresentation of patients with SP compared with those with PP (all differences not significant, P > .110).
Does MOV Reflect Spinal Cord Pathologic Damage?
The correlation coefficient between MOV and UCCV was r = 0.67, whereas that between MOV and BPF was r = 0.45. This difference did not reach statistical significance (Meng test: P = .086; difference in the correlation coefficients = 0.22, confidence interval − 0.05 to 0.67). To put this finding in the context of measurement variability within the cervical spinal cord, we subdivided UCCV into 2 volumes of 3 sections each. The first one contained the volume of spinal cord at the level of C2; the second was the volume of the cord at the level of C3. The correlation between these 2 volumes was r = 0.85.
MOV and Motor Disability
MOV and UCCV showed similar degree of correlation with EDSS (r = −0.49 and r = −0.48, respectively). The correlation coefficient between EDSS and BPF was r = −0.59. The test proposed by Choi32 did not demonstrate a significant difference between these correlation coefficients. The correlations of AI with MOV, UCCV, and BPF were r = −0.52, r = −0.52, and r = −0.58, respectively (on-line Table 2). Again, none of these correlation coefficients were significantly different.
In terms of clinical landmarks of disability, the optimal model for EDSS of more than 4 included only BPF; including MOV (P = .287) or UCCV (P = .125) did not significantly improve the model. The odds ratio for 0.01 U decrease in BPF was 1.53. For AI of more than 3, including MOV in addition to BPF significantly improved model fit (P = .040), but the addition of UCCV did not (P = .066). For the final model with MOV and BPF, the odds ratios for a 0.01 decrease in MOV and BPF, holding the other factor constant, were 1.09 and 1.11, respectively.
Age and Disease Effects on MOV and UCCV
In our patients, both age and disease duration showed statistically significant inverse correlations with MOV (r = −0.47 and r = −0.64, respectively). In a similar fashion, the correlations between UCCV and disease duration (r = −0.61) or BPF and disease duration (r = −0.50) also showed moderate correlation (on-line Table 2).
Difference between Healthy Control Subjects and Patients with MS on MOV
Patients with MS had a significantly smaller MOV (mean ± SD, 1.02 ± 0.17 cm3) than healthy control subjects (1.15 ± 0.15 cm3) (P = .012). In the patients, age-related medulla oblongata atrophy was −0.008 cm3/year of age (P = .001), whereas for healthy subjects, no significant linear relationship between MOV and age was found (0.002 cm3/year of age, P = .324) with use of the linear regression model. Age-related changes of MOV were significantly different between patients and healthy control subjects (P = .003). There was no significant difference between the BPF of the patients (mean ± SD, 0.86 ± 0.05 cm3) and the BPF of the healthy control subjects (mean ± SD, 0.87 ± 0.04 cm3).
Post Hoc Analysis
As this study was primarily designed to validate the measurement of MOV, subjects were not selected according to clinical subtypes. The patients with MS in the study population were mostly in the RR phase (n = 32); 8 had SPMS and 5 had PPMS. For all 3 MR imaging measures, the Kruskal-Wallis test showed significant differences between the groups (P < .001 for MOV, P = .028 for UCCV, and P < .001 for BPF). A preliminary post hoc analysis was performed to evaluate the differences in MOV between the established clinical phenotypes (on-line Table 3 and 4). The largest mean MOV was found in patients with RRMS (1.08 cm3), whereas patients with SPMS or PPMS had a lower mean MOV (SPMS, 0.88 cm3 and PPMS, 0.95 cm3). The difference between the MOV of RRMS and patients with progressive MS (SPMS or PPMS) was statistically significant (on-line Table 4). BPF was also able to differentiate between RRMS and patients with progressive MS, whereas UCCV was not. No significant difference was found between patients with SPMS and those with PPMS or between those with RRMS and healthy control subjects (on-line Table 4).
Reproducibility Analysis
We assessed the intrarater, interrater, and scan-rescan reproducibility of MOV using the ICcoeff. For the intrarater and interrater reproducibility analysis, ICcoeff values of 0.97 and 0.79 were obtained, indicating excellent reproducibility.35 The scan-rescan measurements were also highly reproducible with an ICcoeff of 0.81.
