Abstract
BACKGROUND AND PURPOSE: The traditional paradigm has regarded essential tremor (ET) as a benign disorder. However, recent clinical, neuroimaging, and neuropathologic studies suggest that ET may be a progressive neurologic disorder. Based on clinicopathologic findings that cerebellum and its outflow are the key structures in ET and degeneration of gray matter in cerebellum is followed by consequent wallerian degeneration of white matter (WM) fibers, the aim of the present study was to investigate changes in anisotropy in patients with ET.
MATERIALS AND METHODS: Fractional anisotropy (FA) images were generated from DTI data acquired at 1.5T in 10 patients with ET compared with 8 control subjects by using statistical parametric mapping to make voxel-by-voxel comparisons.
RESULTS: Compared with the control subjects, the patients with ET exhibited significantly reduced FA (Puncorrected < .005) in the anterolateral portion of the right pons and decreased FA in the bilateral cerebellum, left retrorubral area of the midbrain, and bilateral deep WM, including the orbitofrontal, lateral frontal, parietal, and temporal WM.
CONCLUSION: This study demonstrates that structural changes in the WM are extensive in patients with ET, supporting the findings of previous functional neuroimaging and pathologic studies.
Essential tremor (ET) is the most common movement disorder, characterized by a 4- to 12-Hz kinetic and postural tremor affecting the hands, head, or other parts of the body. The traditional paradigm has regarded ET as a benign disorder; however, there is growing evidence that it may be a progressive neurologic disorder.1,2 Although the pathophysiology of ET remains unknown, recent clinical, neuroimaging, and neuropathologic studies suggested that disturbance of cerebellum and its outflow pathway (cerebellothalamocortical loop) might play an important role in the pathogenesis of ET.1–5
Diffusion tensor imaging (DTI) has recently been introduced for the in vivo estimation of white matter (WM) tissue composition.6 The diffusion of water molecules in normal WM is imposed on the ordered structure of axons and myelin sheaths, called anisotropy. WM pathology disrupts the coherent orientation of axons, leading to decreased tissue anisotropy.7 Thus, anisotropy is an index of the organization of axons and allows the demonstration of fiber tracts in vivo.
Based on clinicopathologic findings that cerebellum and its outflow are the key structures in ET and degeneration of gray matter in cerebellum is followed by consequent wallerian degeneration of WM fibers,8 the aim of the present study was to investigate changes in anisotropy in patients with ET. In this study, we used statistical parametric mapping (SPM) to make voxel-by-voxel comparisons between the anisotropic measurements in patients with ET and control subjects.
Materials and Methods
Patients
We prospectively enrolled 10 patients with ET and 8 healthy control subjects. All of the patients with ET were referred to our hospital consecutively for diagnostic evaluation of tremor. All of the patients with ET met the criteria for definite ET according to the diagnostic criteria of the Tremor Investigation Group.9 Age-matched subjects with no history of neurologic diseases were enrolled as a control group.
Data Acquisition
All of the studies were performed with a 1.5T Signa Excite MR imaging scanner (GE Healthcare, Milwaukee, Wis) with an 8-channel head coil. The DTI data were acquired by using spin-echo echo-planar imaging (EPI) pulse sequences with the following imaging parameters: TR/TE, 15,000/94.9 ms; flip angle, 90°; FOV, 200 × 200 mm2; number of sections, 39; acquisition matrix, 128 × 128 (reconstruction matrix, 256 × 256); and section thickness, 3 mm with no gap. Diffusion-weighted images (DWIs) were obtained for 25 different noncollinear diffusion-sensitizing gradients with a b-value of 1000 s/mm−2. All of the data were acquired without the cardiac gating.
Analysis of DTI
The DTI data were analyzed with DTIStudio version 2.03 software (Department of Radiology, Johns Hopkins University, Baltimore, Md) in which the diffusion tensor coefficient was calculated for each voxel using the singular value decomposition method. Fractional anisotropy (FA) was calculated by standard method,10 and FA maps were obtained.
The T2-weighted images (b = 0) and FA maps for all of the participants were normalized to the Montreal Neurologic Institute EPI template supplied with SPM2 (Wellcome Department of Cognitive Neurology, London, United Kingdom),11 and the normalized T2-weighted images were averaged to generate the custom T2 template. FA maps of all of the subjects were normalized to the custom template using the transformation matrix obtained from the normalization of b = 0 images to the costume T2 template. The spatial normalization was performed using nonlinear medium regularization, 16 nonlinear iterations, and no wrap. To include the only WM in our analysis, the binary mask images, which consisted of WM, were made from segmented WM of the custom T2 template. Applying the binary mask image to the FA map, the masked FA map, which included the only WM, was generated and smoothed with an 8-mm full width at half maximum Gaussian kernel.
