Abstract
BACKGROUND AND PURPOSE: CT perfusion is a much more readily accessible imaging method to assess cerebral hemodynamic status than single-photon emission CT. We prospectively assessed quantitative cerebrovascular reserve by using acetazolamide (ACZ)-challenged CT perfusion for evaluating hemodynamic impairment in ischemic adult Moyamoya disease and compared it with angiographic findings.
MATERIALS AND METHODS: Sixteen adult patients with ischemic Moyamoya disease and 12 age-matched normal control subjects underwent both ACZ-challenged CT perfusion and digital subtraction angiography. Normalized baseline hemodynamic parameters and their percent changes (PCs) were calculated in 56 hemispheres. We classified the degrees of distal carotid artery stenosis according to modified Suzuki stage and determined the presence of basal Moyamoya vessels (BMVs). The values of normalized parameters and their PCs were compared with angiographic findings.
RESULTS: Normalized baseline mean transit time (MTT) and PC of normalized cerebral blood flow (CBF) were significantly correlated with angiographic stages in all of the vascular territories; however, the correlation coefficient of the normalized baseline MTT was lower than that of the PC of CBF. In the external borderzone and the middle cerebral arterial territory, the hemispheres with extensive BMVs exhibited significantly lower PC values of CBF and significantly higher normalized baseline MTT values than those in hemispheres with diminished BMVs and in normal control subjects.
CONCLUSION: Among the hemodynamic parameters measured by ACZ-challenged CT perfusion, the PC of CBF correlated highly significantly with angiographic stage; however, the normalized baseline CT perfusion parameters showed weak or no significant correlation.
Moyamoya disease is an idiopathic progressive occlusive cerebrovascular disorder of unknown etiology. This disease has angiographically been characterized by numerous enlarged lenticulostriated and thalamoperforating arteries, as well as dural, leptomeningeal, and pial collateral vessels.1–4 The most common clinical presentation is recurrent transient ischemic attacks or strokes, resulting in significant morbidity. In Moyamoya disease, as in other chronic steno-occlusive cerebrovascular diseases, the steno-occlusive change in the main cerebral arteries decreases the cerebral perfusion pressure, and collateral circulation maintains cerebral blood flow (CBF).4 According to a few earlier reports, patients with Moyamoya diseases have decreased CBF, increased cerebral blood volume (CBV), and delayed mean transit time (MTT).3–6 These hemodynamic changes in Moyamoya disease have been explained by decreases in the cerebral perfusion pressure and associated vasodilation.3 A few studies investigated the relationship between cerebral angiographic findings and hemodynamic status in Moyamoya disease.5–7 Most patients show extensive development of basal Moyamoya vessels (BMV) and present ischemic events.
Acetazolamide (ACZ), a carbonic anhydrase inhibitor, indirectly dilates the cerebrovasculature by increasing carbon dioxide levels in the blood stream.8,9 As a result, ACZ-challenged single-photon emission CT (SPECT) increases the contrast in radioactivity levels observed between regions of adequate vascular reserve and those of inadequate reserve. Cerebrovascular reserve (CVR) obtained by using SPECT provides important information on hemodynamic status in patients with chronic cerebrovascular occlusive diseases.10 However, SPECT usually has to be performed in a 2-day setting due to tracer kinetics. Moreover, it provides less morphologic information than CT or MR imaging. The dynamic CT perfusion (CTP) is a much more readily accessible imaging method to assess cerebral hemodynamic status in patients with cerebral steno-occlusive arterial disease than SPECT, and its perfusion maps can be generated in a short time at a workstation.11
The aim of the present study was to quantitatively evaluate CVR in ischemic adult Moyamoya disease by using ACZ-challenged dynamic CTP (ACZ-CTP) and to compare it with angiographic findings.
Methods
Study Population
Our institutional review board approved this prospective study, and written informed consent was obtained from every participant in accordance with the guidelines of the institutional review board at our institution. Between June 2005 and May 2007, 45 patients with suspected Moyamoya presenting with transient ischemic attack were referred to our institute through neurologic clinics for evaluation of cerebral vascular and perfusion status via digital subtraction angiography (DSA) and CTP. Two patients were excluded because of history of renal disease or allergic reaction to contrast material, and 7 patients were excluded because we did not acquire their informed consent. Five pediatric patients were also excluded due to radiation hazard. Thirty-one patients underwent both DSA and ACZ-CTP, including precontrast CT scan. Both DSA and ACZ-CTP were performed within 3 days of each other. During the period, patients were selected for prospective study if steno-occlusive diseases of the bilateral distal internal carotid arteries (ICAs) around ICA bifurcation were detected on DSA. Stenotic arteries were considered to be diseased according to the modified Suzuki classification.1 Other criteria for inclusion in this study included the normal finding of posterior circulation on DSA. Ten patients who had concomitant proximal posterior cerebral artery stenosis were excluded. MR study including diffusion-weighted imaging was also performed in all of the referred patients to exclude cerebral infarct. Among 21 patients, 5 additional adult patients who had definite territorial infarct on MR images were excluded from the study. Finally, 16 adult patients with ischemic Moyamoya diseases were enrolled in this study. ACZ-CTP was also performed in 12 age-matched normal control subjects who were volunteers for the evaluation of neurologic symptoms such as dizziness. Normal values of baseline hemodynamic parameters, including CBV, CBF, MTT, and percent changes (PCs) of each parameter, were determined based on age-matched normal control subjects. Among 16 study patients, there were 5 men (age range, 32–44 years; mean age, 38 years) and 11 women (age range, 31–66 years; mean age, 45 years). All of the study patients experienced transient ischemic attacks, and 7 patients experienced headache. The clinical features and demographic data of the study patients are summarized in Table 1.