Discussion
We propose a relatively simple method to measure MOV that could serve as a biomarker of spinal cord damage and clinical disability in MS. The correlation between MOV and UCCV (r = 0.67) underscores the relative sensitivity of this metric to spinal cord atrophy. In comparison with our results, a previous study36 reported smaller correlation coefficients between the whole brain stem volume and the upper cervical cord area in patients with RRMS (r = 0.35) and patients with SPMS (r = 0.38) possibly because of the inclusion of brain stem gray matter. In aggregate, these findings support the role of MOV as a biomarker of spinal cord damage in MS.
To assess the strength of associations between the MR imaging measures (UCCV and MOV) and clinical disability, we compared our results with those of previous studies. These studies have reported correlation coefficients between the upper cervical cord area and EDSS ranging between −0.34 and −0.702,12,15,27,37 and a correlation of −0.60 between the upper cervical cord area and AI.27 Our findings are consistent with these results, as the correlation of UCCV with EDSS and AI were −0.48 and −0.52, respectively. MOV showed a similar relationship with clinical disability: the correlation coefficients between MOV and EDSS or AI were r = −0.49 and −0.52, respectively. In summary, the associations of MOV and clinical scores emulate those with previous spinal cord metrics.
Many studies have investigated the use of BPF as a predictor of EDSS.38–41 One previous MR imaging study reported a correlation between BPF and EDSS (r = −0.391) and found that BPF was the best predictor of EDSS.42 Therefore, we included BPF in all prediction models and tested whether the addition of UCCV or MOV would add predictive value to those models. The logistic regression analysis showed that the models predicting EDSS did not improve with the addition of either MOV or UCCV to BPF. However, BPF and MOV predicted AI better than BPF alone, whereas including UCCV instead of MOV did not significantly improve the predictive model of AI. However, it should be noted that our spinal cord measurements were not done on MR images with a single optimized MR imaging protocol, whereas the brain measurements were. This might be an explanation for the slightly better results of MOV compared with UCCV. To assess the fidelity of our UCCV measurements, we compared them to findings reported in the literature. In our study, the mean cross-sectional area was 70.64 mm2 for PPMS, 60.00 mm2 for SPMS, and 74.52 mm2 for RRMS. Previous studies have reported means of cross-sectional areas of the cervical spinal cord (at the level of C2) ranging from 67.82 to 73.1 mm2 for PPMS, 61.2 mm2 for SPMS, and 72.65 to 85.6 mm2 for RRMS.12,26,43 These results are consistent with our results despite some variability in the acquisition parameters of our MR images of the spinal cord.
MR imaging–based studies have reported a correlation of r = −0.281 between disease duration and BPF42 and a correlation of r = −0.75 between disease duration and spinal cord measures.37 In our study, the correlations of disease duration with BPF and UCCV (r = −0.50 and −0.61, respectively) were consistent with those of published findings. A similar relationship was found between disease duration and MOV (r = −0.64). In summary, MOV findings parallel findings in the spinal cord in the detection of degenerative aspects of MS.
To further document the atrophy of the medulla oblongata in MS, we compared the MOV of the patients with that of healthy control subjects and found a significant difference between these groups. In a similar fashion, histopathologic7,8,44 and MR imaging studies12,15,37 have shown that spinal cord measures are smaller in patients with MS than in healthy control subjects. Age-related brain atrophy is considered a normal aspect of aging and is a confounder in the assessment of MS-specific degeneration with BPF. In contrast, previous studies and our results on MOV suggest that the medulla oblongata, the whole brain stem, as well as the spinal cord, do not show significant age-related atrophy in healthy people.24–26,45 Because MOV does not seem to change significantly with age in healthy volunteers but shows disease-dependent atrophy in patients with MS, the decline in MOV might provide a valuable complementary measure of degenerative processes in MS that is not as prone to covariance with age as BPF.
Our preliminary subgroup analysis demonstrates that patients with a progressive disease course (SPMS or PPMS) had a smaller MOV than patients with RRMS or healthy control subjects. Other studies have also demonstrated an increased reduction of the spinal cord in patients with progressive MS compared with patients with RR or healthy control subjects.12,15,37,43 As our results in the MS subgroup comparisons are based on small numbers of patients with SP and PPMS, future studies are required to confirm these preliminary results.
