Abstract
BACKGROUND AND PURPOSE: Our aim was to assess the feasibility of carotid artery stent placement (CAS) for calcified lesions.
MATERIALS AND METHODS: Using embolic protection devices (EPDs), we performed 51 CAS procedures in 43 patients with severe carotid artery stenosis accompanied by plaque calcification. Before intervention, all lesions were subjected to multidetector-row CT. The arc of the circumferential plaque calcification was measured on axial source images at the site of maximal luminal stenosis, and the total volume of the plaque calcification was determined. The angiographic outcome immediately after CAS, and intra- and postoperative complications were recorded.
RESULTS: The mean arc of calcification was 201.1 ± 72.3° (range, 76–352°), and the mean of the total calcification volume was 154.9 ± 35.4 mm3 (range, 92–2680 mm3). Balloon rupture occurred in 1 procedure (2.0%) at predilation angioplasty; all 51 CAS procedures were successful without clinical adverse effects. Although there was a correlation between the arc of plaque calcification and residual stenosis (r = 0.6, P < .001), excellent dilation with residual stenosis ≤30% was achieved in all lesions. There was no correlation between the total volume of calcification and residual stenosis. None of the patients developed stroke or death within 30 days of the CAS procedure.
CONCLUSION: CAS by using EPDs to treat lesions with plaque calcification is feasible even in patients with near-total circumferential plaque calcification.
Carotid artery stent placement (CAS) is being evaluated as an alternative to carotid endarterectomy. CAS procedures are currently performed in high-risk patients, and favorable outcomes have been reported.1 The characteristics of the lesion are important factors influencing technical results and outcomes. Heavily calcified stenoses have been classified as complex lesions because their treatment success rate is lower and the incidence of acute dissection of the coronary artery and other vessels in the peripheral vascular territory is higher.2,3 Severe lesion calcification has been considered a relative contraindication for CAS because it prevents adequate stent expansion.4,5 However, the lesion calcifications had been evaluated only angiographically. A correlation between severe lesion calcification and stroke after CAS was reported6; most of the affected patients were treated without the use of embolic protection devices (EPDs). Therefore, the outcomes of CAS by using EPDs to address calcified lesions have not been well established.
We performed morphologic and quantitative evaluation of plaque calcification by multidetector-row CT (MDCT) before CAS. Here we report the characteristics of plaque calcifications and the short-term angiographic and clinical outcomes after CAS with EPDs.
Materials and Methods
Patient Selection
Between January 2006 and August 2007, we performed 51 CAS procedures to treat 43 nonconsecutive patients with severe carotid stenosis with plaque calcification. Thirty-three of 51 lesions (64.7%) were symptomatic, and 18 (35.3%) were asymptomatic. The presence of calcification on diagnostic carotid angiograms was determined consensually by 2 observers. Before CAS, all lesions were subjected to MDCT for detailed evaluation of plaque calcification. No lesions were excluded from CAS on the basis of preoperative MDCT imaging. Recorded patient baseline characteristics were age, sex, hypertension, diabetes mellitus (medication-dependent including oral hypoglycemic agents and insulin), hypercholesterolemia (medication-dependent or serum cholesterol ≥240 mg/dL), coronary artery disease (history of angina pectoris or myocardial infarction), and smoking habit (still smoking or stopped smoking <6 months before the study). Intra- and postoperative events were also recorded. All patients gave their prior informed consent for participation in our study, which was approved by the ethics committee of our institute.
Endovascular Procedure
All patients received aspirin (100 mg/day) and a thienopyridine drug (ticlopidine 200 mg/day or clopidogrel 75 mg/day) for at least 3 days before CAS. The patients were placed under general anesthesia, a bolus injection of heparin (80 IU/kg) was delivered, and CAS was performed by using the PercuSurge system (Medtronic, Santa Rosa, Calif). Atropine sulfate (0.5 mg) was injected intravenously just before balloon inflation for predilation, which was achieved with a 4 or 5 × 40 mm Symmetry balloon catheter (Boston Scientific, Natick, Mass); the inflation pressure was 6 atm for 60 seconds. A self-expandable stent (10 × 20 mm Wallstent RP, Boston Scientific) was then deployed. Postdilation was with a 6 or 7 × 40 mm Ultra-Soft SV monorail balloon catheter (Boston Scientific); the inflation pressure was 7 atm for 10 seconds. In cases with insufficient stent expansion, the balloon was additionally inflated up to 10 atm.
