Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to report our experience with endovascular treatment of 14 patients with symptomatic intradural vertebral dissecting aneurysms.
Materials AND METHODS: Between January 2000 and January 2006, 14 patients with symptomatic intradural dissecting vertebral aneurysms were treated. A total of 756 (568 ruptured, 188 unruptured) endovascular treated aneurysms (incidence, 1.9%) were treated during this period. There were 7 female and 7 male patients with a mean age of 48 years (age range, 10–64 years). Thirteen patients (93%) presented with subarachnoid hemorrhage (SAH) and 1 (7%) presented with acute symptoms of mass effect on the brain stem.
RESULTS: Treatment consisted of coil occlusion of the dissected arterial segment including the aneurysm (internal coil trapping) in 13 of 14 patients and stent placement over the aneurysm as the only therapy in 1 patient. All aneurysms and occluded arterial segments remained occluded on follow-up imaging at 6 to 13 months, and none of the patients had infarctions in the medulla or territory of the posterior inferior cerebellar artery. Clinical outcome was excellent in 11 patients; 3 had cognitive impairment after SAH but were independent in daily activities. There were no episodes of recurrent hemorrhage.
CONCLUSION: Intradural vertebral dissecting aneurysms presenting with SAH should be treated promptly because of the high risk of recurrent hemorrhage. In our experience, trapping of the dissected segment with coils was straightforward, could be done in most patients, and was effective in preventing rebleeding. In our opinion, only in exceptional circumstances are more sophisticated techniques aimed at preservation of the parent artery necessary.
Vertebral artery dissections most commonly occur in the extradural vertebral artery and usually present with neck pain and symptoms of brain ischemia.1 The wall of the vertebral and basilar arteries consists of 3 well-defined layers: the intima with the internal elastic lamina, the media with the external elastic lamina, and the adventitia. In intracranial dissections, usually both the intima and media are disrupted. Intramural hematomas, caused by extravasations of luminal blood or hemorrhage from a vasa vasorum within the media, are most commonly located between the internal elastic lamina and the media. Pseudoaneurysms may develop when the dissection proceeds through the media to the subadventitial layer and causes dilation of the outer wall of the vessel. With time, evolution is variable: dissecting aneurysms may grow or the dissection may completely heal within several weeks.2 Although in most cases the cause is unknown, several predisposing factors associated with arterial dissection are minor trauma, hypertension, oral contraceptive use, migraine headache, or intrinsic disorders of the vessel wall such as Marfan syndrome and fibromuscular dysplasia. Angiographic findings in vertebral or basilar dissections consist of focal or fusiform aneurysmal dilation with or without proximal or distal stenosis. When the dissection extends inward, the vessel is narrowed or even occluded.3–6 Rarely, vertebral dissections are located on the intradural V4 segment and present with subarachnoid hemorrhage (SAH).1
Because the risk of recurrent hemorrhage is high, prompt surgical or endovascular treatment is indicated.7,8 In this study, we report our experience with endovascular treatment of 14 patients who had symptomatic intradural vertebral dissecting aneurysms.
Patients and Methods
Between January 2000 and January 2006, 14 patients with symptomatic intradural dissecting vertebral aneurysms were treated at the 2 participating hospitals. In the same period, 756 aneurysms were treated with endovascular means, of which 568 were ruptured and 188 were unruptured. The incidence of vertebral artery dissecting aneurysms was 1.9% (14/756). Patient and aneurysmal characteristics are summarized in the accompanying on-line Table. There were 7 female and 7 male patients with a mean age of 48 years (age range, 10–64 years). A total of 13 (93%) patients presented with SAH, and 1 (7%) presented with symptoms of mass effect on the brain stem of acute onset. Of the 13 patients with SAH, clinical condition on admission was Hunt and Hess (HH) grade I–II in 4, HH III in 3, and HH IV–V in 6. Treatment was started within 5 days after SAH in 9 patients and between 14 and 180 days in 4 patients. At the time of treatment, none of the patients had a lateral medullary (Wallenberg) syndrome.