To test whether the control subjects and ET patients showed significant differences in the FA of WM, the smoothed and masked FA maps were analyzed with SPM2 using analysis of covariance, and a statistical map was generated with a significance threshold of P < .005 (uncorrected) and minimal cluster size of 40.
Results
There was no difference in age or sex between the patients with ET (52.8 ± 11.5 years, mean ± SD; 50% female) and the control subjects (51.3 ± 11.1 years; 50% female). All of the ET patients were right handed with tremor involving both hands. The mean duration of tremor in the ET patients was 9.5 ± 7.3 years. The details of the tremor patients are summarized in the Table.
The demographic features in patients with essential tremor
| Pts | Age, y/Sex | Duration, y | Family History | Affected Body Area |
|---|---|---|---|---|
| 1 | 45/M | 20 | + | Hand, R > L |
| 2 | 35/M | 8 | + | Hand, R = L |
| 3 | 32/M | 5 | + | Hand, head, R = L |
| 4 | 62/F | 25 | + | Hand, head, R > L |
| 5 | 61/F | 5 | + | Hand, head, R > L |
| 6 | 59/M | 11 | + | Hand, R = L |
| 7 | 59/M | 8 | − | Hand, head R = L |
| 8 | 55/F | 3 | + | Hand, head, R = L |
| 9 | 55/F | 5 | + | Hand, head, R > L |
| 10 | 65/F | 5 | + | Hand, R > L |
Note:—R indicates right; L, left; Pts, patients; M, male; F, female.
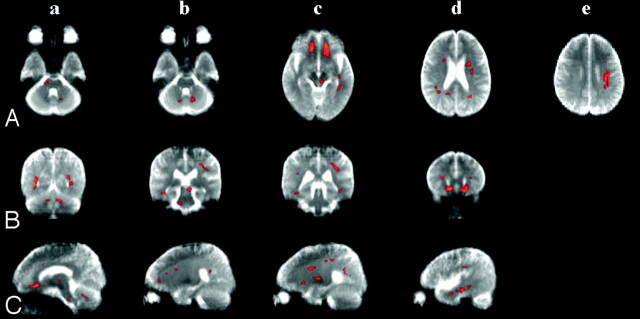
Compared with the control subjects, the patients with ET exhibited significantly reduced FA (Puncorrected < .005) in the anterolateral portion of the right pons (Fig 1A-a, -B-b, and -C-a) and decreased FA in the bilateral cerebellum (Fig 1A-b, -B-a, and -C-a), left retrorubral area of the midbrain (Fig 1A-c, -B-b, and -C-a), and bilateral deep WM, including the orbitofrontal (Fig 1A-c, -B-d, and -C-a), lateral frontal (Fig 1A-d, -B-b, -C-b, and -C-c), parietal (Fig 1A-d, -A-e, -B-c, and -C-c), and temporal WM (Fig 1A-c and -C-d).
Fig 1.
Maps of significant voxels representing regions of reduction in FA in patients with essential tremor compared with control subjects (red) superimposed on the mean of their T2-weighted MR images (b = 0). The smoothed and masked FA maps were analyzed with SPM2, and a statistical map was generated with a significance threshold of Puncorrected < .005. Horizontal (A), coronal (B), and sagittal views (C). Anterolateral portion of the right the pons (A-a, B-b, and C-a); bilateral cerebellum (A-b, B-a, and C-a); left retrorubral area of the midbrain (A-c, B-b, and C-a); orbitofrontal white matter (A-c, B-d, and C-a); lateral frontal white matter (A-d, B-b, C-b, and C-c); parietal white matter (A-d, A-e, B-c, and C-c); and temporal white matter (A-c and C-d).
Discussion
The DTI analysis in the present study showed that the FA in patients with ET was significantly decreased in the bilateral cerebellum, right pons, left retrorubral area of the midbrain, and bilateral deep WM. This study is believed to be the first to demonstrate structural changes of the WM in patients with ET.
In general, it appears that ET might be attributable to an abnormal oscillation of a CNS pacemaker; however, the exact oscillator can only be assumed. Data from previous experiments imply that ET might result from an oscillation within the cerebello-olivary pathway that is transmitted via the cerebellar peduncle to the thalamus and motor cortex.1 According to functional imaging studies in patients with ET, increased blood flow was observed in the olivary nucleus, cerebellum, red nucleus, thalamus, basal ganglia, and primary sensorimotor cortex.12 Interestingly, the WM showing decreased FA in our ET patients corresponded well with the fibers of the cerebello-thalamo-corticocerebellar loop, suggesting that the changes in WM demonstrated in our study may be involved in tremorogenesis pathway.