Immediately after CAS, the lesions were evaluated angiographically in the anteroposterior and lateral directions for residual stenosis. Percentage stenosis was determined according to the criteria of the North American Symptomatic Carotid Endarterectomy Trial.7
MDCT Angiography
All patients underwent MDCT angiography with a 64-section scanner (Somatom Sensation 64; Siemens, Munich, Germany). Images were obtained from the aortic arch to the supraventricular white matter by using a helical acquisition (2.3 mm/rotation and 0.6 × 0.6 mm collimation, 120 kilovolt [peak]). A standard reconstruction kernel/algorithm was used. Iohexol (Omnipaque 300, 100–125 mL; Nycomed, Princeton, NJ) was injected at a rate of 4.0–4.5 mL/s; the delay was determined by an automated bolus-timing program. Image data were transferred to a computer workstation (Aquarius Net Station, Version 1.4; Tera Recon, San Mateo, Calif) for postprocessing.
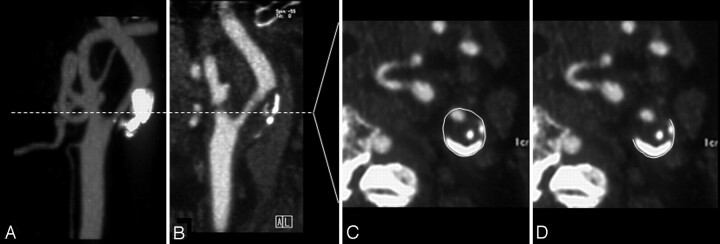
One experienced neuroradiologist, blinded to the clinical information, performed all measurements. Luminal stenosis was evaluated by analysis of axial sections, multiplanar reconstructions, maximum intensity projections, and 3D volume-rendering reconstruction for optimal assessment. Calcium deposits were defined as structures with an attenuation >130 HU within the vessel wall that were hyperattenuated to the contrast-enhanced lumen and surrounding parenchyma. In most cases, we used wide fixed window settings (700-HU window, 250-HU level), though several patients required individual changes in the level and windows for optimal visualization (range, 600-1000 HU window; 150–400 HU level).8,9 To determine the total volume of the plaque calcification, we prepared contiguous axial source images of the index carotid lesion manifesting stenosis. The plaque calcification volume was determined by manually tracing the calcified plaque on each section for the length of the plaque; the sum of the areas of calcium multiplied by the section increment was used to determine the volume in cubic millimeters.8,9 The circumferential distribution of plaque calcifications was evaluated on axial source images at the point of maximal luminal stenosis. To determine the arc of the plaque calcification, we manually traced and measured the outside circumference of the calcification and the vessel circumference. We measured the degree of arc of the calcification: external circumference of the calcified plaque/external circumference of the carotid artery ×360° (Fig 1). If the calcified plaque consisted of several compartments, we evaluated the sum of the arcs.
Fig 1.
Maximum intensity projection (A) and sagittal reformat (B) of a CT angiogram reveal left internal carotid artery (ICA) stenosis with severe calcification. The entire circumference of the ICA (C) and the circumference of the calcified plaque (D) on the axial source image are measured at the narrowest portion of the ICA. The arc (degree) of the calcification is calculated as: arc = external circumference of the calcified plaque/external circumference of the carotid artery ×360°.
Definitions
Clinical Outcome.
Events occurring ≤30 days of the CAS procedure were classified as perioperative complications. Stroke was recorded when symptoms persisted for more than 24 hours. Neurologic deficits lasting less than 24 hours were regarded as transient ischemic attacks. Death was recorded irrespective of cause.
Angiographic Outcome.
Angiographic success was recorded in cases with residual stenosis ≤30%.
Statistical Analysis
Continuous variables were expressed as the mean ± 1 SD. Categoric variables were expressed in terms of percentages. The Pearson correlation test was used to evaluate the relationship between variables. A P value of <.05 was considered statistically significant.