Table 1:
Clinical data of 16 adult patients with ischemic Moyamoya diseases
| Patient No. | Age/Sex | Clinical Symptoms | Duration of TIA | Bypass Surgery |
|---|---|---|---|---|
| 1 | 39/F | TIA | 12 months | EIAB |
| 2 | 44/M | Headache, TIA | 7 months | Negative* |
| 3 | 33/F | Headache, TIA | 8 months | Negative* |
| 4 | 64/F | Headache, TIA | 4 months | Negative* |
| 5 | 66/F | Headache, TIA | 2 months | EIAB |
| 6 | 32/M | Headache, TIA | 24 months | Negative* |
| 7 | 36/M | TIA | 24 months | Negative* |
| 8 | 45/F | TIA | 1 month | Negative* |
| 9 | 42/F | TIA | 7 months | Negative* |
| 10 | 39/F | TIA | 36 months | Negative* |
| 11 | 56/F | Headache, TIA | 1 month | Negative* |
| 12 | 31/F | TIA | 24 months | EIAB |
| 13 | 35/F | Headache, TIA | 12 months | Negative* |
| 14 | 37/M | TIA | 8 months | EIAB |
| 15 | 42/M | TIA | 11 months | Negative* |
| 16 | 48/F | TIA | 1 month | Negative* |
Note:—TIA indicates transient ischemic attack; EIAB, external internal arterial bypass; F, female; M, male.
Negative indicates a patient who did not have vascular bypass surgery.
CTP Imaging Protocol
CT scans were performed in the transverse plane by using a 64-channel multidetector CT scanner (Brilliance 64 Channel CT; Philips Medical Systems, Cleveland, Ohio). The patient's head was fixed in place with a head holder and was positioned by using light beams. CTP consisted of a 60-second series with 30 gantry rotations performed in cine mode during the intravenous administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of 1 image per 2 seconds, resulting in a series of 30 images for each assessed section. After unenhanced CT of the whole brain, 8 adjacent 5-mm-thick sections were selected by starting at the level of the basal ganglia. A 50-mL bolus of nonionic contrast media (Omnipaque, iodine 300 mg/mL; Amersham Health, Princeton, NJ) was administered into an antecubital vein by using a power injector at an injection rate of 4.5 mL/s. At 2 seconds after initiation of the contrast injection, a cine (continuous) scan was initiated with parameters of 80 kVp and 150 mAs. The gantry angle was parallel to and above the orbital roof to avoid radiation exposure to the lens. CTP was repeatedly performed, approximately 20 minutes after intravenous bolus injection of 1000 mg of ACZ (Diamox; Wyeth, Marietta, Pa). All of the rest of the CTP and ACZ-CTP were obtained during the same session with patients remaining in supine position.
Data Processing
CTP data were analyzed using brain perfusion software (Extended Brilliance Workstation v 3.0, Philips Medical Systems). The software relies on the central volume principle to calculate perfusion parameters from the time-concentration curve. It has been reported that this principle is the most accurate for low injection rates of iodinated contrast agent.12 The software first performs a motion correction and then noise reduction was done by using an anisotropic, edge-preserving spatial filter. The software applies curve fitting by a least-mean-squares method to obtain mathematical descriptions of the time-attenuation curves, and the MTT map was calculated by a closed-form (noniterative) deconvolution operation from the time-concentration curve of a particular voxel and the arterial input function (AIF).12 An AIF was selected by placing a small circular region of interest (ROI) within the earliest appearing and most densely enhancing artery (usually one of MCA ipsilateral to a less affected hemisphere). A venous function was selected by placing a circular ROI within a superior sagittal sinus. For each voxel, the CBV map was calculated from the areas under the time-concentration curves. The CBF map for each voxel was finally calculated according to the following equation combining the CBV and MTT value: CBF = CBV/MTT.13
Data Analysis
Two experienced neuroradiologists independently and blindly evaluated the angiographic findings of 56 hemispheres in 16 study patients and 12 normal control subjects who underwent ACZ-CTP. The ICA lesions were evaluated by a 4-stage angiographic classification scale that was modified from that proposed by Suzuki and Takaku1 (Table 2). The development of BMVs was also graded into 4 stages as shown by a previous report (Table 3).3 Our study patients were classified into 2 groups according to the extent of BMV development (poor BMV, stage 1 and 2; extensive BMV, stage 3 and 4). After the blinded study, discrepancies were resolved by consensus. Finally, the consensus data of the angiographic findings were compared with CTP parameters.