Good intraobserver and interobserver agreement was demonstrated for the measurement of MOV. The ICcoeff value was 0.97 for intrarater and 0.79 for interrater reproducibility. An ICcoeff value of greater than 0.75 denotes excellent agreement.35 In this work, we studied the interobserver agreement only between 2 observers. Future studies should assess the interobserver agreement among a larger number of observers to further assess the robustness of this measure. Note that in this work, all medulla oblongata and upper cervical cord measurements were divided by the subject/population ratio of the ICC to normalize these measures. A similar methodology was applied in several previous studies.24–29
Although our MOV measurement technique seems to be adequate for assessing group differences in MOV, further improvement in the measurement technique may be required to assess longitudinal changes in individual patients. The use of higher-resolution sequences could provide a more accurate and robust measurement of MOV. We also did not try to account for partial volume effects, which might improve reproducibility and sensitivity. In future studies, we plan to address these methodologic challenges.
In this study, only patients who received both a brain and spinal cord MR imaging examination were included. Because not every patient at our center undergoes spinal cord MR imaging examination, this may be a potential source of selection bias. To address this issue, we compared the demographic and clinical characteristics of the patients in our study to the overall MS patient population at our center. Because no differences were found between these groups on several characteristics, the study patient population was a good representative sample of our MS patient population. Despite the fact that most patients were undergoing treatment, we were able to find a difference between patients with MS and healthy control subjects. However, the study was not designed to observe treatment effects, and we did not control for this factor. To be considered as a surrogate marker, future studies should assess whether MOV is able to capture specific treatment effects.
A significant correlation between axonal loss and N-acetylaspartate levels was found in spinal cord lesions of patients with MS with severe disability.46 Axonal loss results from both primary (axonal transection) and secondary (wallerian/retrograde/trans-synaptic degeneration) mechanisms.47 Axonal degeneration is likely to be the most significant factor causing spinal cord atrophy, rather than the loss of tissue related to individual cord lesions.8 In addition, local cord atrophy typically does not develop at the site of lesions.3 In our study, we found 14 patients with lesions in the portion of the medulla oblongata that we measured. No significant difference was found when patients with or without lesions were compared (data not shown). It seems reasonable to assume that both the spinal cord and the medulla oblongata are affected in a similar way by the pathologic destructive processes of MS, as they are predominately composed of the same ascending and descending fiber tracts as the ones passing through the spinal cord.
It is not too surprising that we were unable to demonstrate a relationship between spinal cord lesion load and atrophy (UCCV or MOV; data not shown) but did not deem the quality of our images sufficient to conclusively disprove such a relationship. The assessment of spinal cord lesions in clinical practice is still considered challenging and might be a contributing factor to the lack of correlation between spinal lesion load and EDSS, even when spinal cord atrophy and EDSS were correlated.1,13 MOV, as UCCV, may currently represent the only viable alternative to assess spinal cord involvement in clinical practice.
Furthermore, we suggest that the medulla oblongata may provide a unique opportunity to study the neurodegenerative component of MS. One plausible mechanism of reduction in MOV is linked to primary or secondary degeneration of axons superior and inferior to the medulla. The segmental/internodal organization of axonal myelination by oligodendrocytes makes it unlikely that demyelination outside of the medulla would directly contribute to medullary atrophy. Therefore, we suggest that MOV might also provide a simple way to assess axonal degeneration in MS.
MOV is an easily obtainable measure that presents nonnegligible advantages, especially in the context of large-scale clinical trials in MS. Obtaining measures of MOV is relatively easier than obtaining measures of spinal cord volumes because the contrast between the surrounding CSF and the medulla oblongata is high on T2-weighted images. Furthermore, the medulla oblongata has a larger diameter than that of the spinal cord, which facilitates its cross-sectional outlining.
Conclusion
We have proposed a straightforward method for MOV measurement on routine clinical head MR imaging. Preliminary results have demonstrated the use of MOV as a potential biomarker marker of spinal cord damage in MS.