Results
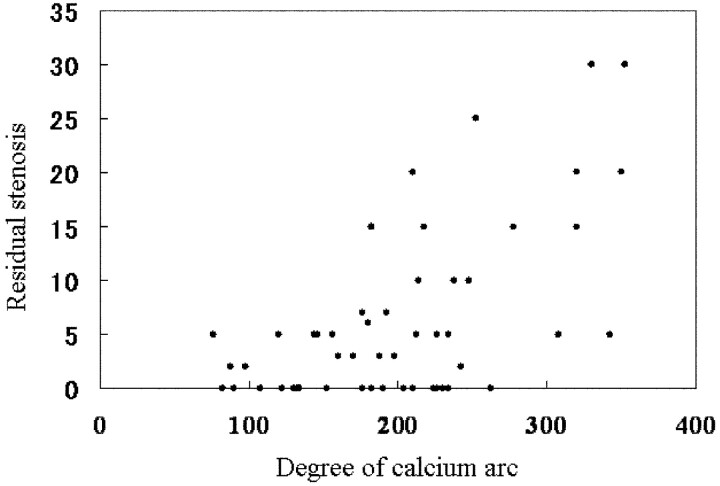
Of the 43 patients, 39 (90.7%) were men and 4 (9.3%) were women; their average age was 73.4 ± 8.3 years (range, 57–95 years). Clinical data included hypertension in 29 (67.4%) patients, diabetes mellitus in 17 (39.5%), hypercholesterolemia in 6 (37.2%), coronary artery disease in 8 (18.6%), and smoking in 21 (48.8%). The arc of plaque calcification ranged from 76° to 352° (mean, 201.1 ± 72.3°). The total volume of the plaque calcifications ranged from 92 to 2680 mm3 (mean, 154.9 ± 35.4 mm3). Balloon rupture occurred during predilation angioplasty in 1 of the 51 lesions (2.0%); the arc of the calcification in this lesion was 320°. All CAS procedures were successful, and excellent lesion dilation was achieved (Fig 2). Residual stenosis ranged from 0% to 30% (mean, 6.3 ± 8.0%). There was a correlation between the arc of calcification and residual stenosis (r = 0.6, P < .001, Fig 3). However, there was no correlation between the total calcification volume and residual stenosis (P = .12). No patient developed stroke or death within 30 days after the CAS procedure.
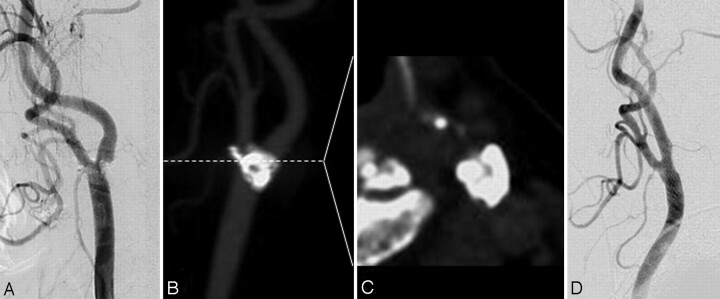
Fig 2.
Illustrative case (an 81-year-old woman). A, Left common carotid artery (CCA) angiogram demonstrates severe stenosis of the internal carotid artery (ICA) with heavy calcification. B, The maximum intensity projection CT angiogram reveals severe calcification at the bifurcation of the carotid artery. C, At the narrowest portion of the ICA, the arc of the plaque calcification is 342°. D, Left CCA angiogram demonstrates excellent dilation. There is a 5% residual stenosis of the lesion after CAS.
Fig 3.
The arc of calcium is plotted against the residual stenosis after CAS. The units represent the degrees of calcium arc by labeling the x-axis. There is a good correlation between these 2 variables (r = 0.6, P < .001).
Discussion
Although severely calcified lesions are a contraindication for CAS because of inadequate stent expansion and high procedural risks,4-6 we encountered no complications in any of our 51 lesions treated by CAS with EPDs. Although residual stenosis was proportionate to the arc of the plaque calcification, we obtained excellent dilation of all 51 lesions regardless of the degree of plaque calcification. Our results suggest that CAS with EPDs is a safe and effective method to address even lesions with near-total circumferential plaque calcification.
The luminal gain achieved by angioplasty and stent placement is mainly attributable to plaque compression and stretching of the vessel wall.10 However, the mechanism of stent expansion in calcified lesions remains unclear. Although some luminal gain may be achieved by plaque compression exerted by the stent strut even in patients with calcified lesions, the hardness of calcified plaques may resist sufficient plaque compression. Some authors 11,12 attributed most of the lumen gain to stretching of the vessel wall at the noncalcified segment. Our finding that the residual stenosis was proportionate to the arc of calcification strengthens this hypothesis. However, in the presence of near-total circumferential calcification, the lumen gain cannot be attributed solely to wall-stretching of the noncalcified segment. Therefore, we posit that stent expansion occurred at the calcified segment of these lesions. To achieve sufficient stent expansion in the presence of lesions with near-total circumferential calcification, the radial force of balloon angioplasty must overcome calcific resistance.10,12 Therefore, high-pressure balloon inflation of more than 16 atm has been recommended in percutaneous coronary intervention (PCI) to address calcified lesions; however, the success rate was moderate at best.10-12 In all our lesions we were able to obtain excellent stent expansion with balloon inflation ≤10 atm. Virmani et al13 reported that the incidence of calcified nodules, a form of calcification that produces irregular calcium nodules, is higher in carotid than in coronary artery disease (6%–7% versus 1%–2%). This pathologic characteristic of calcification, a larger vessel diameter, the devices we used, and other factors may account for the better result we obtained compared with outcomes reported in procedures addressing the PCI territory. Studies are underway in our laboratory to elucidate the mechanisms that underlie stent expansion in calcified lesions treated by CAS.