Table 2:
Modified Suzuki angiographic stages
| Stages | Angiographic Findings |
|---|---|
| I | Mild-to-moderate stenosis around carotid bifurcation with absent or slightly developed ICA Moyamoya:* almost all of both ACA and MCA branches are opacified in antegrade fashion |
| II | Severe stenosis around carotid bifurcation or occlusion of either of proximal ACA or MCA with well-developed ICA Moyamoya:* either ACA or MCA branches or both are clearly defective, but at least several of ACA or MCA branches remain opacified in antegrade fashion |
| III | Occlusion of both proximal ACA and MCA with well-developed ICA Moyamoya:* only a few of either ACA or MCA branches or both are faintly opacified in antegrade fashion through the meshwork of ICA Moyamoya |
| IV | Complete occlusion of both proximal ACA and MCA with absent or small amount of ICA Moyamoya:* without opacification of either ACA or MCA branches in antegrade fashion |
Note:—Data were modified from the staging system proposed by Suzuki and Takaku.1 When the proximal ACA was hypoplastic, stage was determined by evaluating the proximal MCA involvement, opacification of MCA branches, and degree of development of ICA Moyamoya. ICA indicates internal carotid artery; ACA, anterior cerebral artery; MCA, middle cerebral artery.
ICA Moyamoya indicates Moyamoya vessels at or around the terminal part of the ICA.
Table 3:
The stages of basal Moyamoya vessels
| Stages | Angiographic Findings |
|---|---|
| I | No Moyamoya vessels were seen |
| II | Moyamoya vessels were localized in the area around the ICA bifurcation, and each vessel was fine and had little contrast |
| III | Moyamoya vessels had intermediate extension and thickness |
| IV | Moyamoya vessels extended a great deal, and each one was thick and strongly opacified |
Note:—ICA indicates internal carotid artery.
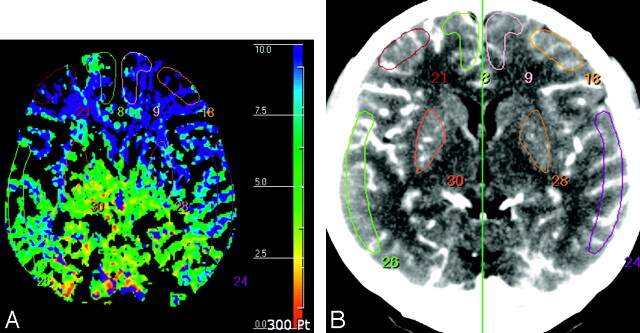
For quantification of the hemodynamic parameters, 2 experienced neuroradiologists independently drew 8 standardized elliptical mirrored ROIs manually on the basal ganglia (BG) section level (around foramen of Monro) of the reference CT image (Fig 1) over the cortical gray matter of the anterior cerebral artery (ACA) distribution, the middle cerebral artery (MCA) distribution, the anterior external borderzone (EBZ), and the putamen in each cerebral hemisphere. The ROI placements in EBZ were performed based on previous reports.14,15 The observer also drew mirrored ROIs in the bilateral cerebellar hemispheres for normalization of the hemodynamic parameters. Large cortical vessels were automatically excluded via brain perfusion software. From each ROI, the absolute values of CBF, CBV, and MTT were calculated. Then, for normalization of each baseline parameter, the ratio of the values of baseline parameters obtained from the ROIs on each cerebral hemisphere to those obtained from the ROIs on the ipsilateral cerebellar hemisphere was calculated.5 These normalized values of baseline parameters were used for quantitative analysis. For the ACZ-CTP study, a section at the same level as the one selected for the baseline study was selected. For the evaluation of vascular reserve capacity, PC was calculated as follows: PC (%) = (NVACZ − NVBaseline) ÷ NVBaseline × 100, where NVBaseline and NVACZ represented normalized values of the hemodynamic parameters before and after intravenous injection of ACZ, respectively. Interobserver variability was calculated for all of the quantitative analyses.
Fig 1.