Although the pathophysiology of ET is not well understood, there are several lines of evidence suggesting that ET is a neurodegenerative disease. ET is clinically progressive, with complex clinical characteristics accompanied by cerebellar, as well as extracerebellar, signs, which may include olfactory deficits and cognitive dysfunction.13,14 Based on these data, Louis1 argued that pathologic lesions in ET may eventually spread over time to involve multiple brain regions, as in Parkinson disease or Alzheimer disease. Regarding the pathologic substrate of ET, a recent study reported that pathologic findings in ET patients were heterogenous and clustered into 2 groups: those with cerebellar changes and those with brain stem Lewy bodies.2 This finding agrees with that of an MR spectroscopic study showing that the N-acetyl-l-aspartate/creatine ratio within the cerebellum was decreased in patients with ET.2,3
Our results from a voxel-by-voxel comparison using SPM, which is a more objective analysis than a comparison of regions of interest, indicated that the WM pathology extended beyond the previously pathologic data, where WM pathology was restricted to efferent fibers in the cerebellum. There were few reports studying the WM abnormalities in patients with ET. Recently, Martinelli et al15 reported in a study with DWI that apparent diffusion coefficient (ADC) values in cerebellar and frontal WM and middle cerebellar peduncle were not significantly different from controls, arguing against major structural changes in the ET. However, DWI only measured in one direction can lead to an underestimation of diffusion-related pathologic changes, because the fiber tracts are not oriented in the same direction, whereas the trace of diffusion tensor is given by the average of ADCs measured in the 3 orthogonal directions.16,17 As pointed out by the authors, therefore, it is possible that DWI may not be sensitive enough to detect subtle structural changes in the ET.
The main neuropsychologic abnormality observed in patients with ET was associated with relative dysfunction of frontal lobe-mediating processes. Because similar neuropsychologic patterns have been observed with disruption of the frontosubcortical circuits or cerebellar disorders, it is hypothesized that cognitive dysfunction in ET patients may be related to an abnormality of the frontocerebellar circuit function.14 However, the exact anatomic and pathologic basis for the cognitive dysfunction observed in ET patients is unknown. Based on our data showing widespread WM pathology involving primarily the frontal area with some temporoparietal area, it is possible that WM pathology is the anatomic substrate for frontal executive dysfunction in ET patients. In addition, the decreased FA in the orbitofrontal WM may be associated with olfactory dysfunction in patients with ET13 via the disruption of the central processing of olfaction. Therefore, our results support the concept of ET as a degenerative disease. A larger study using DTI to analyze the association analysis between disease duration and laterality of ET and degree of WM changes would be helpful to further clarify the nature of ET.
In summary, DTI analysis in ET patients supports the findings of previous functional neuroimaging and pathologic studies. Moreover, the present study may suggest extensive structural changes of the WM in patients with ET.
Acknowledgments
This study was supported by grant 0412-DB00-010-0007 of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea.
Footnotes
D.H.S. and P.H.L. contributed equally.
References
- 1.Louis ED. Essential tremor. Lancet Neurol 2005;4:100–10 [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Shungu DC, Chan S, et al. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett 2002;333:17–20 [DOI] [PubMed] [Google Scholar]
- 3.Pagan FL, Butman JA, Dambrosia JM, et al. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology 2003;60:1344–47 [DOI] [PubMed] [Google Scholar]
- 4.Louis ED, Vonsattel JP, Honig LS, et al. Neuropathologic findings in essential tremor. Neurology 2006;66:1756–59 [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, Vonsattel JP, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol 2006;63:1189–93 [DOI] [PubMed] [Google Scholar]
- 6.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247–54 [DOI] [PubMed] [Google Scholar]
- 7.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–19 [DOI] [PubMed] [Google Scholar]
- 8.Padovani A, Borroni B, Brambati SM, et al. Diffusion tensor imaging and voxel based study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 2006;77:457–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findley LJ. Classification of tremors. J Clin Neurophysiol 1996;13:122–32 [DOI] [PubMed] [Google Scholar]
- 10.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–46 [DOI] [PubMed] [Google Scholar]
- 11.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995;2:189–10 [Google Scholar]
- 12.Bucher SF, Seelos KC, Dodel RC, et al. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol 1997;41:32–40 [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Bromley SM, Jurewicz EC, et al. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology 2002;59:1631–33 [DOI] [PubMed] [Google Scholar]
- 14.Lombardi WJ, Woolston DJ, Roberts WJ, et al. Cognitive deficits in patients with essential tremor. Neurology 2001;57:785–90 [DOI] [PubMed] [Google Scholar]
- 15.Martinelli P, Rizzo G, Manners D, et al. Diffusion-weighted imaging study of patients with essential tremor. Mov Disord 2007;22:1182–85 Epub Apr 27 [DOI] [PubMed] [Google Scholar]
- 16.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48 [DOI] [PubMed] [Google Scholar]
- 17.Hsu EW, Mori S. Analytical expressions for the NMR apparent diffusion coefficients in an anisotropic system and a simplified method for determining fiber orientation. Magn Reson Med 1995;34:194–200 [DOI] [PubMed] [Google Scholar]