Balloon rupture is a rare complication of balloon angioplasty in PCI for calcified lesions.14-16 We encountered balloon rupture at predilation angioplasty in 1 of our 51 lesions (2.0%). Angioplasty may cause various plaque dissections. The sharp edges on dissected calcified plaques may produce balloon rupture.14 Balloon rupture has been reported as a relatively benign event,16 though it carries the risk for cerebral embolism. Therefore, we recommend that EPDs be used at predilation angioplasty in patients undergoing CAS for calcified lesions. Although stents can be placed without predilation balloon angioplasty,17 the irregular surface of hard calcified plaques may result in internal stent strut protrusion, and the protruding sharp edges of the stent strut may prevent the delivery of devices for postdilation angioplasty.3 Because the radial force of self-expandable stents cannot overcome calcific resistance,17 we subjected all lesions to predilation angioplasty.
In addition, we used Wallstent RP stents in all lesions. These stents are wire-braided, and their structure is a meshwork and closed-cell design. Closed-cell stents are easier for inserting the devices for postdilation angioplasty than open-cell stents featuring an interrupted mesh pattern.3 We encountered no difficulties in the delivery of the balloon catheter for postdilation. Moreover, closed-cell stents cover a greater percentage of the vascular wall within the stented region than open-cell stents.18 The presence of many stent struts between the postdilation balloon and the plaque may achieve high focal stress and result in excellent stent expansion.19,20 On the basis of these considerations, we regard closed-cell stents as more suitable than open-cell stents for the treatment of calcified lesions.
Preoperative plaque evaluation is important for a favorable CAS outcome. MDCT is useful for plaque evaluation in patients with carotid artery stenosis.21 It facilitates characterization and quantification of the plaque burden, fibrous tissue, and the lipid core in atherosclerotic carotid plaques.22,23 It is also an excellent method for assessing calcification, which correlates well with the histopathology of the carotid artery.8,9,22 Intravascular sonography (IVUS) imaging has been established as a useful technique for evaluating calcifications, and it can be used to estimate plaque calcification circumferentially and quantitatively.6,10-12 However, it raises the risk for cerebral embolism.6 On the other hand, IVUS with EPDs is thought to be safe. Because we used EPDs of the balloon-occlusion type in all lesions, we did not perform IVUS routinely to avoid the prolonged occlusion of the internal carotid artery. Rather, we performed MDCT scanning before CAS to obtain details on the plaque calcification. Although we found no correlation between the total calcification volume and residual stenosis, there was a correlation between the arc of the plaque calcification and residual stenosis. The correlation was not strong (r = 0.6) because we obtained angiographic confirmation of excellent dilation and residual stenosis was ≤30% in all lesions. In calcified lesions, the circumferential distribution of the calcification played a role in residual stenosis. Our results indicate that a single massive calcification on preoperative angiograms may not be a contraindication for CAS. To develop a treatment strategy in patients with carotid artery stenosis with plaque calcification, we suggest that MDCT be used to evaluate circumferential calcifications.
Study Limitations
Our study has some limitations. First, the number of carotid lesions with moderate-to-severe calcification was relatively small. The arc of calcification ranged up to 352°; however, the true total circumference of the calcifications was not determined. Second, we assessed only the circumferential distribution and total volume of plaque calcifications but did not determine the thickness or length of the calcifications. Third, we evaluated only the acute procedural results of CAS without collecting follow-up data. To determine the true efficacy of CAS in patients with calcified lesions, we will carry out a prospective large cohort study of CAS, in which we will compare the long-term outcomes in patients with calcified and noncalcified lesions.
Conclusion
We showed that CAS procedures with EPDs are feasible in patients with calcified carotid artery stenoses, even those with near-total circumferential plaque calcification. We obtained excellent dilation of residual angiographic stenoses in ≤30% of all lesions and found that the degree of circumferential plaque calcification had the greatest effect on the residual stenosis.
Acknowledgments
We thank Ms. Ursula Petralia for assistance in preparing this article.