ROIs drawn in a reference CT image (A) and vessel-removed MTT map (B). The ROIs were placed on cortical regions in the MCA territory, EBZ, ACA territory, and putamen in the section of BG level around the foramen of Monro.
Modified Suzuki angiographic stage was compared with the normalized baseline hemodynamic parameter and the PCs. In addition, the patients were dichotomized into BMV stages 1 and 2 (poor BMV group) versus 3 and 4 (extensive BMV group), with additional 1-way analysis of variance (ANOVA) tests to compare differences of normalized hemodynamic values among the poor BMV group, extensive BMV group, and normal control subjects. Finally, by using 12 normal control subjects, a range of normal values was developed, and patients were categorized as being abnormal if baseline values and their PCs were 2 SDs beyond the mean.
Statistical Analysis
Interobserver variability for quantitative CTP analysis was evaluated by intraclass correlation coefficients. The correlation coefficient between the values of the hemodynamic parameters and the modified Suzuki angiographic stages was calculated by using the Pearson correlation coefficient. The ANOVA, including the Tukey honestly significant difference test and the Duncan method for pairwise multiple comparison procedures, was used to determine differences among quantitative hemodynamic values in the extensive BMV group, poor BMV group, and normal control subjects. A P value less than .05 was considered to be statistically significant.
Results
The angiographic stages ipsilateral to ICA occlusive lesions of the 32 hemispheres in 16 adult Moyamoya patients were as follows: stage 1, 7 hemispheres; stage 2, 8 hemispheres; stage 3, 11 hemispheres; and stage 4, 6 hemispheres. For all of the vascular territories (BG, ACA, MCA, and EBZ), normal values (%) of the PCs of absolute CBV, CBF, and MTT in the 24 cerebral hemispheres of normal control subjects were 19.22 ± 17.53, 25.51 ± 12.74, and −15.45 ± 9.59, respectively. Normal values (%) of the PCs of normalized CBV, CBF, and MTT in the same hemispheres were 1.42 ± 2.31, −2.12 ± 3.25, and −2.24 ± 3.57, respectively. The PCs of absolute CTP values for normal control subjects are absolutely positive (eg, 10% and 20%). However, the PCs of normalized CTP values for normal control subjects are usually close to 0%, because ACZ increases CBF of both cerebral and cerebellar hemispheres. The intraclass correlation coefficients were 0.79 (95% confidence intervals, 0.67–0.82), 0.84 (95% confidence intervals, 0.79–0.87), 0.82 (95% confidence intervals, 0.77–0.89), and 0.87 (95% confidence intervals, 0.83–0.93) for the normalized CBV, CBF, and MTT, and PC of CBF ROIs, respectively.
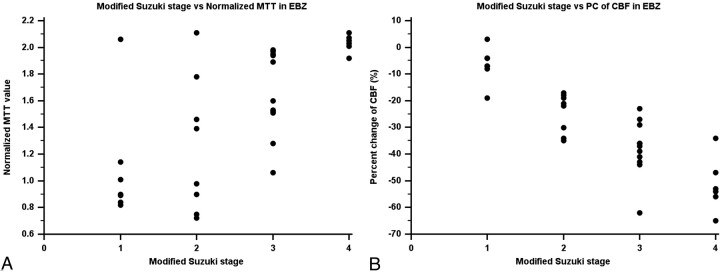
Among normalized baseline and ACZ-challenged CTP parameters, the PC of CBF showed significant good or excellent correlation with the angiographic stage in the ROIs of BG, MCA, and EBZ. The correlation in the ACA territory was significant but fair (Table 4 and Fig 2). The normalized baseline MTT also showed significant correlation with the angiographic stage in the ROIs of all of the vascular territories; however, the correlation coefficients were lower than those of the PCs of CBF (Table 4 and Fig 3). The normalized baseline CBV displayed significant correlation with the angiographic stage in the EBZ and also the normalized baseline CBF with the angiographic stage in the MCA territory. The PC of MTT showed significant pair correlation with the angiographic stage in the EBZ (Table 4).