Results
Treatment
Treatment consisted of coil occlusion of the dissected arterial segment including the aneurysm (internal coil trapping) in 13 of 14 patients and stent placement over the aneurysm as the only treatment in 1 patient. Balloon test occlusion of the dominant affected vertebral artery preceded internal coil trapping in 2 patients. In the 2 patients with dissecting vertebral artery aneurysms with involvement of the posterior inferior cerebellar artery (PICA) origin, internal coil trapping included the PICA origin in both patients without clinical symptoms and without PICA infarctions on follow-up CT or MR imaging. There were no complications of treatment.
Follow-up
Follow-up imaging consisted of 6 to 13 months of follow-up angiograms in combination with CT or MR imaging in 8 patients and CT or MR imaging only in 6 patients. All aneurysms and occluded arterial segments remained occluded on follow-up imaging studies, and none of the patients had infarctions in the medulla or PICA territory. Clinical outcome was excellent in 11 patients; 3 patients had cognitive impairment and memory disturbances after SAH but were independent in daily activities. There were no episodes of recurrent hemorrhage.
Representative Cases
Case 1.
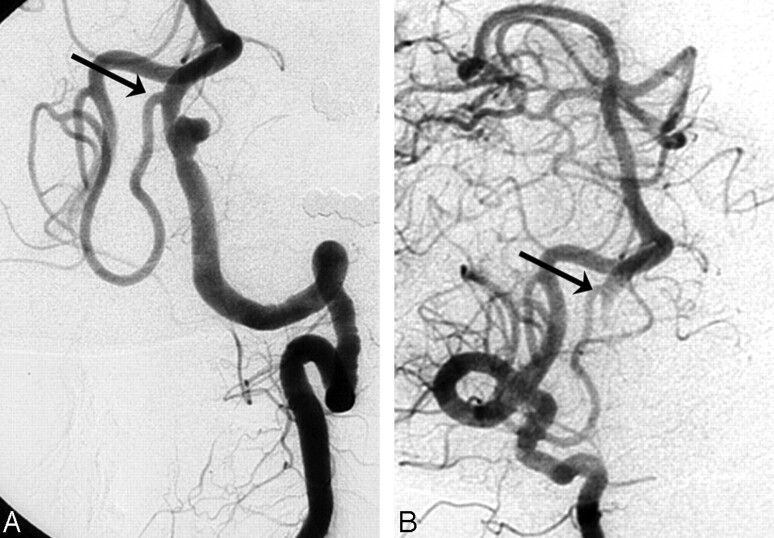
A 47-year-old man (patient #3) was admitted with SAH grade III. Left vertebral angiograms revealed a small vertebral aneurysm proximal to the PICA origin on a narrowed segment consistent with dissection. Both vertebral arteries had a normal caliber. The dissecting aneurysm, including the vertebral artery, was completely occluded with coils. After occlusion of the left vertebral artery, the left PICA remained patent via the right vertebral artery (Fig 1).
Fig 1.
Acutely ruptured left vertebral dissecting aneurysm proximal to the PICA origin in a 47-year-old man (patient #3). A, Left vertebral angiogram shows dissecting aneurysm on a narrowed segment proximal to the PICA origin (arrow). B, Right vertebral angiogram after internal coil trapping of the aneurysm and dissecting aneurysm demonstrates retrograde filling of the left distal vertebral artery and PICA (arrow).