References
- 1.Yadav JS, Wholey MH, Kuntz RE, et al, for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501 [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty: an observational study using intravascular ultrasound. Circulation 1992;86:64–70 [DOI] [PubMed] [Google Scholar]
- 3.Onal B, Ilgit ET, Yücel C, et al. Primary stenting for complex atherosclerotic plaques in aortic and iliac stenoses. Cardiovasc Intervent Radiol 1998;21:386–92 [DOI] [PubMed] [Google Scholar]
- 4.Roubin GS, Iyer S, Halkin A, et al. Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation 2006;113:2021–30 [DOI] [PubMed] [Google Scholar]
- 5.Choi HM, Hobson RW, Goldstein J, et al. Technical challenges in a program of carotid artery stenting. J Vasc Surg 2004;40:746–51 [DOI] [PubMed] [Google Scholar]
- 6.Clark DJ, Lessio S, O'Donoghue M, et al. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv 2004;63:355–62 [DOI] [PubMed] [Google Scholar]
- 7.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 8.Nandalur KR, Baskurt E, Hagspiel KD, et al. Carotid artery calcification on CT may independently predict stroke risk. AJR Am J Roentgenol 2006;186:547–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandalur KR, Hardie AD, Raghavan P, et al. Composition of the stable carotid plaque: insights from a multidetector computed tomography study of plaque volume. Stroke 2007;38:935–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht D, Kaspers S, Füssl R, et al. Coronary plaque morphology affects stent deployment: assessment by intracoronary ultrasound. Cathet Cardiovasc Diagn 1996;38:229–35 [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann R, Mintz GS, Popma JJ, et al. Treatment of calcified coronary lesions with Palmaz-Schatz stents: an intravascular ultrasound study. Eur Heart J 1998;19:1224–31 [DOI] [PubMed] [Google Scholar]
- 12.Vavuranakis M, Toutouzas K, Stefanadis C, et al. Stent deployment in calcified lesions: can we overcome calcific restraint with high-pressure balloon inflations? Catheter Cardiovasc Interv 2001;52:164–72 [DOI] [PubMed] [Google Scholar]
- 13.Virmani R, Ladich ER, Burke AP, et al. Histopathology of carotid atherosclerotic disease. Neurosurgery 2006;59 (5 Suppl 3):S219–27 [DOI] [PubMed] [Google Scholar]
- 14.Carell ES, Schroth G, Ali A. Circumferential balloon rupture and catheter fracture due to entrapment in a calcified coronary stenosis. Cathet Cardiovasc Diagn 1994;32:346–48 [DOI] [PubMed] [Google Scholar]
- 15.Asakura Y, Furukawa Y, Ishikawa S, et al. Successful predilation of a resistant, heavily calcified lesion with cutting balloon for coronary stenting: a case report. Cathet Cardiovasc Diagn 1998;44:420–22 [DOI] [PubMed] [Google Scholar]
- 16.Simpfendorfer CC, Dimas AP, Zaidi A, et al. Balloon rupture during coronary angioplasty. Angiology 1986;37:828–31 [DOI] [PubMed] [Google Scholar]
- 17.Lownie SP, Pelz DM, Lee DH, et al. Efficacy of treatment of severe carotid bifurcation stenosis by using self-expanding stents without deliberate use of angioplasty balloons. AJNR Am J Neuroradiol 2005;26:1241–48 [PMC free article] [PubMed] [Google Scholar]
- 18.Hart JP, Peeters P, Verbist J, et al. Do device characteristics impact outcome in carotid artery stenting? J Vasc Surg 2006;44:725–30 [DOI] [PubMed] [Google Scholar]
- 19.Stillabower ME. Longitudinal force focused coronary angioplasty: a technique for resistant lesions. Cathet Cardiovasc Diagn 1994;32:196–98 [DOI] [PubMed] [Google Scholar]
- 20.Yazdanfar S, Ledley GS, Alfieri A, et al. Parallel angioplasty dilatation catheter and guide wire: a new technique for the dilatation of calcified coronary arteries. Cathet Cardiovasc Diagn 1993;28:72–75 [DOI] [PubMed] [Google Scholar]
- 21.Bartlett ES, Walters TD, Symons SP, et al. Quantification of carotid stenosis on CT angiography. AJNR Am J Neuroradiol 2006;27:13–19 [PMC free article] [PubMed] [Google Scholar]
- 22.de Weert TT, Ouhlous M, Meijering E, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26:2366–72 [DOI] [PubMed] [Google Scholar]
- 23.Hardie AD, Kramer CM, Raghavan P, et al. The impact of expansive arterial remodeling on clinical presentation in carotid artery disease: a multidetector CT angiography study. AJNR Am J Neuroradiol 2007;28:1067–70 [DOI] [PMC free article] [PubMed] [Google Scholar]