Table 4:
Correlation coefficient between normalized baseline- and ACZ-challenged CT perfusion parameters and angiographic stages in each vascular territory
| Variable | BG* | ACA* | MCA* | EBZ* |
|---|---|---|---|---|
| Observer 1 | ||||
| CBV vs AS | 0.39† | 0.29 | 0.29 | 0.69† |
| CBF vs AS | −0.16 | −0.24 | −0.50† | −0.33 |
| MTT vs AS | 0.47† | 0.39† | 0.52† | 0.68† |
| PC of CBV vs AS | −0.36 | 0.11 | −0.29 | −0.19 |
| PC of CBF vs AS | −0.65† | −0.40† | −0.81† | −0.82† |
| PC of MTT vs AS | 0.25 | 0.32 | 0.33 | 0.48† |
| Observer 2 | ||||
| CBV vs AS | 0.37 | 0.26 | 0.31 | 0.67† |
| CBF vs AS | −0.14 | −0.27 | −0.47† | −0.31 |
| MTT vs AS | 0.46† | 0.38† | 0.53† | 0.64† |
| PC of CBV vs AS | −0.33 | 0.14 | −0.29 | −0.19 |
| PC of CBF vs AS | −0.69† | −0.45† | −0.83† | −0.79† |
| PC of MTT vs AS | 0.28 | 0.34 | 0.31 | 0.49† |
Note:—CBV indicates normalized cerebral blood volume; CBF, normalized cerebral blood flow; MTT, normalized mean transit time; PC, percent change; AS, angiographic stage; BG, basal ganglia; ACA, anterior cerebral artery; MCA, middle cerebral artery; EBZ, anterior external border zone.
Values are Pearson correlation coefficient (r).
Values are statistically significant (P < .05).
Fig 2.
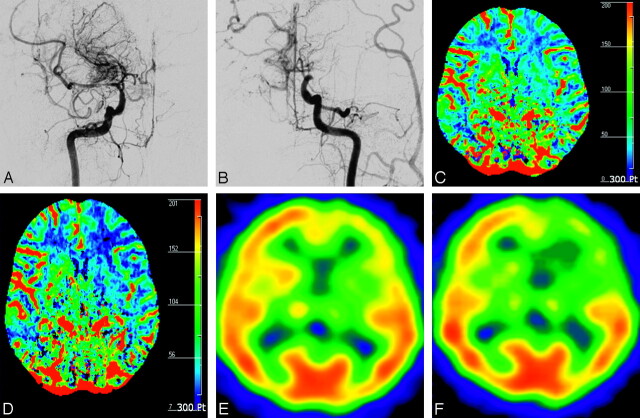
A 48-year-old woman with transient ischemic attack (case 16). A, Anteroposterior view of right internal carotid arteriogram shows severe stenosis in the proximal M1 portion of MCA with developed BMVs (modified Suzuki stage II). B, Anteroposterior view of left internal carotid arteriogram shows occlusion of distal ICA without antegrade flow (modified Suzuki stage IV). CBF maps before (C) and after (D) ACZ administration show decreased CVR in the left anterior EBZ (white arrow) and MCA territory (black arrow) ipsilateral to the hemisphere with higher modified Suzuki stage compared with contralateral hemisphere with lower modified Suzuki stage. Corresponding SPECT before (E) and after (F) ACZ administration show decreased CVR in the same areas.
Fig 3.
Scatterplot between baseline MTT and PCs of CBF and angiographic stages in the anterior EBZ. A, Angiographic stage versus MTT in the EBZ. B, Angiographic stage versus PC of CBF in the EBZ.
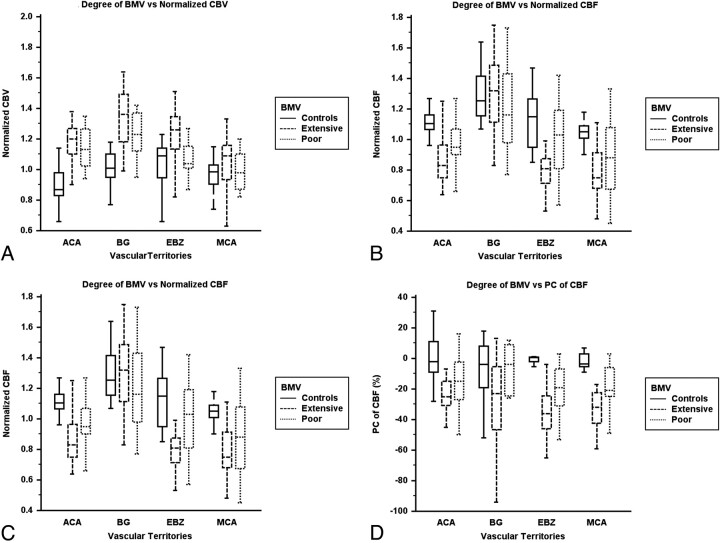
The presence of BMV development in 32 hemispheres consisted of 19 extensive BMV hemispheres and 13 poor BMV hemispheres. In the ROIs of the EBZ and the MCA territory, the PCs of CBF in the extensive BMV hemispheres were significantly lower than those in diminished BMV hemispheres and normal control subjects (Table 5 and Fig 4). In the ROIs of the MCA and the ACA territories, the normalized baseline MTT values in the hemispheres with extensive BMV were significantly higher than those in the hemispheres of diminished BMV and normal control subjects (Table 5 and Fig 4). The values of all of the normalized baseline hemodynamic parameters and the PC of CBF were significantly different between the extensive BMV and poor BMV groups in the EBZ (Table 5).