Supplementary Material
Footnotes
This study was supported in part by funding provided by the National Multiple Sclerosis Society grant as well as the National Institutes of Health. Z. Liptak and A.M. Berger contributed equally to the study.
Paper previously presented in part as a poster at: Meeting of American Academy of Neurology, April 28–May 5, 2007; Boston, Mass.
Indicates article with supplemental on-line tables.
References
- 1.Bakshi R, Dandamudi VS, Neema M, et al. Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging 2005;15:30S–45S. [DOI] [PubMed] [Google Scholar]
- 2.Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain 1998;121 (Pt 4):687–97 [DOI] [PubMed] [Google Scholar]
- 3.Lycklama G, Thompson A, Filippi M, et al. Spinal-cord MRI in multiple sclerosis. Lancet Neurol 2003;2:555–62 [DOI] [PubMed] [Google Scholar]
- 4.Bot JC, Barkhof F, Polman CH, et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology 2004;62:226–33 [DOI] [PubMed] [Google Scholar]
- 5.Bergers E, Bot JC, van der Valk P, et al. Diffuse signal abnormalities in the spinal cord in multiple sclerosis: direct postmortem in situ magnetic resonance imaging correlated with in vitro high-resolution magnetic resonance imaging and histopathology. Ann Neurol 2002;51:652–56 [DOI] [PubMed] [Google Scholar]
- 6.Gilmore CP, DeLuca GC, Bo L, et al. Spinal cord atrophy in multiple sclerosis caused by white matter volume loss. Arch Neurol 2005;62:1859–62 [DOI] [PubMed] [Google Scholar]
- 7.DeLuca GC, Ebers GC, Esiri MM. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain 2004;127:1009–18 [DOI] [PubMed] [Google Scholar]
- 8.Evangelou N, DeLuca GC, Owens T, et al. Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain 2005;128:29–34 [DOI] [PubMed] [Google Scholar]
- 9.Simon JH. Brain atrophy in multiple sclerosis: what we know and would like to know. Mult Scler 2006;12:679–87 [DOI] [PubMed] [Google Scholar]
- 10.Agosta F, Benedetti B, Rocca MA, et al. Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology 2005;64:631–35 [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Tench CR, Evangelou N, et al. Measurement of spinal cord atrophy in multiple sclerosis. J Neuroimaging 2004;14:20S–26S. [DOI] [PubMed] [Google Scholar]
- 12.Losseff NA, Webb SL, O'Riordan JI, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996;119 (Pt 3):701–08 [DOI] [PubMed] [Google Scholar]
- 13.Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology 1993;43:2632–37 [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Tench CR, Turner B, et al. Spinal cord atrophy and disability in multiple sclerosis over four years: application of a reproducible automated technique in monitoring disease progression in a cohort of the interferon beta-1a (Rebif) treatment trial. J Neurol Neurosurg Psychiatry 2003;74:1090–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippi M, Campi A, Colombo B, et al. A spinal cord MRI study of benign and secondary progressive multiple sclerosis. J Neurol 1996;243:502–05 [DOI] [PubMed] [Google Scholar]
- 16.Zivadinov R, Bakshi R. Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging 2004;14:27S–35S. [DOI] [PubMed] [Google Scholar]
- 17.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–27 [DOI] [PubMed] [Google Scholar]
- 18.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120 (Pt 11):2059–69 [DOI] [PubMed] [Google Scholar]
- 19.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–11 [DOI] [PubMed] [Google Scholar]
- 20.Thompson AJ, Montalban X, Barkhof F, et al. Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol 2000;47:831–35 [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52 [DOI] [PubMed] [Google Scholar]
- 22.Hauser SL, Dawson DM, Lehrich JR, et al. Intensive immunosuppression in progressive multiple sclerosis. A randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med 1983;308:173–80 [DOI] [PubMed] [Google Scholar]
- 23.Guttmann CR, Kikinis R, Anderson MC, et al. Quantitative follow-up of patients with multiple sclerosis using MRI: reproducibility. J Magn Reson Imaging 1999;9:509–18 [DOI] [PubMed] [Google Scholar]