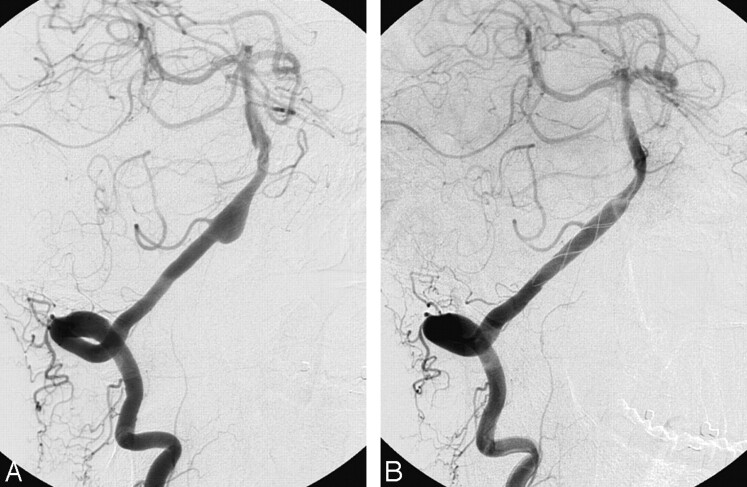
Case 2.
A 51-year-old man (patient #5) was referred 4 weeks after SAH from a right vertebral dissecting aneurysm involving the origin of the right PICA. The contralateral left vertebral artery showed a dissection in the V3 segment; therefore, it was decided to preserve the right vertebral artery. A Leo stent (Balt, Montmorency, France) was placed over the aneurysm and PICA origin as the only treatment. Follow-up angiograms after 6 months showed complete occlusion of the aneurysm with a patent right PICA (Fig 2).
Fig 2.
Right vertebral dissecting aneurysm involving the origin of the PICA in a 51-year-old man referred 4 weeks after SAH (patient #5). A, Right vertebral angiogram shows dissecting aneurysm. B, Follow-up angiogram 6 months after stent placement demonstrates complete occlusion of the aneurysm with a patent right PICA.
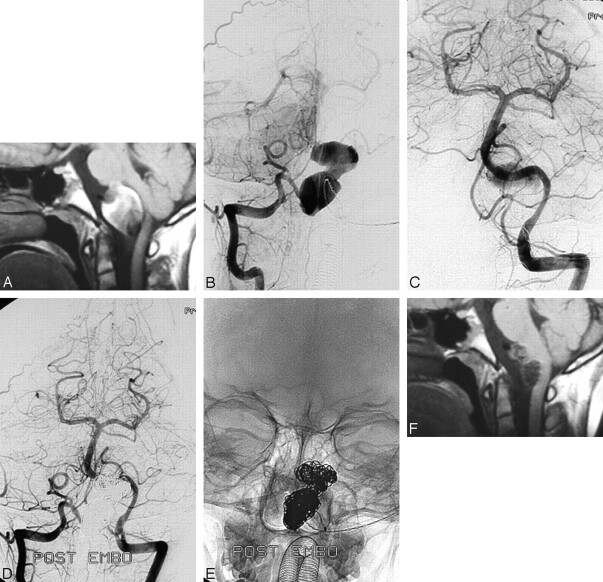
Case 3.
A 10-year-old boy (patient #8) complained of sudden headache and difficulty in swallowing. MR imaging and angiograms revealed a giant dissecting aneurysm of the right vertebral artery distal to the PICA with an intramural thrombus compressing the brain stem. The lumen of the aneurysm, including the dissected vertebral segment, was occluded with coils. At follow-up MR imaging 3 and 6 months later, the aneurysm had substantially decreased in size. From a clinical standpoint, the patient was free of symptoms (Fig 3).
Fig 3.
Giant, partially thrombosed right vertebral dissecting aneurysm distal to the PICA origin occurring with acute compression of the brain stem in a 10-year-old boy (patient #8). A, MR imaging shows dissecting aneurysm compressing the brain stem. B,C, Right (B) and left (C) vertebral angiogram shows giant aneurysm of the right vertebral artery distal to the PICA origin. D,E, Bilateral vertebral angiograms (D) and corresponding radiograph (E) with complete coil occlusion of the aneurysm and parent vertebral segment. F, MR imaging 6 months later demonstrates shrinkage of the aneurysm with decreased compression of the brain stem.