Table 5:
Differences of the mean values of normalized hemodynamic parameters according to the presence of BMV
| Variable | BG |
ACA |
MCA |
EBZ |
||||
|---|---|---|---|---|---|---|---|---|
| BMV(+) | BMV(−) | BMV(+) | BMV(−) | BMV(+) | BMV(−) | BMV(+) | BMV(−) | |
| Observer 1 | ||||||||
| CBV | 1.34 | 1.22 | 1.21 | 1.14 | 1.06 | 0.98 | 1.24* | 1.07* |
| CBF | 1.27 | 1.21 | 0.85 | 0.97 | 0.75 | 0.89 | 0.78* | 0.99* |
| MTT | 1.10 | 1.04 | 1.45* | 1.21* | 1.46 | 1.20 | 1.76* | 1.20* |
| PC of CBV | −2% | 5% | −5% | −6% | −3% | 4% | −11% | −5% |
| PC of CBF | −29% | −15% | −24% | −17% | −33%* | −18%* | −39%* | −21%* |
| PC of MTT | 25% | 20% | 31% | 22% | 30% | 22% | 37% | 26% |
| Observer 2 | ||||||||
| CBV | 1.33 | 1.20 | 1.22 | 1.17 | 1.09 | 1.01 | 1.27* | 1.10* |
| CBF | 1.29 | 1.19 | 0.92 | 0.95 | 0.77 | 0.89 | 0.82* | 0.96* |
| MTT | 1.11 | 1.01 | 1.45* | 1.24* | 1.42 | 1.22 | 1.71* | 1.22* |
| PC of CBV | −4% | 5% | −7% | −9% | −6% | 1% | −9% | −4% |
| PC of CBF | −32% | −16% | −21% | −13% | −32%* | −19%* | −33%* | −19%* |
| PC of MTT | 27% | 24% | 35% | 27% | 27% | 19% | 34% | 27% |
Note:—CBV indicates normalized cerebral blood volume; CBF, normalized cerebral blood flow; MTT, normalized mean transit time; PC, percent change; BG, basal ganglia; ACA, anterior cerebral artery; MCA, middle cerebral artery; EBZ, anterior external border zone; BMV, basal Moyamoya vessel.
Values are statistically significant (P < .05) by using analysis of variance and the multiple comparison test.
Fig 4.
Clustered box-and-whisker graphs show the differences in the mean values of hemodynamic parameters according to the presence of BMVs in each vascular territory. A, BMV versus normalized CBV. B, BMV versus normalized CBF. C, BMV versus normalized MTT. D, BMV versus PC of CBF.
Discussion
Our present study of ACZ-CTP in patients with ischemic adult Moyamoya diseases showed 2 major findings. First, the CTP is suitable for quantitative evaluation of CVR after ACZ challenge and certainly provides information similar to that described previously with positron-emission tomography (PET) or SPECT.16,17 Second, the PC of CBF is more correlated with angiographic stages than the baseline MTT, which has been shown previously to have positive correlation with the degrees of arterial stenosis in patients with Moyamoya disease.3 In our study, there was no case presenting any major or minor complications associated with ACZ administration. SPECT usually requires 2-day protocol to evaluate cerebral perfusion, whereas ACZ-CTP study in our imaging protocol was performed within 30 minutes. Moreover, SPECT or PET does not routinely provide a quantitative CVR parameter. On the other hand, quantitative analysis using ACZ-CTP could be made immediately after image processing. Nevertheless, radiation exposure is still a drawback of CTP imaging (ACZ-CTP: 0.2 mSv × 2 = 0.4 mSv versus Diamox SPECT: 0.011 mSv × 2 = 0.022 mSv); therefore, we excluded pediatric patients from this study and simplified our imaging protocol, including precontrast CT and ACZ-CTP. Our second major finding indicates that, among the resting and stress hemodynamic parameters, the PC of CBF was correlated most significantly with angiographic stages, suggesting that the PC of CBF is a reliable parameter of cerebral hemodynamics in patients with Moyamoya disease. Previous report showed that MTT correlates positively with angiographic stages.3 MTT can be measured by using CTP or MR perfusion imaging. By using dynamic susceptibility contrast-enhanced MR imaging, Kikuchi et al18 showed that CVR impairment could be evaluated with MTT. However, our present study showed weak or fair correlation between baseline MTT value and angiographic stage. Baseline CBF value is used mostly in clinical practice; however, the regional CBF value is an insensitive indicator for the severity of occlusive cerebrovascular disease, because the value does not vary with a small change of cerebral perfusion pressure.19 In our study, the baseline CBF did not significantly correlate with angiographic stages. Previous studies showed increased baseline CBV value distal to stenotic or occluded cerebral arteries20,21; however, we found that this parameter was variable compared with angiographic stage and clinical symptoms.