- 24.Luft AR, Skalej M, Schulz JB, et al. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex 1999;9:712–21 [DOI] [PubMed] [Google Scholar]
- 25.Murshed KA, Ziylan T, Seker M, et al. Morphometric assessment of brain stem und cerebellar vermis with midsagittal MRI: the gender differences and effects of age. Neuroanatomy 2003;2:35–38 [Google Scholar]
- 26.Rashid W, Davies GR, Chard DT, et al. Increasing cord atrophy in early relapsing-remitting multiple sclerosis: a 3 year study. J Neurol Neurosurg Psychiatry 2006;77:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaithianathar L, Tench CR, Morgan PS, et al. Magnetic resonance imaging of the cervical spinal cord in multiple sclerosis–a quantitative T1 relaxation time mapping approach. J Neurol 2003;250:307–15 [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Edwards S, Gong Q, et al. Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 1999;66:323–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashid W, Davies GR, Chard DT, et al. Upper cervical cord area in early relapsing-remitting multiple sclerosis: cross-sectional study of factors influencing cord size. J Magn Reson Imaging 2006;23:473–76 [DOI] [PubMed] [Google Scholar]
- 30.Wei X, Warfield SK, Zou KH, et al. Quantitative analysis of MRI signal abnormalities of brain white matter with high reproducibility and accuracy. J Magn Reson Imaging 2002;15:203–09 [DOI] [PubMed] [Google Scholar]
- 31.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psych Bull 1992;111:172–75 [Google Scholar]
- 32.Choi SC. Tests of equality of dependent correlation coefficients. Biometrika 1977;64:645–47 [Google Scholar]
- 33.Sokal RR, Rohlf FJ. Biometry. New York: W.H. Freeman;1995
- 34.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psych Methods 1996;1:30–46 [Google Scholar]
- 35.Rosner B. Fundamentals of Biostatistics. 6th ed. Duxbury Press;2005
- 36.Lin X, Blumhardt LD, Constantinescu CS. The relationship of brain and cervical cord volume to disability in clinical subtypes of multiple sclerosis: a three-dimensional MRI study. Acta Neurol Scand 2003;108:401–06 [DOI] [PubMed] [Google Scholar]
- 37.Stevenson VL, Leary SM, Losseff NA, et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology 1998;51:234–38 [DOI] [PubMed] [Google Scholar]
- 38.Rudick RA, Fisher E, Lee JC, et al. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler 2000;6:365–72 [DOI] [PubMed] [Google Scholar]
- 39.Fisher E, Rudick RA, Cutter G, et al. Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult Scler 2000;6:373–77 [DOI] [PubMed] [Google Scholar]
- 40.Bakshi R, Benedict RH, Bermel RA, et al. Regional brain atrophy is associated with physical disability in multiple sclerosis: semiquantitative magnetic resonance imaging and relationship to clinical findings. J Neuroimaging 2001;11:129–36 [DOI] [PubMed] [Google Scholar]
- 41.Sanfilipo MP, Benedict RH, Sharma J, et al. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage 2005;26:1068–77 [DOI] [PubMed] [Google Scholar]
- 42.Bermel RA, Sharma J, Tjoa CW, et al. A semiautomated measure of whole-brain atrophy in multiple sclerosis. J Neurol Sci 2003;208:57–65 [DOI] [PubMed] [Google Scholar]
- 43.Bieniek M, Altmann DR, Davies GR, et al. Cord atrophy separates early primary progressive and relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2006;77:1036–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganter P, Prince C, Esiri MM. Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol Appl Neurobiol 1999;25:459–67 [DOI] [PubMed] [Google Scholar]
- 45.Raininko R, Autti T, Vanhanen SL, et al. The normal brain stem from infancy to old age. A morphometric MRI study. Neuroradiology 1994;36:364–68 [DOI] [PubMed] [Google Scholar]
- 46.Bjartmar C, Kidd G, Mork S, et al. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 2000;48:893–901 [PubMed] [Google Scholar]
- 47.Perry VH, Anthony DC. Axon damage and repair in multiple sclerosis. Philos Trans R Soc Lond B Biol Sci 1999;354:1641–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.