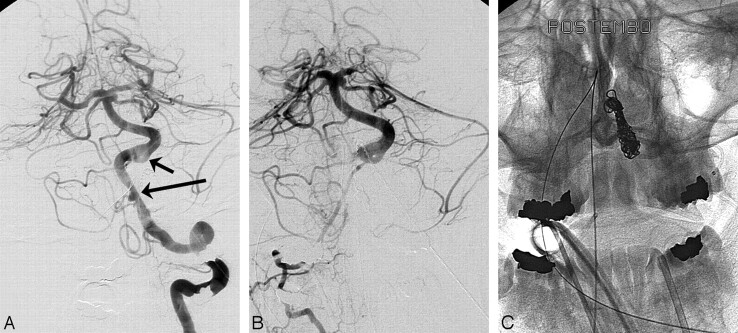
Case 4.
A 36-year-old woman (patient #9) was admitted with SAH grade IV from a dissecting aneurysm of the dominant left vertebral artery distal to the PICA. The right vertebral artery was of small caliber. With the patient under general anesthesia, a balloon test occlusion of the left vertebral artery was performed, and angiograms of the right vertebral artery during the test occlusion demonstrated adequate filling of the posterior circulation. The dissected segment of the left vertebral artery, including the aneurysm, was occluded with coils. The patient had an uneventful recovery (Fig 4).
Fig 4.
Acutely ruptured left vertebral dissecting aneurysm of a dominant vertebral artery in a 36-year-old woman presenting in HH IV (patient #9). A, Angiogram of the dominant left vertebral artery demonstrates a dissecting aneurysm distal to the PICA origin (long arrow) with focal narrowing. Note proximal basilar fenestration (short arrow). B, Right vertebral angiogram during test occlusion of the left vertebral artery shows adequate filling of posterior circulation vessels despite small caliber. C, Unsubstracted radiograph after internal coil trapping of the distal left vertebral artery.
Discussion
Dissections located on the intradural V4 segment are uncommon, and presentation with SAH is rare. In a study by Arnold et al1 comprising 169 patients with 195 vertebral dissections, only 22 dissections (11%) were located in the intradural V4 segment and 6 (4%) occurred with SAH. When vertebrobasilar dissections occur with SAH, the risk of recurrent hemorrhage is extremely high (30% to 70%)7,8 and prompt treatment is indicated. In a study by Yamada et al9 of 24 conservatively treated patients with ruptured vertebral dissecting aneurysms, 14 (58%) experienced a total of 35 rebleeding episodes. Of these 14 patients, 13 died and 11 of these deaths were directly attributable to rebleeding.
Surgical treatment of vertebral dissecting aneurysms may consist of proximal vertebral occlusion, trapping, bleb clipping, or wrapping and when the dissection involves the PICA origin, with or without PICA revascularization.10–16 In recent years, endovascular treatment of vertebrobasilar dissecting aneurysms has evolved as an alternative to surgical treatment and may consist of proximal vertebral occlusion, internal coil trapping, stent-assisted coiling, or stent placement as the only therapy.17–23
Results of multiple series6,10–23 suggest surgical or internal coil trapping to occlude the affected segment of the artery as the appropriate therapy for vertebral artery dissecting aneurysms. In some instances, PICA revascularization procedures are considered required.15,16 Vessel wrapping for intradural lesions was ineffective in the small number of documented cases.14 Proximal surgical or endovascular balloon or coil occlusion is not effective in a substantial proportion of cases17 because of retrograde filling of the dissecting aneurysm from the contralateral vertebral artery with outflow in the ipsilateral PICA. Also, stent-assisted coiling or stent placement with preservation of the vascular patency18,23 is mostly not effective in excluding dissecting aneurysms from the circulation. In a study by Ahn et al,23 10 of 13 dissecting aneurysms were incompletely occluded after stent placement and at 6 to 12 months follow-up, 6 of 12 aneurysms were still not occluded. In addition, the required antiplatelet medication may have been a major drawback.