As shown previously with PET and SPECT,4 the hemodynamic status of the hemispheres with extensive BMV is different from that of hemispheres with diminished BMV. Patients with hemispheres with extensive BMV exhibited impaired perfusion in the cortices, whereas patients with hemispheres with diminished BMV did not, suggesting that intracerebral anastomoses may not provide an adequate blood supply to the cerebral cortices, even if they are fully developed. Piao et al4 reported that an extensive development of BMV is a sign of severe hemodynamic impairment in adult patients with ischemic Moyamoya disease. Moyamoya vessels would develop to compensate for the misery cerebral perfusion. In our study, hemispheres with extensive BMV exhibited significantly lower PC of CBF and significantly higher baseline MTT than those in hemispheres with diminished BMV and normal control, indirectly suggesting that the CVR parameters obtained by CTP are comparable with those obtained by SPECT or PET.
Previous study found that the values of hemodynamic parameters obtained from CTP before and after the ACZ challenge are correlated with those obtained from PET.16 However, there are different underlying physiologic mechanisms between CTP and PET imaging. Moreover, both methods have certain restrictions: CTP measurements depend on injection rate and cardiac output, whereas PET measurements offer low spatial resolution and assume that the time-activity curve of radiotracer in the radial artery is same as in the intracranial arteries, which may not hold true in patients with carotid stenosis or occlusion and collateral flows. Consequently, the data obtained with the 2 methods may not exactly be interchangeable. Previous studies with ACZ-CTP lack normal data from healthy subjects, thus providing no reference values to determine abnormal findings. On the other hand, we determined normal values of baseline hemodynamic parameters and PCs of the parameters based on the data of 12 normal control subjects in our institution. Therefore, these normal values of hemodynamic parameters were the references in our study.
There are several limitations regarding the quantification of CTP. First, in the normal clinical setting, CTP commonly leads to the selection of major cerebral vessels for the AIF. These vessels lie over the circle of Willis and, therefore, are influenced by collateral recruitment. It is reasonable to expect that an AIF on the affected hemisphere will be subjected to more dispersion than that on the unaffected or slightly affected hemisphere because of increase of length of the collateral pathways supplying this region. However, the problem with using a single unaffected artery as a reference artery for the whole brain is that CBF values on the affected side may be underestimated, whereas MTT values may be overestimated. In Moyamoya disease, the presence of steno-occlusion of the main cerebral arteries and collateral vessels always leads to the delay and dispersion of the bolus of the contrast agent. We used the AIF obtained from an MCA branch ipsilateral to the less affected hemisphere at the level of the BG, which is fine and narrow in patients with Moyamoya disease, and, therefore, would be susceptible to partial volume averaging. A new method that can compensate for or resolve this problem is desirable. Second, the above limitations may result in a wide range of absolute measurements, making the detection of abnormal values difficult. Intrasubject normalization of data has been used frequently to deal with these problems. The use of hemispheric ratios takes advantage of the basic symmetry of the brain to improve sensitivity for identifying localized disease. Moreover, intraobserver and interobserver variability for absolute hemodynamic parameter values can significantly be improved if the values are normalized. We used the baseline parameters and their PCs obtained from the hemispheric ratio to cerebellum, and a previous report indicates that the calculation of ratios eliminates variations caused by the choice of AIF, and it may also reduce the variability in the proportion of gray and white matter that is included in the regional analysis.22 Finally, there are no standardized guidelines for placing ROIs. Larger ROIs may result in greater volume averaging of gray and white matters, thus lowering quantitative values for CBF compared with results obtained by using smaller ROIs centered in the cortex. In the present study, an observer manually drew ROIs over the cortical gray matter of the expected territory of ACA, MCA, EBZ, and BG, with care not to involve substantial parts of the cerebral white matter. Large cortical blood vessels were automatically excluded from the ROIs.
Conclusion
The CTP is feasible for the evaluation of quantitative CVR and certainly provides information that is similar to that described previously with PET or SPECT. Moreover, the CTP is a much more readily accessible imaging method than PET or SPECT, and the quantitative analysis using CTP could be made immediately after image processing. The quantitative CVR (PC of CBF), measured by ACZ-CTP, is more significantly correlated with angiographic stage than normalized baseline CTP parameters in adult ischemic Moyamoya disease. Therefore, CTP evaluation for the CVR in patients with Moyamoya disease requires ACZ challenge. Future evaluation of this technique in prebypass and postbypass patients is warranted to determine the potential clinical use.
Acknowledgments
We acknowledge Scott Pohlman of the Philips Medical Systems for his cooperation and valuable advice.
Footnotes
This study was supported by a 2007 grant from Ajou University School of Medicine.
References
- 1.Suzuki J, Takaku A. Cerebrovascular Moyamoya disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99 [DOI] [PubMed] [Google Scholar]
- 2.Calamante F, Ganesa V, Kirkham FJ, et al. MR perfusion imaging in Moyamoya syndrome: potential implications for clinical evaluation of occlusive cerebrovascular disease. Stroke 2001;32:2810–16 [DOI] [PubMed] [Google Scholar]
- 3.Togao O, Mihara F, Yoshiura T, et al. Cerebral hemodynamics in Moyamoya disease: correlation between perfusion-weighted MR imaging and cerebral angiography. AJNR Am J Neuroradiol 2006;27:391–97 [PMC free article] [PubMed] [Google Scholar]
- 4.Piao R, Oku N, Kitagawa K, et al. Cerebral hemodynamics and metabolism in adult Moyamoya disease: comparison of angiographic collateral circulation. Ann Nucl Med 2004;18:115–21 [DOI] [PubMed] [Google Scholar]
- 5.Yamada I, Himeno Y, Suzuki S. SPECT and MRI evaluations of the posterior circulation in Moyamoya disease. J Nucl Med 1996;37:1613–17 [PubMed] [Google Scholar]
- 6.Mugikura S, Rakahashi S, Higano S. The relationship between cerebral infarction and angiographic characteristics in childhood Moyamoya disease. AJNR Am J Neuroradiol 1999;20:336–43 [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada I, Himeno Y, Nagaoka T, et al. Moyamoya disease: evaluation with diffusion-weighted and perfusion echo-planar MR imaging. Radiology 1999;212:340–47 [DOI] [PubMed] [Google Scholar]
- 8.Vorstrup S, Henriksen L, Paulson OB. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest 1984;74:1634–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan HG, Kingsbury TB 4th, Morgan ME, et al. The rCBF response to Diamox in normal subjects and cerebrovascular disease patients. J Neurosurg 1987;67:525–34 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, Watanabe M, Uematsu T, et al. Correlation of angiographic circulation time and cerebrovascular reserve by acetazolamide-challenged single photon emission CT. AJNR Am J Neuroradiol 2004;25:242–47 [PMC free article] [PubMed] [Google Scholar]
- 11.Hoeffner EG, Case I, Jain R, et al. Cerebral perfusion CT: technique and clinical applications. Radiology 2004;231:632–44 [DOI] [PubMed] [Google Scholar]
- 12.Wintermark M, Maeder P, Thiran JP, et al. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 2001;11:1220–30 [DOI] [PubMed] [Google Scholar]
- 13.Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol 1983;8:94–99 [DOI] [PubMed] [Google Scholar]
- 14.Van der Zwan A, Hillen B, Tulleken CAF, et al. Variability of the territories of the major cerebral arteries. J Neurosurg 1992;77:927–40 [DOI] [PubMed] [Google Scholar]
- 15.Hupperts RMM, Lodder J, Heuts-van Raak, et al. Borderzone brain infarcts on CT taking into account the variability in vascular supply areas. Cerebrovasc Dis 1996;6:294–300 [Google Scholar]
- 16.Bisdas S, Nemitz O, Berding G, et al. Correlative assessment of cerebral blood flow obtained with perfusion CT and positron emission tomography in symptomatic stenotic carotid disease. Eur Radiol 2006;16:2220–28 [DOI] [PubMed] [Google Scholar]
- 17.Honda M, Ezaki Y, Kitagawa N, et al. Quantification of the regional cerebral blood flow and vascular reserve in Moyamoya disease using split-dose iodoamphetamine I 123 single-photon emission computed tomography. Surg Neurol 2006;66:155–59 [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi K, Murase K, Miki H, et al. Quantitative evaluation of mean transit times obtained with dynamic susceptibility contrast-enhanced MR imaging and with 133 Xe SPECT in occlusive cerebrovascular disease. AJR Am J Roentgenol 2002;179:229–35 [DOI] [PubMed] [Google Scholar]
- 19.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 1991;29:231–40 [DOI] [PubMed] [Google Scholar]
- 20.Chen A, Shyr MH, Chen TY, et al. Dynamic CT perfusion imaging with acetazolamide challenge for evaluation of patients with unilateral cerebrovascular steno-occlusive disease. AJNR Am J Neuroradiol 2006;27:1876–81 [PMC free article] [PubMed] [Google Scholar]
- 21.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 2002;125:595–607 [DOI] [PubMed] [Google Scholar]
- 22.Waaijer A, van der Schaaf IC, Velthuis BK, et al. Reproducibility of quantitative CT brain perfusion measurements in patients with symptomatic unilateral carotid artery stenosis. AJNR Am J Neuroradiol 2007;28:927–32 [PMC free article] [PubMed] [Google Scholar]