In internal coil trapping, several theoretical and technical considerations can be made. First, there may be a concern of occluding perforating arteries or a contribution to the anterior spinal artery arising from the dissected segment. Because small perforators usually are invisible on angiograms, this cannot be avoided with certainty. On the other hand, it seems unlikely that small vessels would still be patent in a dissected segment; thus, occluding the affected segment can be considered safe.
Second, there may be a concern of perforation of the aneurysm or vessel wall, as it is well known that dissections may harbor extremely thin and fragile walls prone to recurrent hemorrhage. In addition, the stability of the first inserted coil should be guaranteed in a high-flow state. In this respect, we found it helpful to use coils of 0.018-inch thickness and oversize the first coil relative to the maximum diameter of the dissecting aneurysm. After insertion of the first coil loop in the aneurysm, we deliberately allow the second coil loop to herniate into the parent vessel. In this way, the coil conducts its force to the microcatheter instead of to the fragile aneurysmal wall, and the risk of perforation is thus reduced. At the same time, maximum stability of the coil is obtained because both the parent vessel and the aneurysm serve as an anchor. After creating this framework, we insert additional smaller size coils that finally completely occlude both the aneurysm and the parent vertebral artery. In this way, coiling of dissecting aneurysms is less challenging and more straightforward than traditional selective coiling of the aneurysmal sac because maneuvers to keep the parent vessel open are not necessary.
In our study, we applied internal coil trapping of the dissected vertebral segment in 12 of 13 patients, with excellent clinical and angiographic results. When the dissection involved the dominant vertebral artery, balloon test occlusion was valuable in the evaluation of the collateral circulation via the contralateral vertebral artery or posterior communicating arteries. In 2 patients with acute hemorrhagic dissections involving the PICA origin, coil occlusion including the PICA origin did not lead to clinical symptoms or infarctions on imaging studies. In 1 patient with a dissection involving the PICA origin and a long delay in referral, the vertebral artery and PICA patency was preserved with stent placement. From our results, and from those of others, we believe that in the acute phase of SAH after vertebral dissection, immediate internal coil trapping is the preferred therapy. This also holds true for dissections involving the PICA origin because prevention of recurrent SAH outweighs the risk of ischemia in the PICA territory. In patients with a single vertebral artery and insufficient posterior communicating arteries, stent-assisted coiling or surgical treatment is indicated.
When patients present in a delayed time course, and the risk of recurrent hemorrhage is low, more sophisticated treatment options to preserve patency of the parent vessel may be considered, including stent-assisted coiling, single stent placement or stent in stent, neurosurgical bypass and trapping, combined procedures, or conservative close follow-up. Balloon test occlusion in the patient who is awake may be helpful. Although revascularization procedures of the PICA are technically possible, this type of therapy is technically challenging and is not available in all centers. In our experience with deliberate PICA origin occlusion (also for PICA aneurysms), clinical outcome is usually good.24 Nevertheless, when expertise is available, in selected cases PICA revascularization may be a valuable treatment.
Conclusion
Intradural vertebral dissecting aneurysms that occur with SAH should be treated promptly because of the high risk of recurrent hemorrhage. In our experience, trapping of the dissected segment with coils was straightforward, could be done in most patients, and was effective in preventing rebleeding. In our opinion, only in exceptional circumstances are more sophisticated techniques aimed at preservation of the parent artery necessary.
Supplementary Material
Footnotes
Indicates article with supplemental on-line tables.
References
- 1.Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome [published erratum appears in Stroke 2007;38:208]. Stroke 2006;37:2499–503 [DOI] [PubMed] [Google Scholar]
- 2.Mizutani T, Kojima H, Asamoto S. Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery 2004;54:342–47; discussion 347–48 [DOI] [PubMed] [Google Scholar]
- 3.Sasaki O, Ogawa H, Koike T, et al. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 1991;75:874–82 [DOI] [PubMed] [Google Scholar]
- 4.Mizutani T, Kojima H, Asamoto S, et al. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg 2001;94:712–17 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson IM. The vertebral artery. Extracranial and intracranial structure. Arch Neurol 1972;27:392–96 [DOI] [PubMed] [Google Scholar]
- 6.Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 1990;72:183–88 [DOI] [PubMed] [Google Scholar]
- 7.Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke 1990;21:1628–31 [DOI] [PubMed] [Google Scholar]
- 8.Mizutani T, Aruga T, Kirino T, et al. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 1995;36:905–11; discussion 912–13 [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Kitahara T, Kurata A, et al. Intracranial vertebral artery dissection with subarachnoid hemorrhage: clinical characteristics and outcomes in conservatively treated patients. J Neurosurg 2004;101:25–30 [DOI] [PubMed] [Google Scholar]
- 10.Amin-Hanjani S, Ogilvy CS, Buonanno FS, et al. Treatment of dissecting basilar artery aneurysm by flow reversal. Acta Neurochir (Wien) 1997;139:44–51 [DOI] [PubMed] [Google Scholar]
- 11.Yonas H, Agamanolis D, Takaoka Y, et al. Dissecting intracranial aneurysms. Surg Neurol 1977;8:407–15 [PubMed] [Google Scholar]
- 12.Shimoji T, Bando K, Nakajima K, et al. Dissecting aneurysm of the vertebral artery. Report of seven cases and angiographic findings. J Neurosurg 1984;61:1038–46 [DOI] [PubMed] [Google Scholar]
- 13.Berger MS, Wilson CB. Intracranial dissecting aneurysms of the posterior circulation. Report of six cases and review of the literature. J Neurosurg 1984;61:882–94 [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara S, Fujii K, Nishio S, et al. Long-term results of wrapping of intracranial ruptured aneurysms. Acta Neurochir (Wien) 1990;103:27–29 [DOI] [PubMed] [Google Scholar]
- 15.Takikawa S, Kamiyama H, Nomura M, et al. [Vertebral dissecting aneurysm treated with trapping and bilateral posterior inferior cerebellar artery side-to-side anastomosis; case report]. No Shinkei Geka 1991;19:571–76 [PubMed] [Google Scholar]
- 16.Lemole GM, Henn J, Javedan S, et al. Cerebral revascularization performed using posterior inferior cerebellar artery-posterior inferior cerebellar artery bypass. Report of four cases and literature review. J Neurosurg 2002;97:219–23 [DOI] [PubMed] [Google Scholar]
- 17.Rabinov JD, Hellinger FR, Morris PP, et al. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 2003;24:1421–28 [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta B, Burke T, Kole M, et al. Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. AJNR Am J Neuroradiol 2003;24:1814–18 [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukahara T, Wada H, Satake K, et al. Proximal balloon occlusion for dissecting vertebral aneurysms accompanied by subarachnoid hemorrhage. Neurosurgery 1995;36:914–19; discussion 919–20 [DOI] [PubMed] [Google Scholar]
- 20.Leibowitz R, Do HM, Marcellus ML, et al. Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. AJNR Am J Neuroradiol 2003;24:902–07 [PMC free article] [PubMed] [Google Scholar]
- 21.Halbach VV, Higashida RT, Dowd CF, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg 1993;79:183–91 [DOI] [PubMed] [Google Scholar]
- 22.Yamaura A, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg 1999;90:853–56 [DOI] [PubMed] [Google Scholar]
- 23.Ahn JY, Han IB, Kim TG, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol 2006;27:1514–20 [PMC free article] [PubMed] [Google Scholar]
- 24.Peluso JP, van Rooij WJ, Sluzewski M, et al. Posterior inferior cerebellar artery aneurysms: incidence, clinical presentation and outcome of endovascular treatment. AJNR Am J Neuroradiol 2008;29:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.